Mineral Coating Enhances the Carbon Sequestration Capacity of Biochar Derived from Paulownia Biowaste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biowaste of Paulownia Branches
2.2. Preparation of Limewater
2.3. Experimental Design
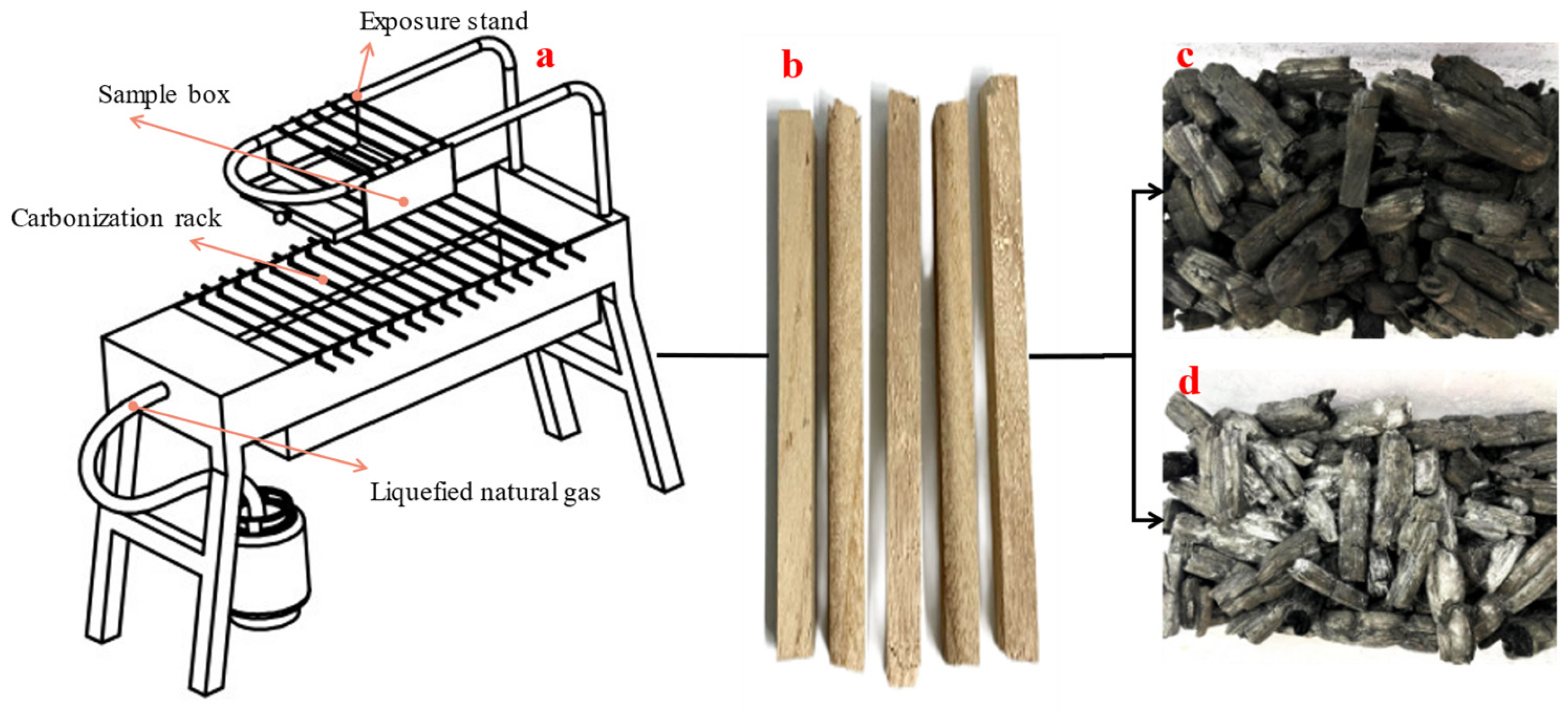
2.4. Aerobic Carbonization via Water–Fire Coupled Method
2.5. Sample Collection and Analysis
2.6. Data Processing
3. Results
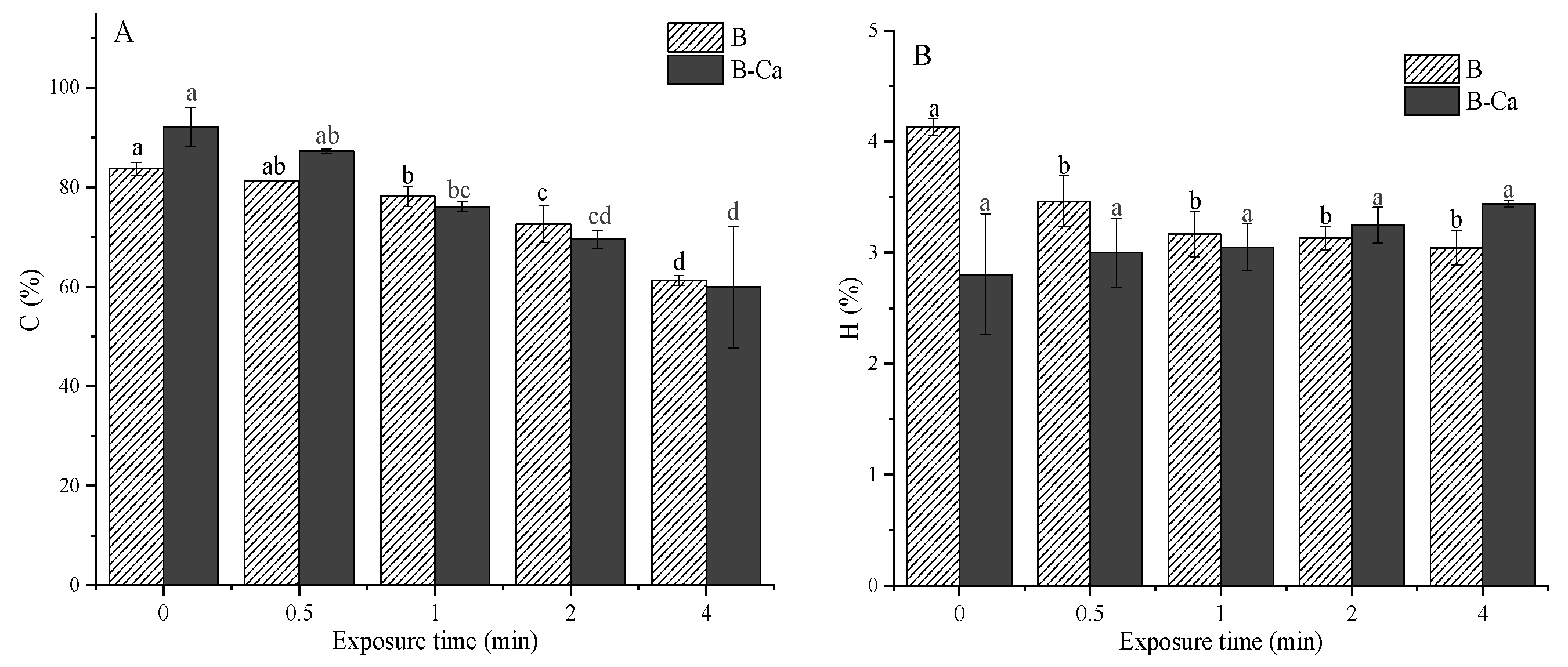
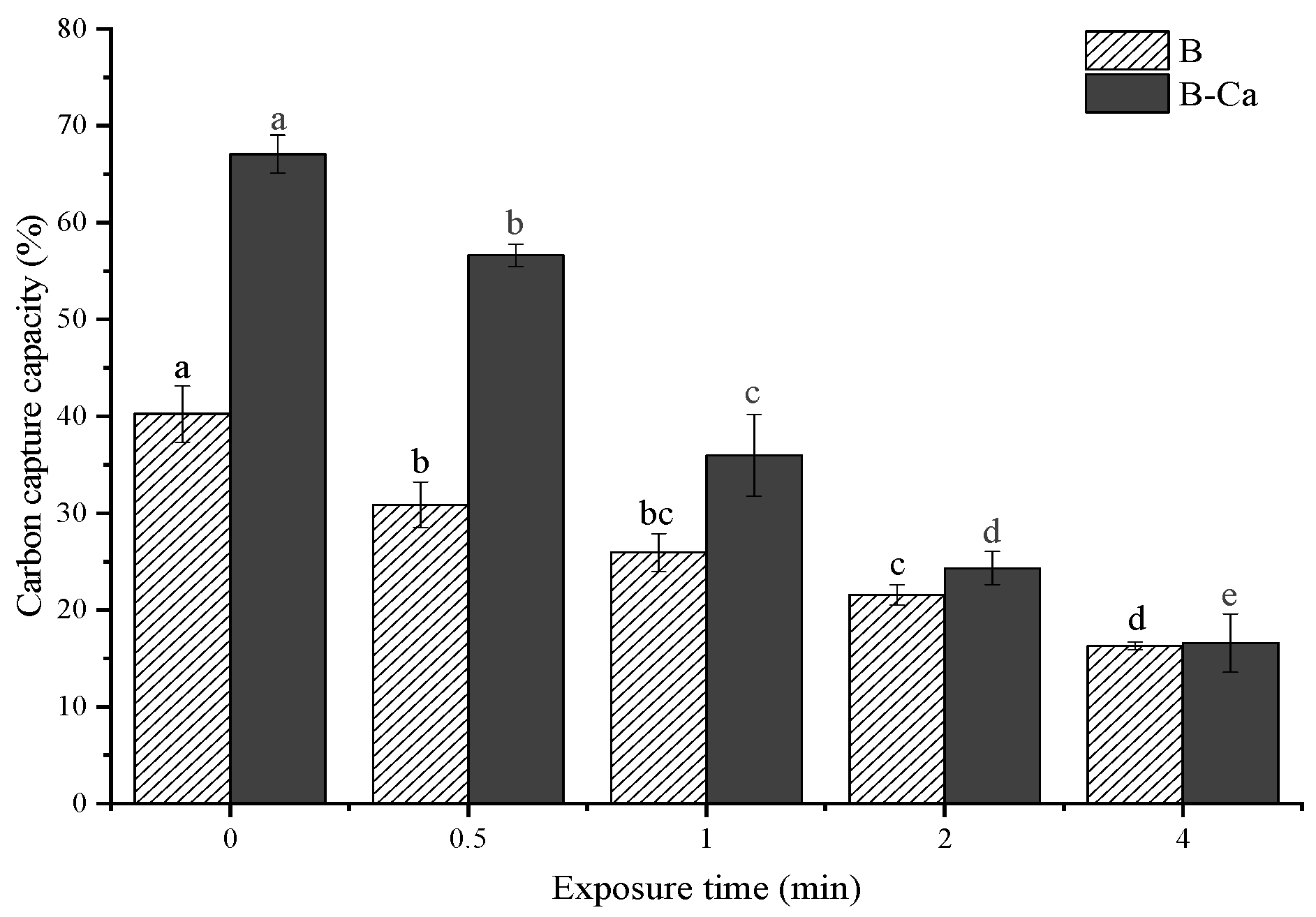
3.1. Opposite Effects of Exposure Time and Mineral Coating on Carbon Content and Carbon Capture Capacity of Paulownia Biochar
3.2. Mineral Coating and Exposure Time Affected Ash Content and pH of Paulownia Biochar
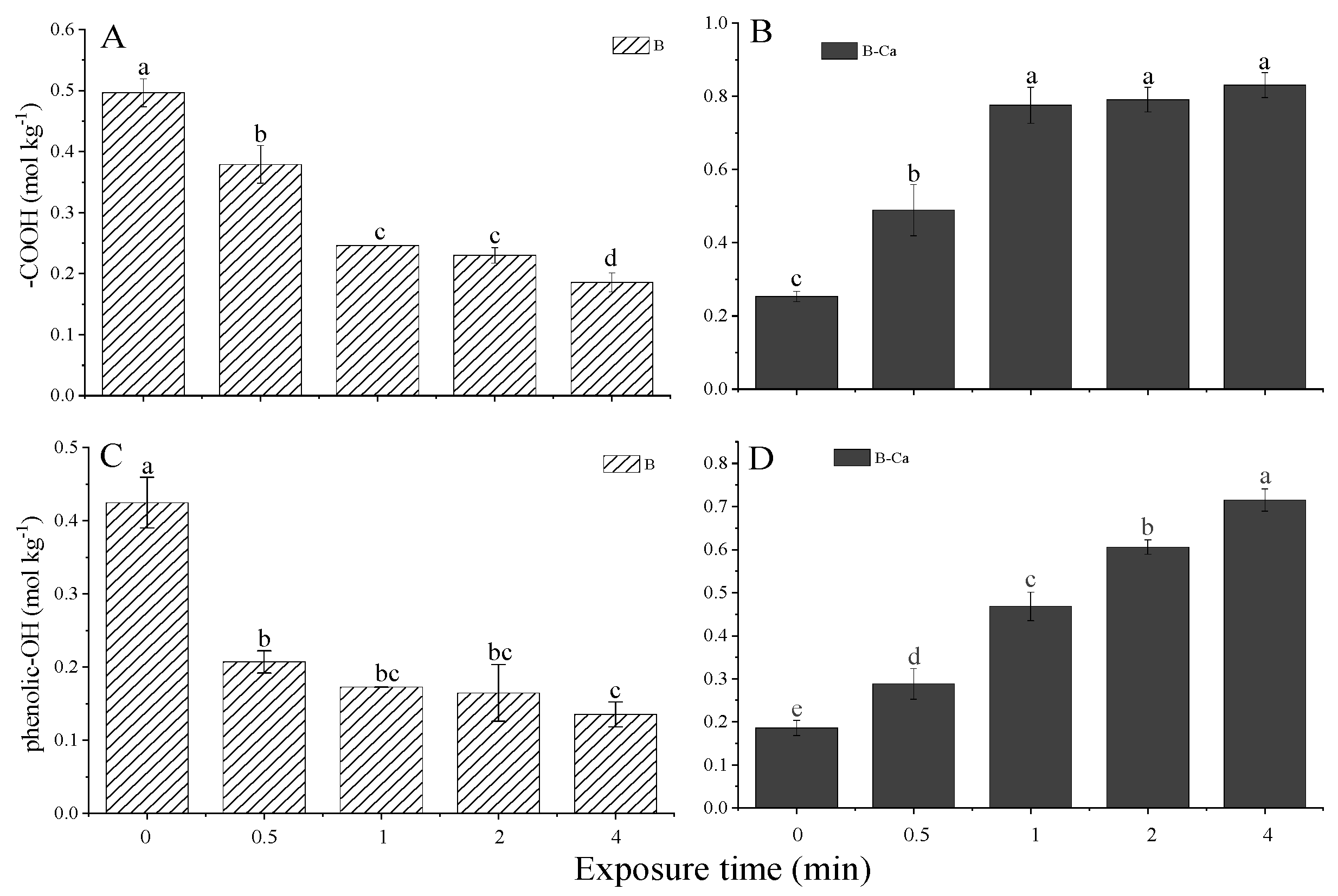
3.3. Mineral Coating and Exposure Time Affected the Specific Surface Area and Functional Groups of Paulownia Biochar
3.4. Correlation between Carbon Capture Capacity and Other Properties of Paulownia Biochar under 0 Exposure Time
4. Discussion
4.1. Exposure Time Deteriorates Paulownia Biochar Properties
4.2. Mineral Coating Enhances Biochar Properties of Paulownia Biochar
4.3. Innovative Techniques Improve the Carbon Sequestration Potential of Biochar
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boguta, P.; Cybulak, M.; Sokołowska, Z.; Zarzycki, R.; Kacprzak, A.; Kobyłecki, R. Quality and quantity of humic-like and fulvic-like acids entrapped in biochars–The effect of various forestry feedstock and pyrolysis temperature of biochars. Fuel 2023, 333, 126405. [Google Scholar] [CrossRef]
- Sadowska, U.; Zaleski, T.; Kuboń, M.; Latawiec, A.; Klimek-Kopyra, A.; Sikora, J.; Gliniak, M.; Kobyłecki, R.; Zarzycki, R. Effect of the application of sunflower biochar and leafy trees biochar on soil hydrological properties of fallow soils and under soybean cultivation. Materials 2023, 16, 1737. [Google Scholar] [CrossRef] [PubMed]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Hamidzadeh, Z.; Ghorbannezhad, P.; Ketabchi, M.R.; Yeganeh, B. Biomass-derived biochar and its application in agriculture. Fuel 2023, 41, 127701. [Google Scholar] [CrossRef]
- Dwibedi, S.K.; Pandey, V.C.; Divyasree, D.; Bajpai, O. Biochar-based land development. Land Degrad. Dev. 2022, 338, 1139–1158. [Google Scholar] [CrossRef]
- Safarian, S. To what extent could biochar replace coal and coke in steel industries? Fuel 2023, 339, 127401. [Google Scholar] [CrossRef]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in climate change mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Yang, H.; Doran, J.; Rooney, D.W. Industrial biochar systems for atmospheric carbon removal: A review. Environ. Chem. Lett. 2021, 194, 3023–3055. [Google Scholar] [CrossRef]
- Batista, R.; Jensen-Fellows, A.; Richard, B.; Sheridan, T. Biochar Market Profile Report; Worcester Polytechnic Institute: Worcester, MA, USA, 2021; pp. 1–62. [Google Scholar]
- Qifa, Z.; Houge, B.A.; Zhaohui, T.; Bin, G.; Guodong, L. An in-situ technique for producing low-cost agricultural biochar. Pedosphere 2018, 28, 690–695. [Google Scholar]
- Campbell, R.M.; Anderson, N.M.; Daugaard, D.E.; Naughton, H.T. Financial viability of biofuel and biochar production from forest biomass in the face of market price volatility and uncertainty. Appl. Energy 2018, 230, 330–343. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Ajumobi, O.; Dzidzienyo, P. Pyrolysis of date palm waste to biochar using concentrated solar thermal energy: Economic and sustainability implications. Waste Manag. 2019, 93, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Marousěk, J.; Vochozka, M.; Plachý, J.; Źák, J. Glory and misery of biochar. Clean Technol. Environ. Policy 2017, 19, 311–317. [Google Scholar] [CrossRef]
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar]
- Campion, L.; Bekchanova, M.; Malina, R.; Kuppens, T. The costs and benefits of biochar production and use: A systematic review. J. Clean. Prod. 2023, 408, 137138. [Google Scholar] [CrossRef]
- Wu, P.; Ata-UI-Karim, S.T.; Singh, B.P.; Wang, H.L.; Wu, T.; Liu, C.; Fang, G.; Zhou, D.; Wang, Y.; Chen, W. A scientometric review of biochar research in the past 20 years (1998–2018). Biochar 2019, 1, 23–43. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, D.; Wang, M.; Gong, T.; Sun, M.; Yang, T. A scientometric review of biochar preparation research from 2006 to 2019. Biochar 2021, 3, 283–298. [Google Scholar] [CrossRef]
- Gao, P.; Liu, Y.; Huang, X.; Abulaiti, A.; Yang, S. Effect of wet torrefaction on the physicochemical characteristics and gasification behavior of biochar. Ind. Crops Prod. 2023, 197, 116544. [Google Scholar] [CrossRef]
- Marris, E. Putting the carbon back: Black is the new green. Nature 2006, 442, 624–626. [Google Scholar] [CrossRef]
- Wardle, D.A.; Nilsson, M.C.; Zackrisson, O. Fire-derived charcoal causes loss of forest humus. Sci. Rep. 2008, 320, 629. [Google Scholar] [CrossRef]
- Xiao, L.; Feng, L.; Yuan, G.; Wei, J. Low-cost field production of biochars and their properties. Environ. Geochem. Health 2020, 42, 1569–1578. [Google Scholar] [CrossRef]
- Xiao, L.; Yuan, G.D.; Bi, D.X.; Wei, J.; Shen, G.H. Equipment and technology of field preparation of biochars from agricultural and forest residues under aerobic conditions with water-fire coupled method. Trans. Chin. Soc. Agric. Eng. 2019, 35, 239–244. [Google Scholar]
- Zhuo, S.N.; Dai, T.C.; Ren, H.Y.; Liu, B.F. Simultaneous adsorption of phosphate and tetracycline by calcium modified corn stover biochar: Performance and mechanism. Bioresour. Technol. 2022, 359, 127477. [Google Scholar] [CrossRef]
- Nan, H.; Yin, J.; Yang, F.; Luo, Y.; Zhao, L.; Cao, X. Pyrolysis temperature-dependent carbon retention and stability of biochar with participation of calcium: Implications to carbon sequestration. Environ. Pollut. 2021, 287, 117566. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into multiple and multilevel structures of biochars and their potential environmental applications: A critical review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef]
- Balmuk, G.; Videgain, M.; Manyà, J.J.; Duman, G.; Yanik, J. Effects of pyrolysis temperature and pressure on agronomic properties of biochar. J. Anal. Appl. Pyrolysis 2023, 169, 105858. [Google Scholar] [CrossRef]
- IHSS. Available online: https://ihss.humicsubstances.org/ (accessed on 1 July 2023).
- Song, S.; Cong, P.; Wang, C.; Li, P.; Liu, S.; He, Z.; Zhou, C.; Liu, Y.; Yang, Z. Properties of biochar obtained from tropical crop wastes under different pyrolysis temperatures and its application on acidic soil. Agronomy 2023, 13, 921. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; Van Hooijdonk, G.; Faaij, A.P. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Shafizadeh, F. Introduction to pyrolysis of biomass. J. Anal. Appl. Pyrolysis 1982, 3, 283–305. [Google Scholar] [CrossRef]
- Shanmugam, V.; Sreenivasan, S.N.; Mensah, R.A.; Försth, M.; Sas, G.; Hedenqvist, M.S.; Neisiany, R.E.; Tu, Y.; Das, O. A review on combustion and mechanical behaviour of pyrolysis biochar. Mater. Today Commun. 2022, 31, 103629. [Google Scholar] [CrossRef]
- Wang, R.; He, Q.; Guo, C.; Bao, Y.; He, X.; Cao, Y.; Li, P.; Huangpu, X. Adsorption characteristics of Fe/Ca loaded on rice straw biochar for phosphorus in septic tanks. J. Civil Environ. Eng. 2022, 45, 205–214. [Google Scholar]
- Ou, W.; Lan, X.; Guo, J.; Cai, A.; Liu, P.; Liu, N.; Liu, Y.; Lei, Y. Preparation of iron/calcium-modified biochar for phosphate removal from industrial wastewater. J. Clean. Prod. 2023, 383, 135468. [Google Scholar] [CrossRef]
- Pan, L.; Mao, L.; Zhang, H.; Wang, P.; Wu, C.; Xie, J.; Muhammad, U.S.; Zhang, L.; Zhang, Y.; Zhu, L.; et al. Modified biochar as a more promising amendment agent for remediation of pesticide-contaminated soils: Modification methods, mechanisms, applications, and future perspectives. Appl. Sci. 2022, 12, 11544. [Google Scholar] [CrossRef]
- Iamsaard, K.; Weng, C.H.; Yen, L.T.; Tzeng, J.H.; Poonpakdee, C.; Lin, Y.T. Adsorption of metal on pineapple leaf biochar: Key affecting factors, mechanism identification, and regeneration evaluation. Bioresour. Technol. 2022, 344, 126131. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Y.; Song, J.Z.; Peng, P.A.; Yu, C.L.; Li, K.M. Characterization of pyrolysis products in biochar prepared at different temperatures. Geochimica 2019, 48, 511–520. [Google Scholar]
- Liu, M.; Zhao, Z.; Lu, Q.; Yu, W. Release of dissolved organic carbon from biochar and formation of humic-like component during photoreaction: Effects of Ca2+ and pH. Water Res. 2022, 219, 118616. [Google Scholar] [CrossRef]
- Chen, Q.; Qin, J.; Cheng, Z.; Huang, L.; Sun, P.; Chen, L.; Shen, G. Synthesis of a stable magnesium-impregnated biochar and its reduction of phosphorus leaching from soil. Chemosphere 2018, 199, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z. China carbon neutral framework roadmap research. Chin. Ind. Inf. Technol. 2021, 8, 54–61. [Google Scholar]
- Wang, Y.J.; Wang, F.; Shi, Z.L.; Gao, C.Y.; Wang, H.Y.; Bi, Y.Y. Straw resources and its utilization in China from the perspective of agricultural supply-side structural reform. Chin. J. Agric. Resour. Reg. Plan. 2017, 38, 921. [Google Scholar]

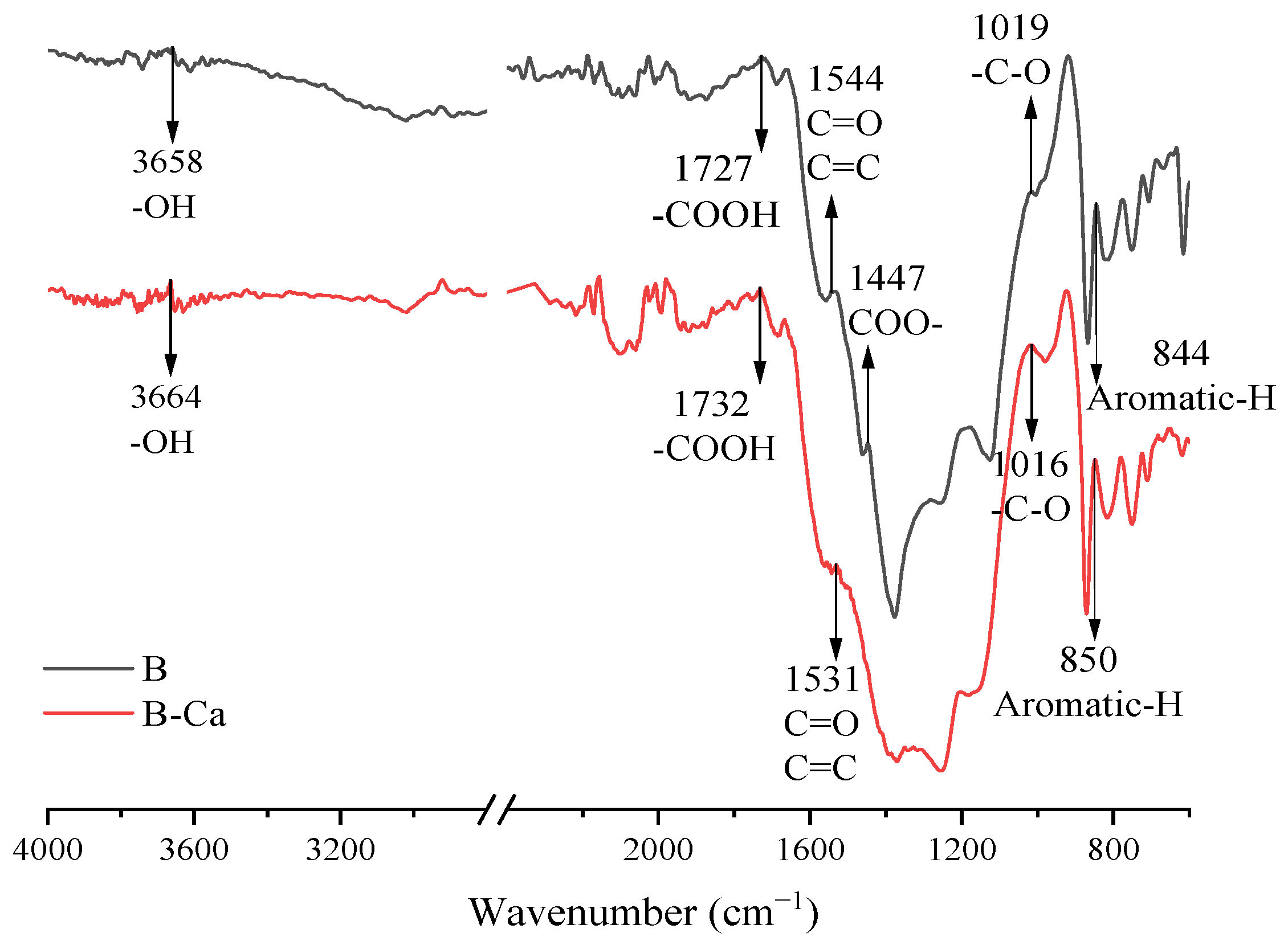
| Index | Treatments | Exposure Time (min) | ||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | 4 | ||
| Ash content (%) | B | 1.03 ± 0.23 c,* | 1.29 ± 0.10 c | 1.52 ± 0.07 c | 3.30 ± 0.98 b | 6.10 ± 0.81 a |
| B-Ca | 6.93 ± 1.48 c | 9.49 ± 0.29 bc | 11.59 ± 0.55 b | 16.81 ± 2.81 a | 18.49 ± 1.15 a | |
| pH | B | 7.96 ± 0.06 b | 8.13 ± 0.23 ab | 8.17 ± 0.26 ab | 8.23 ± 0.15 ab | 8.58 ± 0.38 a |
| B-Ca | 8.39 ± 0.24 a | 8.12 ± 0.04 ab | 8.05 ± 0.11 ab | 8.01 ± 0.04 ab | 7.97 ± 0.04 b | |
| Pyrolysis temperature (°C) | B | 564.9 ± 27.6 a | 457.6 ± 28.4 b | 385.9 ± 5.1 c | 143.7 ± 23.1 d | 47.3 ± 7.9 e |
| B-Ca | 623.6 ± 16.1 a | 457.3 ± 14.0 b | 424.5 ± 7.0 b | 347.5 ± 25.0 c | 117.8 ± 13.8 d | |
| Treatments | Dimension | Carbon Capture Capacity (%) | Ash Content (%) | pH | Specific Surface Areas (m2/g) | Phenolic-OH (mol/kg) | -COOH (mol/kg) |
|---|---|---|---|---|---|---|---|
| B | Carbon capture capacity (%) | 1 | |||||
| Ash content (%) | −0.819 ** | 1 | |||||
| pH | −0.698 * | 0.789 ** | 1 | ||||
| Specific surface areas (m2/g) | 0.938 ** | −0.906 ** | −0.770 ** | 1 | |||
| phenolic-OH (mol/kg) | 0.880 ** | −0.578 | −0.53 | 0.761 * | 1 | ||
| -COOH (mol/kg) | 0.958 ** | −0.699 * | −0.668 * | 0.907 ** | 0.930 ** | 1 | |
| B-Ca | Carbon capture capacity (%) | 1 | |||||
| Ash content (%) | −0.934 ** | 1 | |||||
| pH | 0.772 ** | −0.794 ** | 1 | ||||
| Specific surface areas (m2/g) | 0.964 ** | −0.905 ** | 0.812 ** | 1 | |||
| phenolic-OH (mol/kg) | −0.983 ** | 0.953 ** | −0.785 ** | −0.974 ** | 1 | ||
| -COOH (mol/kg) | −0.944 ** | 0.856 ** | −0.829 ** | −0.941 ** | 0.938 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Wu, J.; Li, W.; Yuan, G.; Xu, Q.; Wei, J.; Han, F. Mineral Coating Enhances the Carbon Sequestration Capacity of Biochar Derived from Paulownia Biowaste. Agronomy 2023, 13, 2361. https://doi.org/10.3390/agronomy13092361
Xiao L, Wu J, Li W, Yuan G, Xu Q, Wei J, Han F. Mineral Coating Enhances the Carbon Sequestration Capacity of Biochar Derived from Paulownia Biowaste. Agronomy. 2023; 13(9):2361. https://doi.org/10.3390/agronomy13092361
Chicago/Turabian StyleXiao, Liang, Jinghua Wu, Wenhan Li, Guodong Yuan, Qing Xu, Jing Wei, and Fengxiang Han. 2023. "Mineral Coating Enhances the Carbon Sequestration Capacity of Biochar Derived from Paulownia Biowaste" Agronomy 13, no. 9: 2361. https://doi.org/10.3390/agronomy13092361






