Exploring the Potential of Wood Vinegar: Chemical Composition and Biological Effects on Crops and Pests
Abstract
:1. Introduction
- Wood vinegar would exhibit acidity, with a chemical composition rich in biologically active compounds, including acetic acid, phenols, and cresol.
- The application of wood vinegar would result in a reduction in the infestation levels of B. oleae within olive orchards.
- Wood vinegar would exhibit concentration-dependent effects on crops, manifesting phytotoxicity at elevated concentrations and biostimulation when significantly diluted.
2. Materials and Methods
2.1. Wood Vinegar Production
2.2. Chemical and Metabolomic Analysis
2.3. Effect of Wood Vinegar on Bactrocera oleae under Field Conditions
2.4. Effect of Wood Vinegar on Strawberry Plants
2.5. Effect of Wood Vinegar on Meloidogyne incognita in the Strawberry Field
2.6. Crop Phytotoxicity Bioassay
2.7. Statistical Analysis
3. Results
3.1. Wood Vinegar Chemistry and Metabolomics
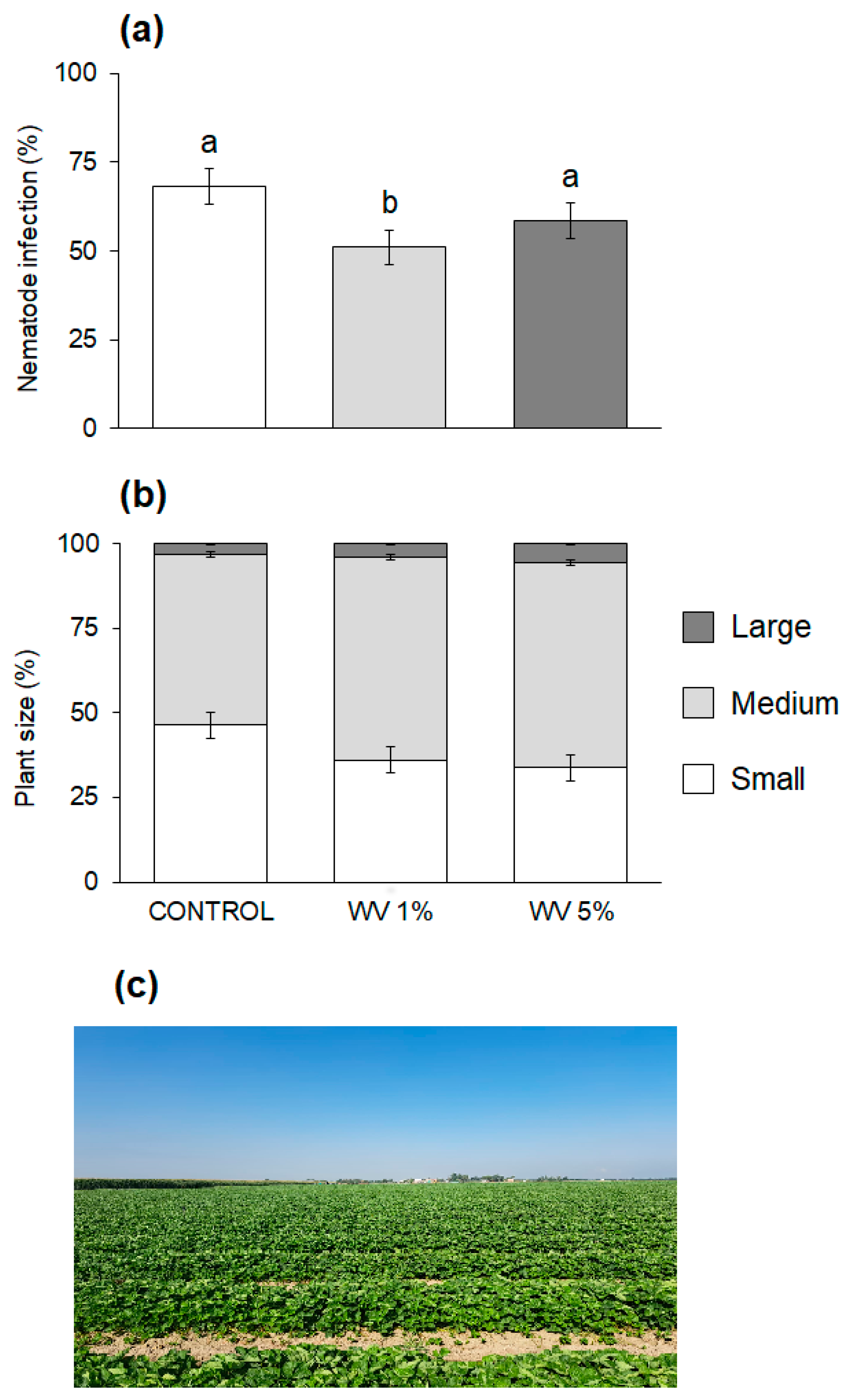
3.2. Effects of Wood Vinegar on Bactrocera oleae
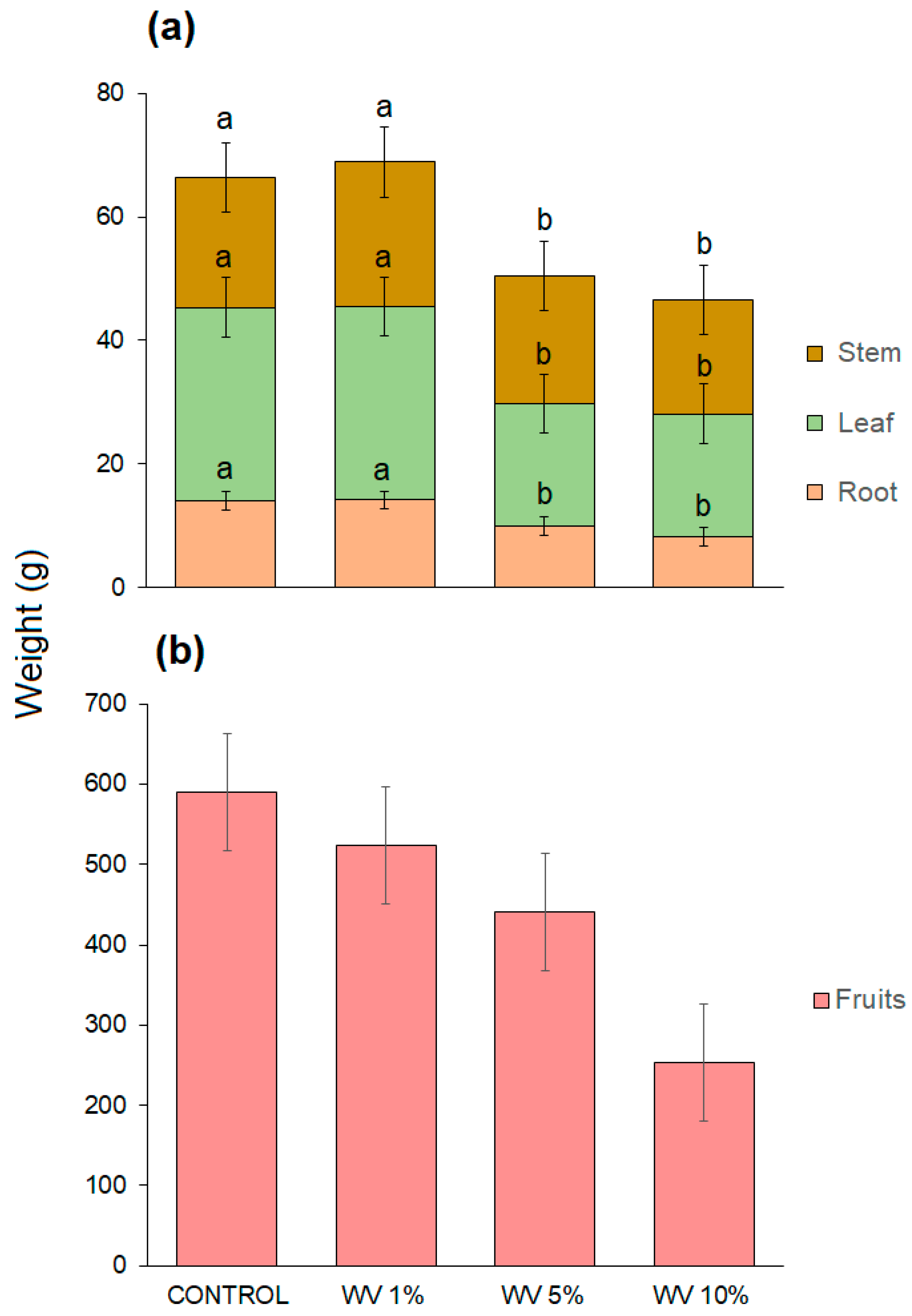
3.3. Effects of Wood Vinegar on Strawberry Plants
3.4. Effects of Wood Vinegar on Meloidogyne incognita
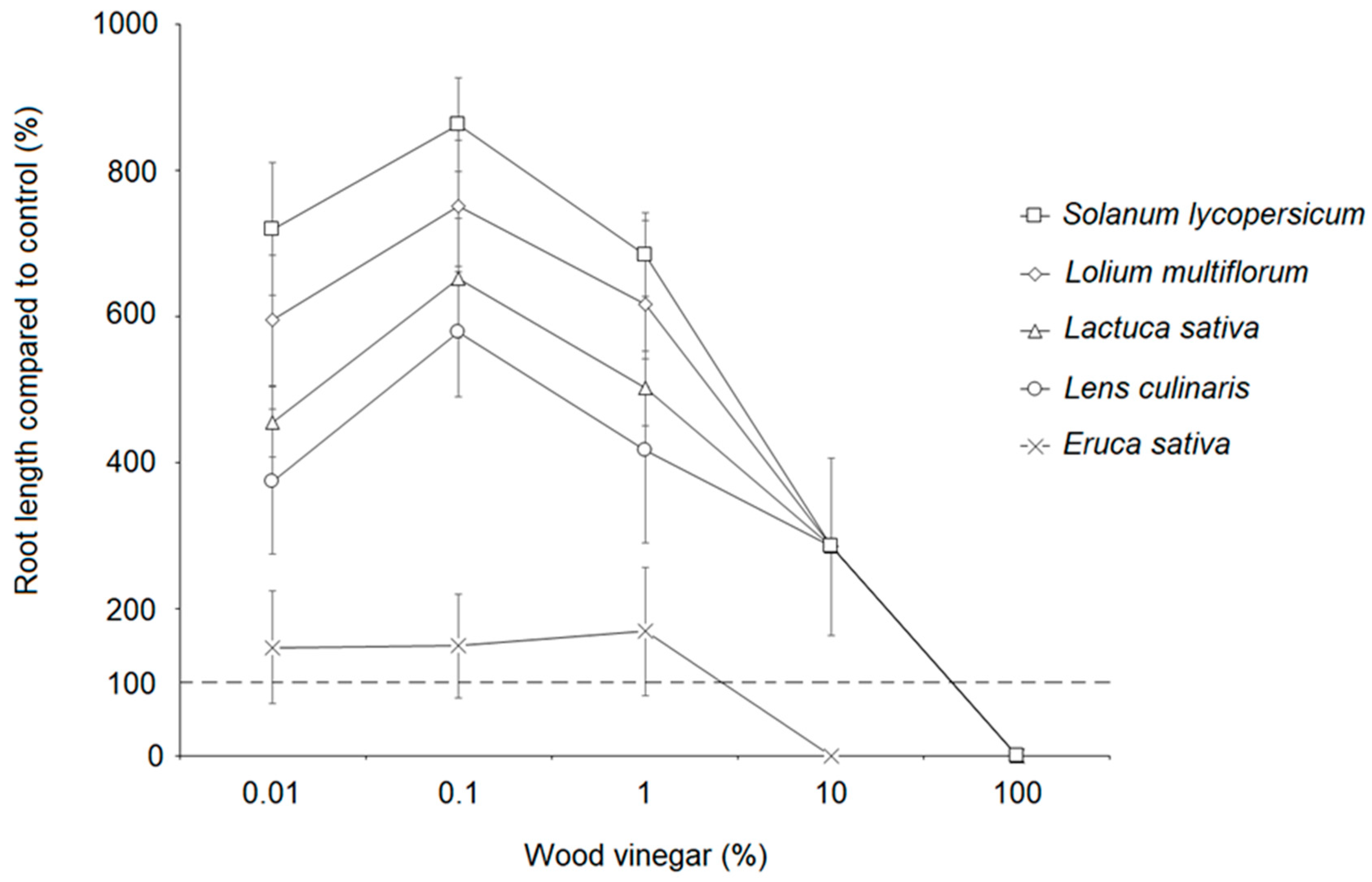
3.5. Plant Bioassay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Abingdon-on-Thames, UK, 2015. [Google Scholar]
- Pennise, D.M.; Smith, K.R.; Kithinji, J.P.; Rezende, M.E.; Raad, T.J.; Zhang, J.; Fan, C. Emissions of greenhouse gases and other airborne pollutants from charcoal making in Kenya and Brazil. J. Geophys. Res. 2001, 106, 143–155. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenerg. 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Vamvuka, D. Bio-oil, solid and gaseous biofuels from biomass pyrolysis processes—An overview. Int. J. Energy Res. 2011, 35, 835–862. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Saravanan, A.; Varjani, S.; Ramamurthy, R. Bioconversion of municipal solid waste into bio-based products: A review on valorisation and sustainable approach for circular bioeconomy. Sci. Total Environ. 2020, 748, 141312. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Light, M.E.; Van Staden, J. Plant-derived smoke: Old technology with possibilities for economic applications in agriculture and horticulture. S. Afr. J. Bot. 2011, 77, 972–979. [Google Scholar] [CrossRef]
- Sparg, S.G.; Kulkarni, M.G.; Van Staden, J. Aerosol smoke and smoke-water stimulation of seedling vigor of a commercial maize cultivar. Crop Sci. 2006, 46, 1336–1340. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A compound from smoke that promotes seed germination. Science 2004, 305, 977. [Google Scholar] [CrossRef]
- Van Staden, J.; Jager, A.K.; Light, M.E.; Burger, B.V. Isolation of the major germination cue from plant-derived smoke. S. Afr. J. Bot. 2004, 70, 654–659. [Google Scholar] [CrossRef]
- Soós, V.; Sebestyén, E.; Juhász, A.; Pintér, J.; Light, M.E.; Van Staden, J.; Balázs, E. Stress-related genes define essential steps in the response of maize seedlings to smoke-water. Funct. Integr. Genom. 2009, 9, 231–242. [Google Scholar] [CrossRef]
- De Lange, J.H.; Boucher, C. Autecological studies on Audouinia capitata (Bruniaceae). I. Plant-derived smoke as a seed germination cue. S. Afr. J. Bot. 1990, 56, 700–703. [Google Scholar] [CrossRef]
- Bonanomi, G.; Jesu, M.; Zotti, M.; Idbella, G.; D’Errico, S.; Laudonia, F.; Vinale, A. Abd-ElGawad Biochar-derived smoke-water exerts biological effects on nematodes, insects, and higher plants but not fungi. Sci. Total Environ. 2021, 750, 142307. [Google Scholar] [CrossRef] [PubMed]
- Desvita, H.; Faisal, M. Antimicrobial potential of wood vinegar from cocoa pod shells (Theobroma cacao L.) against Candida albicans and Aspergillus niger. Mater. Today Proc. 2022, 63, S210–S213. [Google Scholar] [CrossRef]
- Oramahi, H.A.; Yoshimura, T.; Diba, F.; Setyawati, D. Antifungal and antitermitic activities of wood vinegar from oil palm trunk. J. Wood Sci. 2018, 64, 311–317. [Google Scholar] [CrossRef]
- Lingbeck, J.M.; Cordero, P.; O’Bryan, C.A.; Johnson, M.G.; Ricke, S.C.; Crandall, P.G. Functionality of liquid smoke as an all-natural antimicrobial in food preservation. Meat Sci. 2014, 97, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Saloko, S.; Darmadji, P.; Setiaji, B.; Pranoto, Y. Antioxidative and antimicrobial activities of liquid smoke nanocapsules using chitosan and maltodextrin and its application on tuna fish preservation. Food Biosci. 2014, 7, 71–79. [Google Scholar] [CrossRef]
- Imaningsih, W.; Junaidi, A.B.; Adventaria, D. Inhibitory effect of ulin wood liquid smoke and gogo rice endophytic fungi against pathogen Pyricularia oryzae. BIOTROPIA-Southeast Asian J. Trop. Biol. 2022, 29. [Google Scholar] [CrossRef]
- Faisa, M.; Chamzurni, T.; Daimon, H. A study on the effectiveness of liquid smoke produced from palm kernel shells in inhibiting black pod disease in cacao fruit in vitro. GEOMATE J. 2018, 14, 36–41. [Google Scholar]
- Adfa, M.; Hattori, Y.; Yoshimura, T.; Koketsu, M. Antitermite activity of 7-alkoxycoumarins and related analogs against Coptotermes formosanus Shiraki. Int. Biodeterior. Biodegrad. 2012, 74, 129–135. [Google Scholar] [CrossRef]
- Pangnakorn, U.; Kanlaya, S.; Kuntha, C. Efficiency of wood vinegar and extracts from some medicinal plants on insect control. Adv. Environ. Biol. 2011, 47, 477–483. [Google Scholar]
- Abbas, M.N.; Sajeel, M.; Kausar, S. House fly (Musca domestica), a challenging pest; biology, management and control strategies. Elixir Entomol. 2013, 64, 19333–19338. [Google Scholar]
- UNI EN 13037; Soil Improvers and Growing Media. Determination of pH. UNI Ente Italiano di Normazione: Milano, Italy, 2012.
- UNI EN 13038; Soil Improvers and Growing Media. Determination of Electrical Conductivity. UNI Ente Italiano di Normazione: Milano, Italy, 2012.
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. Identification of alkyl substituted 2 H-furo [2,3-c] pyran-2-ones as germination stimulants present in smoke. J. Agric. Food Chem. 2009, 57, 9475–9480. [Google Scholar] [CrossRef] [PubMed]
- Staropoli, A.; Vassetti, A.; Salvatore, M.M.; Andolfi, A.; Prigigallo, M.I.; Bubici, G.; Scagliola, M.; Salerno, P.; Vinale, F. Improvement of nutraceutical value of parsley leaves (Petroselinum crispum) upon field applications of beneficial microorganisms. Horticulturae 2021, 7, 281. [Google Scholar] [CrossRef]
- Staropoli, A.; Iacomino, G.; De Cicco, P.; Woo, S.L.; Di Costanzo, L.; Vinale, F. Induced secondary metabolites of the beneficial fungus Trichoderma harzianum M10 through OSMAC approach. Chem. Biol. Technol. Agric. 2023, 10, 28. [Google Scholar] [CrossRef]
- Saour, G.; Makee, H. A kaolin-based particle film for suppression of the olive fruit fly Bactrocera oleae Gmelin (Dip., Tephritidae) in olive groves. J. Appl. Entomol. 2004, 128, 28–31. [Google Scholar] [CrossRef]
- Hallman, J.; Viaene, N. PM 7/119(1) Nematode extraction. EPPO Bull. 2013, 43, 471–495. [Google Scholar]
- Scarpati, M.L.; Scalzo, R.L.; Vita, G. Olea europaea volatiles attractive and repellent to the olive fruit fly (Dacus oleae, Gmelin). J. Chem. Ecol. 1993, 19, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Liscia, A.; Angioni, P.; Sacchetti, P.; Poddighe, S.; Granchietti, A.; Setzu, M.D.; Belcari, A. Characterization of olfactory sensilla of the olive fly: Behavioral and electrophysiological responses to volatile organic compounds from the host plant and bacterial filtrate. J. Insect Physiol. 2013, 59, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Jesu, G.; Laudonia, S.; Bonanomi, G.; Flematti, G.; Germinara, S.G.; Pistillo, M.; Giron, D.; Bèzier, A.; Vinale, F. Biochar-Derived Smoke Waters Affect Bactrocera oleae Behavior and Control the Olive Fruit Fly under Field Conditions. Agronomy 2022, 12, 2834. [Google Scholar] [CrossRef]
- Stanczyk, N.M.; Brookfield, J.F.Y.; Field, L.M.; Logan, J.G. Aedes aegypti Mosquitoes Exhibit Decreased Repellency by DEET following Previous Exposure. PLoS ONE 2013, 8, e54438. [Google Scholar] [CrossRef]
- Graber, E.R.; Meller Harel, Y.; Kolton, M.; Cytryn, E.; Silber, A.; Rav David, D.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Chiwocha, S.D.; Dixon, K.W.; Flematti, G.R.; Ghisalberti, E.L.; Merritt, D.J.; Nelson, D.C.; Stevens, J.C. Karrikins: A new family of plant growth regulators in smoke. Plant Sci. 2009, 177, 252–256. [Google Scholar] [CrossRef]
- Mahler, R.L.; McDole, R.E. Effect of soil pH on crop yield in northern idaho. Agron. J. 1987, 79, 751–755. [Google Scholar] [CrossRef]
- Armstrong, J.; Armstrong, W. Phragmites die-back: Toxic effects of propionic, butyric and caproic acids in relation to pH. New Phytol. 1999, 142, 201–217. [Google Scholar] [CrossRef]
- Bolan, N.S.; Duraisamy, V.P. Role of inorganic and organic soil amendments on immobilisation and phytoavailability of heavy metals: A review involving specific case studies. Soil Res. 2003, 41, 533–555. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, Y.; Li, X.; Li, Y.; Feng, X.; Bagavathiannan, M.; Yu, J. The use of wood vinegar as a non-synthetic herbicide for control of broadleaf weeds. Ind. Crops Prod. 2021, 173, 114105. [Google Scholar] [CrossRef]
- Chu, L.; Liu, H.; Zhang, Z.; Zhan, Y.; Wang, K.; Yang, D.; Liu, Z.; Yu, J. Evaluation of Wood Vinegar as an Herbicide for Weed Control. Agronomy 2022, 12, 3120. [Google Scholar] [CrossRef]
- Hao, Z.; Bagavathiannan, M.; Li, Y.; Qu, M.; Wang, Z.; Yu, J. Wood vinegar for control of broadleaf weeds in dormant turfgrass. Weed Technol. 2021, 35, 901–907. [Google Scholar] [CrossRef]
- Marra, R.; Vinale, F.; Cesarano, G.; Lombardi, N.; d’Errico, G.; Crasto, A.; Mazzei, P.; Piccolo, A.; Incerti, G.; Woo, S.L.; et al. Biochars from olive mill waste have contrasting effects on plants, fungi and phytoparasitic nematodes. PLoS ONE 2018, 13, e0198728. [Google Scholar] [CrossRef]

| Parameter | Unit | Result |
|---|---|---|
| Acetic acid | mg L−1 | 27,840.1 |
| Propionic acid | mg L−1 | 72,447.0 |
| Phenols | mg L−1 | 54.1 |
| pH | - | 3.2 |
| Electrical conductivity | µS cm−1 | 1389.1 |
| Total suspended solids | mg L−1 | 26.0 |
| Ammonia nitrogen | mg L−1 | 8.3 |
| Sulfates | mg L−1 | 53.7 |
| Sulfites | mg L−1 | 0.32 |
| Sulfides | mg L−1 | 0.12 |
| Chlorides | mg L−1 | 29.2 |
| Total phosphorus | mg L−1 | 0.97 |
| Nitrates | mg L−1 | 5.2 |
| Aluminum | mg L−1 | 0.92 |
| Arsenic | mg L−1 | 0.02 |
| Iron | mg L−1 | 183 |
| Manganese | mg L−1 | 1.11 |
| Total organic carbon | mg L−1 | 15,600.4 |
| Hydrocarbons | mg L−1 | 77.1 |
| Acetone | mg L−1 | 320.0 |
| Name | Formula | Monoisotopic Mass (Da) | RT (min) |
|---|---|---|---|
| Nicotinic acid | C6H5NO2 | 123.0318 | 1.475 |
| Malvalic acid | C18H32O2 | 280.2424 | 6.697 |
| Name | RT (min) |
|---|---|
| Phenol, TMS derivative | 5.617 |
| o-Cresol, TMS derivative | 6.673 |
| m-Cresol, TMS derivative | 6.8 |
| p-Cresol, TMS derivative | 6.938 |
| Guaiacol, TMS derivative | 8 |
| Diethylene glycol, 2TMS derivative | 8.231 |
| Glycerol, 3TMS derivative | 8.699 |
| 2-Methyl-4-oxo-3,4-dihydro-2H-pyran-3-yl isobutyrate | 8.834 |
| Catechol, 2TMS derivative | 9.279 |
| 2,5-Dimethyl-4-pyrimidinamine, TMS derivative | 9.575 |
| 4-Methylcatechol, 2TMS derivative | 10.242 |
| Vanillin, TMS derivative | 10.271 |
| Syringol, TMS derivative | 10.374 |
| Protocatechuic aldehyde, 2TMS derivative | 11.141 |
| 4-Ethylsyingol, TMS derivative | 12.035 |
| Eugenol, TMS derivative | 12.161 |
| m-Ethoxycarbonylaniline | 12.301 |
| 2-Methoxymandelic acid, ethyl ester, TMS derivative | 13.429 |
| Syringaldehyde, TMS derivative | 13.785 |
| Vanillic acid, 2TMS derivative | 13.838 |
| Propyl vanillate, TMS derivative | 14.586 |
| Palmitic acid, TMS derivative | 15.24 |
| Oleic acid, (Z)-, TMS derivative | 15.301 |
| Stearic acid, TMS derivative | 16.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacomino, G.; Idbella, M.; Staropoli, A.; Nanni, B.; Bertoli, T.; Vinale, F.; Bonanomi, G. Exploring the Potential of Wood Vinegar: Chemical Composition and Biological Effects on Crops and Pests. Agronomy 2024, 14, 114. https://doi.org/10.3390/agronomy14010114
Iacomino G, Idbella M, Staropoli A, Nanni B, Bertoli T, Vinale F, Bonanomi G. Exploring the Potential of Wood Vinegar: Chemical Composition and Biological Effects on Crops and Pests. Agronomy. 2024; 14(1):114. https://doi.org/10.3390/agronomy14010114
Chicago/Turabian StyleIacomino, Giuseppina, Mohamed Idbella, Alessia Staropoli, Bruno Nanni, Tomaso Bertoli, Francesco Vinale, and Giuliano Bonanomi. 2024. "Exploring the Potential of Wood Vinegar: Chemical Composition and Biological Effects on Crops and Pests" Agronomy 14, no. 1: 114. https://doi.org/10.3390/agronomy14010114








