Response of Nitrification and Crop Yield to the Presence of NBPT and DCD in a Wheat-Corn Double Cropping System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Experimental Design
2.2. Soil Sample Analysis
2.3. DNA Extraction and Quantitative PCR (qPCR)
2.4. Data Processing
3. Results
3.1. Soil -N and -N Content
3.2. Abundance of Ammonia-Oxidizing Microorganisms
3.2.1. Abundance of the AOA amoA Gene
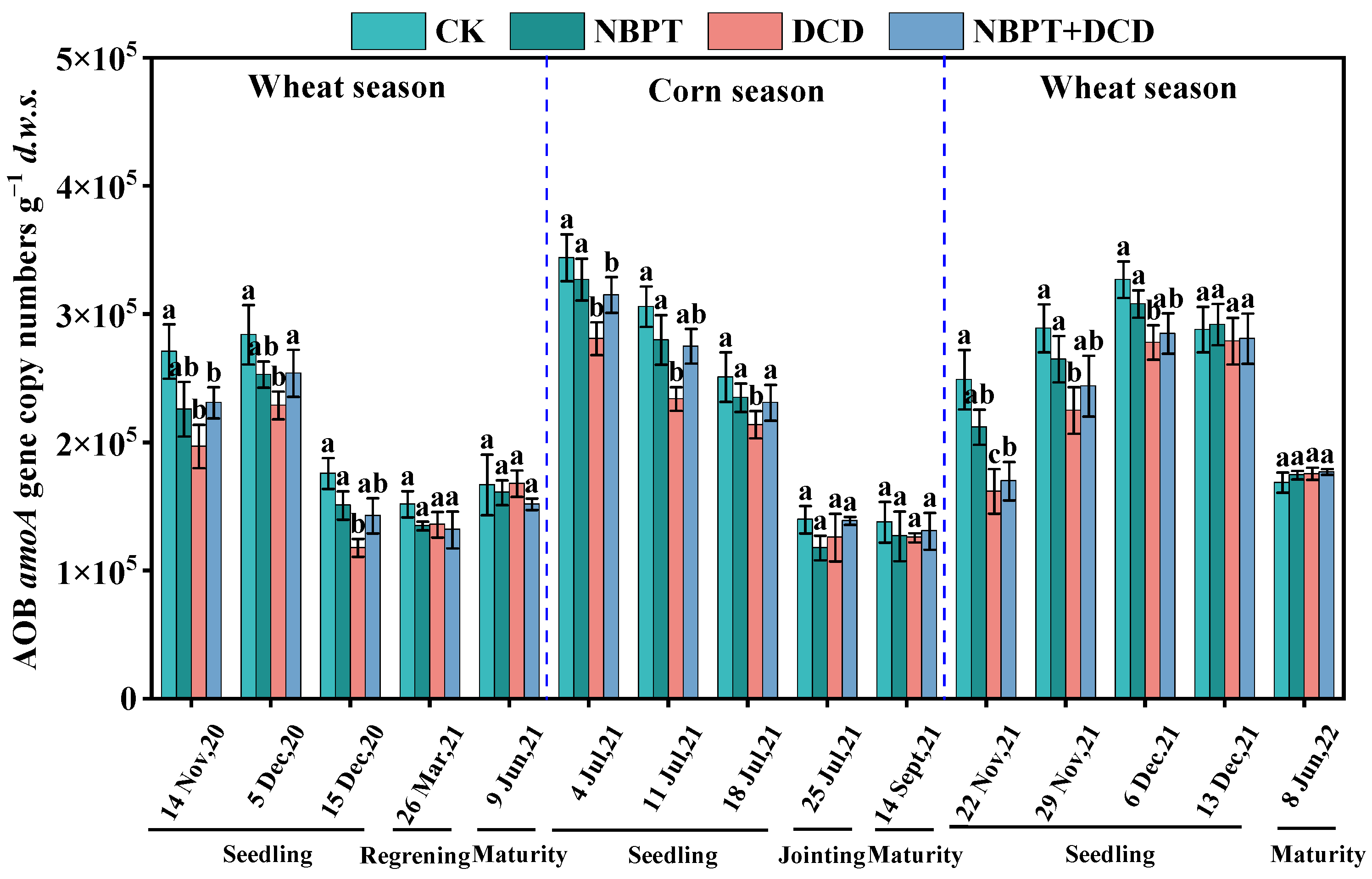
3.2.2. Abundance of the AOB amoA Gene
3.2.3. Abundance of the comammox amoA Gene
3.3. Relationships between Nitrification Activity and the Abundance of Ammonia Oxidizers
3.4. Crop Yield
4. Discussion
4.1. Effects of Nitrification Inhibitors on Soil Mineral N
4.2. Effects of NBPT and DCD on Ammonia-Oxidizing Microorganisms
4.3. Correlation between Nitrifying Communities and Nitrification Activity
4.4. Effect of Fertilizer Inhibitors on Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kits, K.; Sedlacek, C.; Lebedeva, E.; Han, P.; Bulaev, A.; Pjevac, P.; Daebeler, A.; Romano, S.; Albertsen, M.; Stein, L.; et al. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature 2017, 549, 269–272. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, Y.; Zhu-Barker, X.; Nicol, G.; Wright, A.; Jia, Z.; Jiang, X. Comammox Nitrospira clade B contributes to nitrification in soil. Soil Biol. Biochem. 2019, 135, 392–395. [Google Scholar] [CrossRef]
- Ssenyonga, P.; Umemiya, Y. Amount of nitrogen and phosphorus fertilizer required to optimize growth and yield of rice. Afr. J. Agric. Res. 2021, 17, 829–835. [Google Scholar] [CrossRef]
- Singh, B.; Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Ren, B.; Huang, Z.; Liu, P.; Zhao, B.; Zhang, J. Urea ammonium nitrate solution combined with urease and nitrification inhibitors jointly mitigate NH3 and N2O emissions and improves nitrogen efficiency of summer maize under fertigation. Field Crops Res. 2023, 296, 108909. [Google Scholar] [CrossRef]
- Abdo, A.; Xu, Y.; Shi, D.; Li, J.; Li, H.; El-Sappah, A.; Elrys, A.; Alharbi, S.; Zhou, C.; Wang, L.; et al. Nitrogen transformation genes and ammonia emission from soil under biochar and urease inhibitor application. Soil Tillage Res. 2022, 223, 105491. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Yao, H.; Wang, J.; Dai, F.; Wu, Y.; Chapman, S. Nitrification and urease inhibitors improve rice nitrogen uptake and prevent denitrification in alkaline paddy soil. Appl. Soil Ecol. 2020, 154, 103665. [Google Scholar] [CrossRef]
- Luchibia, A.; Suter, H.C.; Hu, H.; Lam, S.; He, J.-Z. Responses of ureolytic and nitrifying microbes to urease and nitrification inhibitors in selected agricultural soils in Victoria, Australia. J. Soils Sediments 2020, 20, 1309–1322. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Ma, S.; Zheng, X.; Wang, Z.; Lu, C. Effects of commonly used nitrification inhibitors—Dicyandiamide (DCD), 3,4-dimethylpyrazole phosphate (DMPP), and nitrapyrin—On soil nitro-gen dynamics and nitrifiers in three typical paddy soils. Geoderma 2020, 380, 114637. [Google Scholar] [CrossRef]
- Avrahami, S.; Conrad, R.; Braker, G. Effect of Soil Ammonium Concentration on N2O Release and on the Community Structure of Ammonia Oxidizers and Denitrifiers. Appl. Environ. Microbiol. 2002, 68, 5685–5692. [Google Scholar] [CrossRef]
- Kelliher, F.; van Koten, C.; Kear, M.J.; Sprosen, M.S.; Ledgard, S.; De Klein, C.; Letica, S.; Luo, J.; Rys, G. Effect of temperature on dicyandiamide (DCD) longevity in pastoral soils under field conditions. Agric. Ecosyst. Environ. 2014, 186, 201–204. [Google Scholar] [CrossRef]
- Kim, D.G.; Giltrap, D.; Saggar, S.; Palmada, T.; Berben, P.; Drysdale, D. Factors controlling disappearance of nitrification inhibitor, dicyandiamide (DCD) in a grazed pasture soil in Manawatu. In Proceedings of the Workshop “Advanced Nutrient Management: Gains from the Past–Goals for the Future, Palmerston North, New Zealand, 7–9 February 2012. [Google Scholar]
- Fu, Q.; Abadie, M.; Blaud, A.; Carswell, A.; Misselbrook, T.; Clark, I.; Hirsch, P. Effects of urease and nitrification inhibitors on soil N, nitrifier abundance and activity in a sandy loam soil. Biol. Fertil. Soils 2020, 56, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Lehtovirta-Morley, L.E.; Verhamme, D.T.; Nicol, G.W.; Prosser, J.I. Effect of nitrification inhibitors on the growth and activity of Nitrosotalea devanaterra culture and soil. Soil Biol. Biochem. 2013, 62, 129–133. [Google Scholar] [CrossRef]
- Duff, A.; Forrestal, P.; Ikoyi, I.; Brennan, F. Assessing the long-term impact of urease and nitrification inhibitor use on microbial community composition, diversity and function in grassland soil. Soil Biol. Biochem. 2022, 170, 108709. [Google Scholar] [CrossRef]
- Li, C.; Hu, H.; Chen, Q.; Chen, D.; He, J.-Z. Growth of comammox Nitrospira is inhibited by nitrifica-tion inhibitors in agricultural soils. J. Soils Sediments 2020, 20, 621–628. [Google Scholar] [CrossRef]
- Zaman, M.; Saggar, S.; Blennerhassett, J.D.; Singh, J. Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biol. Biochem. 2009, 41, 1270–1280. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Z.; Du, J.; He, A.; Yang, H.; Xue, G.; Yu, C.; Zhang, Y. Adding NBPT to urea increases N use efficiency of maize and decreases the abundance of N-cycling soil microbes under reduced fertilizer-N rate on the North China. Plain PLoS ONE 2020, 15, e0240925. [Google Scholar] [CrossRef] [PubMed]
- Rotthauwe, J.; Witzel, K.; Liesack, W. The Ammonia Monooxygenase Structural Gene amoA as a Functional Marker: Molecular Fine-Scale Analysis of Natural Ammonia-Oxidizing Populations. Appl. Environ. Microbiol. 1998, 63, 4704–4712. [Google Scholar] [CrossRef]
- Long, X.-E.; Chen, C.; Xu, Z.; Oren, R.; He, J.-Z. Abundance and community structure of ammo-nia-oxidizing bacteria and archaea in a temperate forest ecosystem under ten-years elevated CO2. Soil Biol. Biochem. 2012, 46, 163–171. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, G.; Wang, M.; Zhou, N.; He, S.; Dang, C.; Wang, J.; Zheng, M. Abundance and community composition of comammox bacteria in different ecosystems by a universal primer set. Sci. Total Environ. 2019, 691, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.; Sören, T.-B. Major metabolites of NBPT degradation pathways contribute to urease inhibition in soil. Chemosphere 2022, 303, 135163. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Suter, H.C.; Mosier, A.; Chen, D. Using nitrification inhibitors to mitigate agricultural N2O emission: A double-edged sword? Glob. Chang. Biol. 2016, 23, 485–489. [Google Scholar] [CrossRef]
- Tufail, M.; Naeem, A.; Arif, M.; Farooq, T.; Shahzad, S.; Dar, A.A.; Albasher, G.; Shakoor, A. Unraveling the efficacy of nitrification inhibitors (DCD and DMPP) in reducing nitrogen gases emissions across agroecosystems: A three-decade global data synthesis (1993–2021). Fuel 2022, 324, 124725. [Google Scholar] [CrossRef]
- Barth, G.; Otto, R.; Ferraz-Almeida, R.; Cardoso, E.; Cantarella, H.; Vitti, G. Conversion of ammonium to nitrate and abundance of ammonium-oxidizing-microorganism in Tropical soils with nitrification inhibitor. Sci. Agric. 2020, 77. [Google Scholar] [CrossRef]
- Gilsanz, C.; Báez, D.; Misselbrook, T.H.; Dhanoa, M.S.; Cárdenas, L.M. Development of emission factors and efficiency of two nitrification inhibitors, DCD and DMPP. Agric. Ecosyst. Environ. 2016, 216, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wang, S.; Hou, W.; Yan, L. The Combined Use of Liquid Fertilizer and Ure-ase/Nitrification Inhibitors on Maize Yield, Nitrogen Loss and Utilization in the Mollisol Region. Plants 2023, 12, 1486. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Ali, R.; Latif, Z.; Ishaque, W. Dicyandiamide increases the fertilizer N loss from an alkaline calcareous soil treated with 15N-labelled urea under warm climate and under different crops. Biol. Fertil. Soils 2011, 47, 619–631. [Google Scholar] [CrossRef]
- Chen, D.; Suter, H.C.; Islam, A.; Edis, R.; Freney, J.; Walker, C. Prospects of improving efficiency of fertiliser nitrogen in Australian agriculture: A review of enhanced efficiency fertilisers. Aust. J. Soil Res. 2008, 46, 289–301. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; Menegale, M.L.d.C. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol. Biochem. 2012, 52, 82–89. [Google Scholar] [CrossRef]
- Klimczyk, M.; Siczek, A.; Schimmelpfennig, L. Improving the efficiency of urea-based fertilization leading to reduction in ammonia emission. Sci. Total Environ. 2021, 771, 145483. [Google Scholar] [CrossRef]
- Fan, X.; Chen, H.; Yan, G.; Ye, M.; Yin, C.; Li, T.; Wakelin, S.; Liang, Y. Niche Differentiation Among Canonical Nitrifiers and N2O Reducers Is Linked to Varying Effects of Nitrification Inhibitors DCD and DMPP in Two Arable Soils. Microb. Ecol. 2022, 85, 1434–1447. [Google Scholar] [CrossRef]
- Guo, J.; Ling, N.; Chen, H.; Zhu, C.; Kong, Y.; Wang, M.; Shen, Q.; Guo, S. Distinct drivers of activity, abundance, diversity and composition of ammonia-oxidizers: Evidence from a long-term field experiment. Soil Biol. Biochem. 2017, 115, 403–414. [Google Scholar] [CrossRef]
- Yin, Z.; Bi, X.; Xu, C. Ammonia-Oxidizing Archaea (AOA) Play with Ammonia-Oxidizing Bacteria (AOB) in Nitrogen Removal from Wastewater. Archaea 2018, 2018, 8429145. [Google Scholar] [CrossRef] [PubMed]
- Sakoula, D.; Koch, H.; Frank, J.; Jetten, M.; Kessel, M.; Lücker, S. Enrichment and physiological characterization of a novel comammox Nitrospira indicates ammonium inhibition of complete nitrification. ISME J. 2020, 15, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- van de Leemput, I.; Veraart, A.; Dakos, V.; De Klein, J.; Strous, M.; Scheffer, M. Erratum to Predicting microbial nitrogen pathways from basic principles. Environ. Microbiol. 2014, 16, 1477–1487. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Kawakami, E.M.; Oosterhuis, D.M.; Snider, J.L.; Mozaffari, M. Physiological and yield responses of field-grown cotton to application of urea with the urease inhibitor NBPT and the nitrification inhibitor DCD. Eur. J. Agron. 2012, 43, 147–154. [Google Scholar] [CrossRef]





| Target Gene | Primer Set | Sequence (5′–3′) | Annealing Temperature | Reference |
|---|---|---|---|---|
| Ammonia-oxidizing archaea (AOA) | crenamoA23f crenamoA616r | ATGGTCTGGCTWAGACG GCCATCCATCTGTATGTCCA | 59 °C | [20] |
| Ammonia-oxidizing bacteria (AOB) | amoA-1F amoA-2R | GGGGTTTCTACTGGTGGT CCCCTCKGSAAAGCCTTCTTC | 55 °C | [21] |
| Complete ammonia oxidizer (comammox) | comamoA F comamoA R | AGGNGAYTGGGAYTTCTGG CGGACAWABRTGAABCCCAT | 53 °C | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Z.; Xia, X.; Liu, D.; Dong, H.; Pan, T.; Feng, H.; Lou, Y.; Wang, H.; Yang, Q.; Yang, Z.; et al. Response of Nitrification and Crop Yield to the Presence of NBPT and DCD in a Wheat-Corn Double Cropping System. Agronomy 2024, 14, 285. https://doi.org/10.3390/agronomy14020285
Qu Z, Xia X, Liu D, Dong H, Pan T, Feng H, Lou Y, Wang H, Yang Q, Yang Z, et al. Response of Nitrification and Crop Yield to the Presence of NBPT and DCD in a Wheat-Corn Double Cropping System. Agronomy. 2024; 14(2):285. https://doi.org/10.3390/agronomy14020285
Chicago/Turabian StyleQu, Zhaoqi, Xuejing Xia, Dan Liu, Huimin Dong, Tingliang Pan, Haojie Feng, Yanhong Lou, Hui Wang, Quangang Yang, Zhongchen Yang, and et al. 2024. "Response of Nitrification and Crop Yield to the Presence of NBPT and DCD in a Wheat-Corn Double Cropping System" Agronomy 14, no. 2: 285. https://doi.org/10.3390/agronomy14020285





