Physiological Nitrogen Uptake and Utilisation Responses in Two Native Plants from the Qinghai-Tibet Plateau under Different Water and Fertiliser Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Measurement of Indicators and Methods
2.3.1. Soil Nutrient and Enzyme Activity Measurement
2.3.2. Soluble Sugar, Starch, and Proline Measurement
2.3.3. Measuring Root Indicators
2.4. Statistical Analysis
3. Results
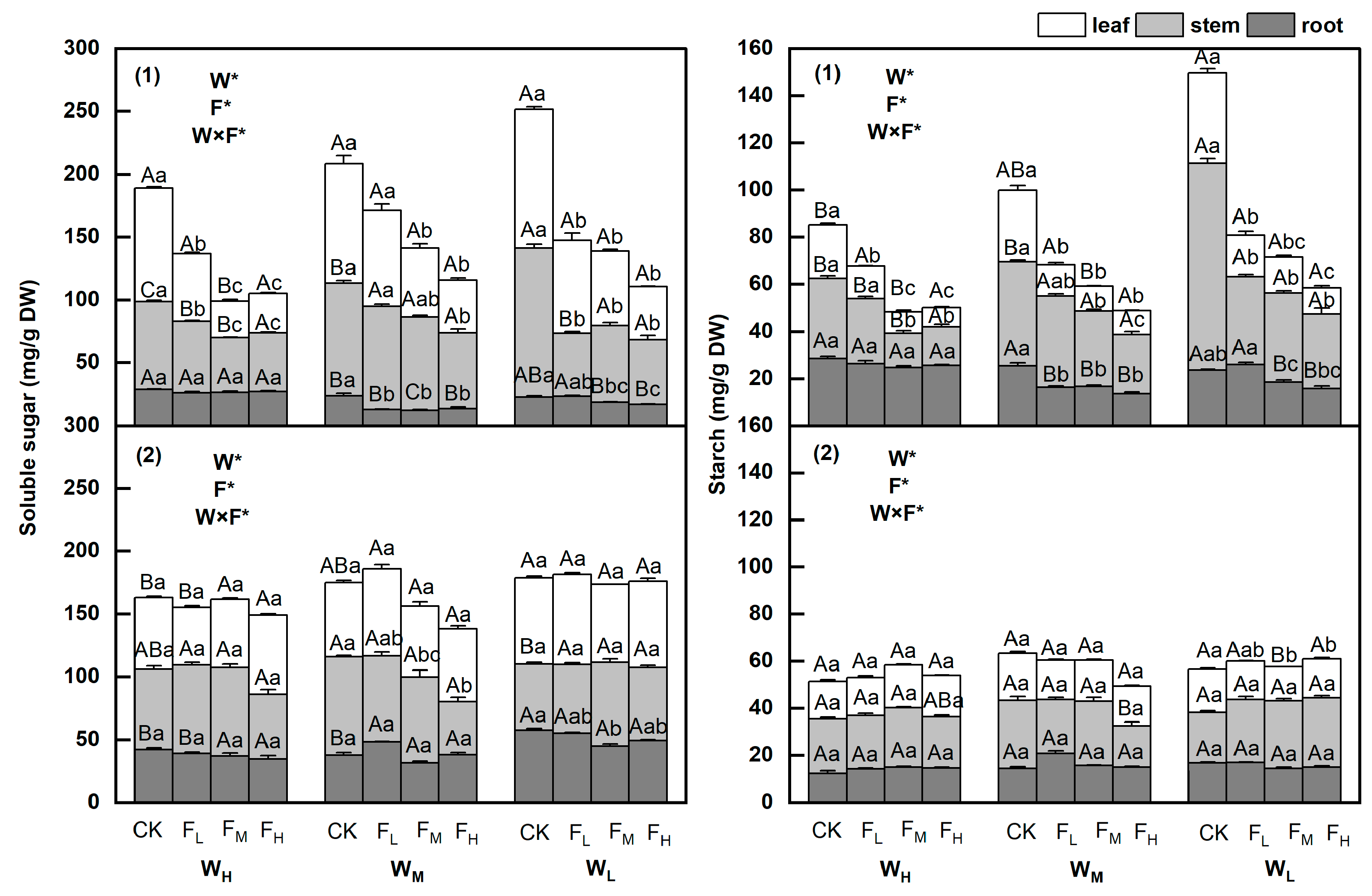
3.1. Changes in Soluble Sugars and Starch Contents
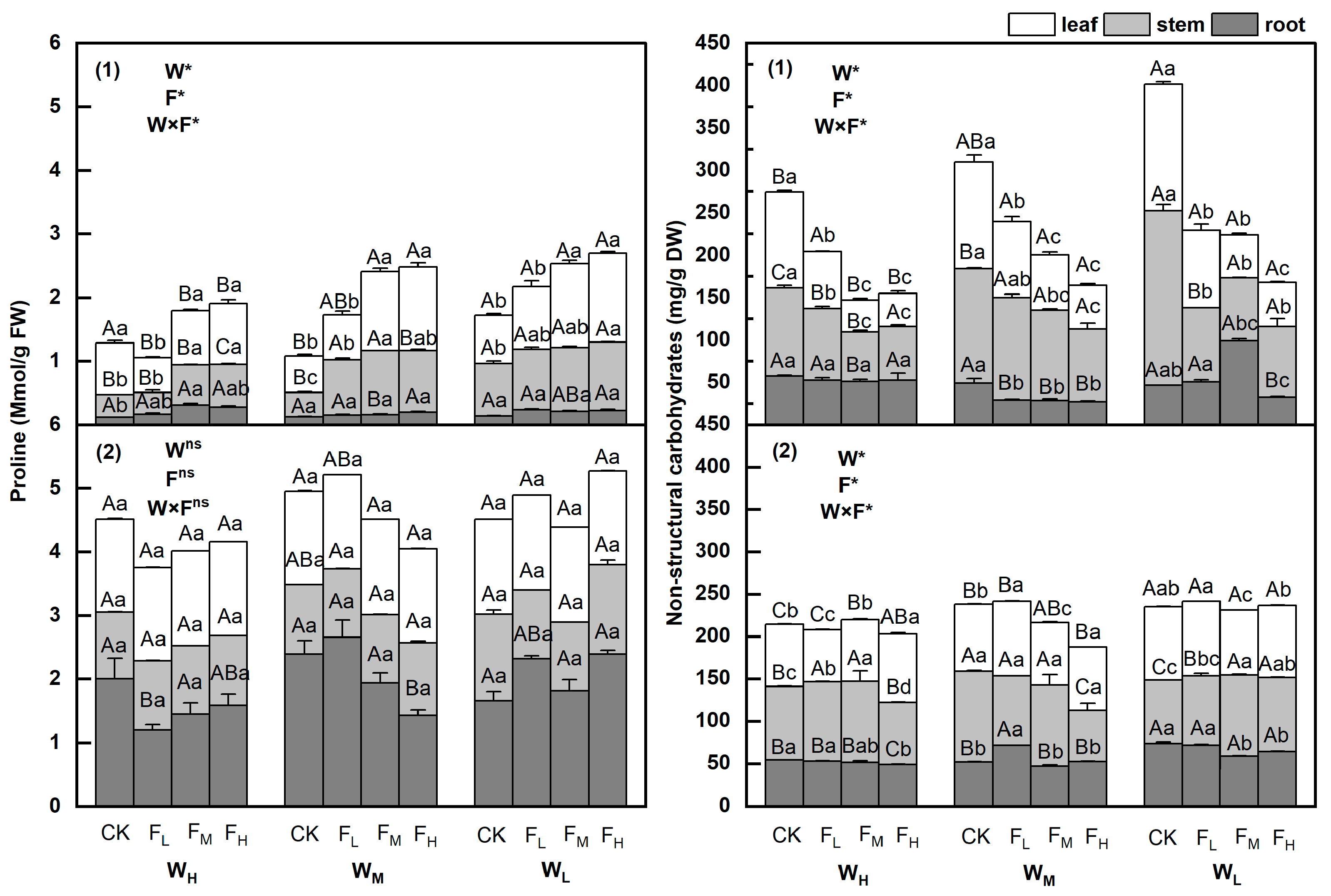
3.2. Changes in Proline and Nonstructural Carbohydrate Contents
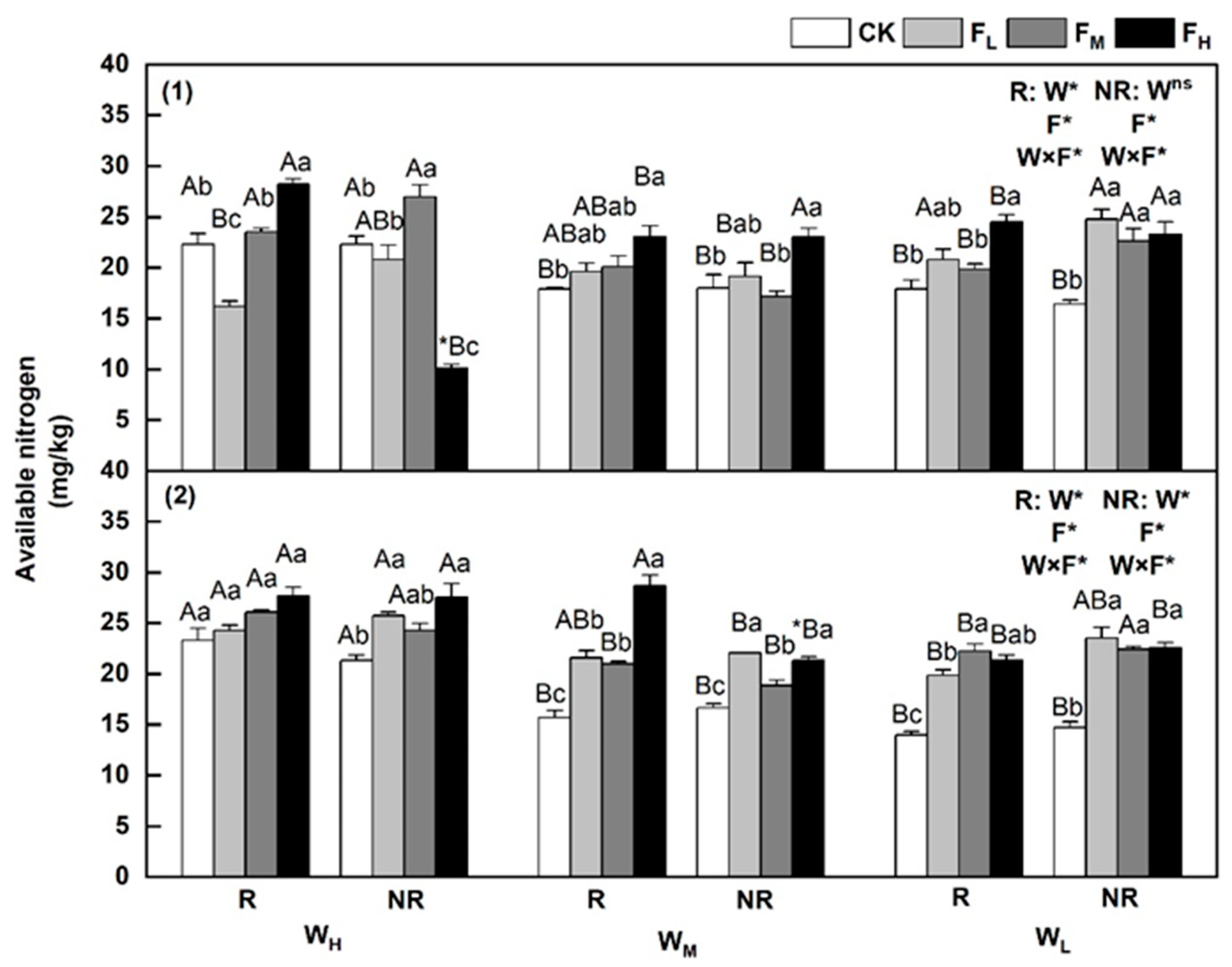
3.3. Changes in Alkaline-Dissolved Nitrogen Content
3.4. Changes in Urease Activity
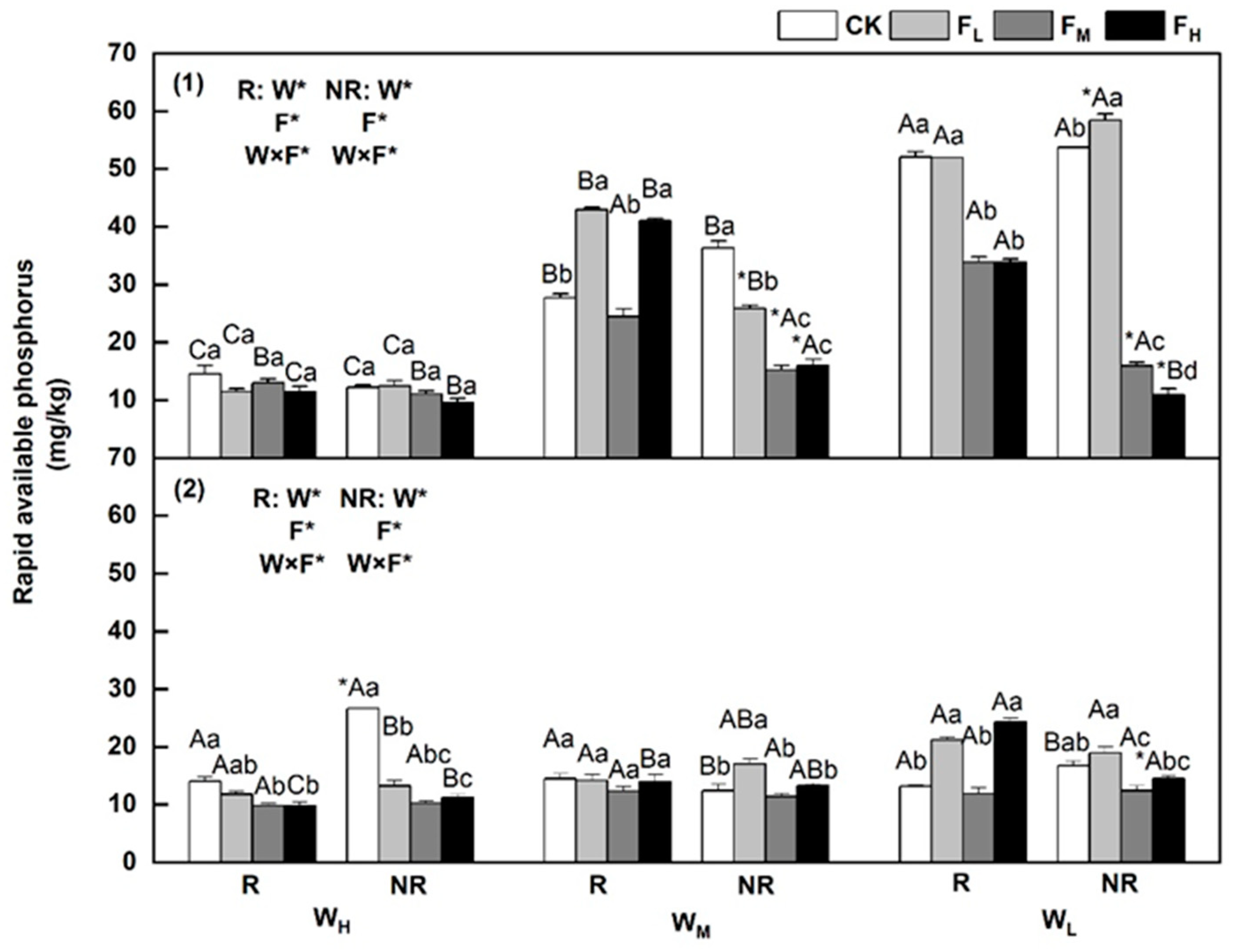
3.5. Changes in Rapidly Available Phosphorus Content
3.6. Changes in Alkaline Phosphatase
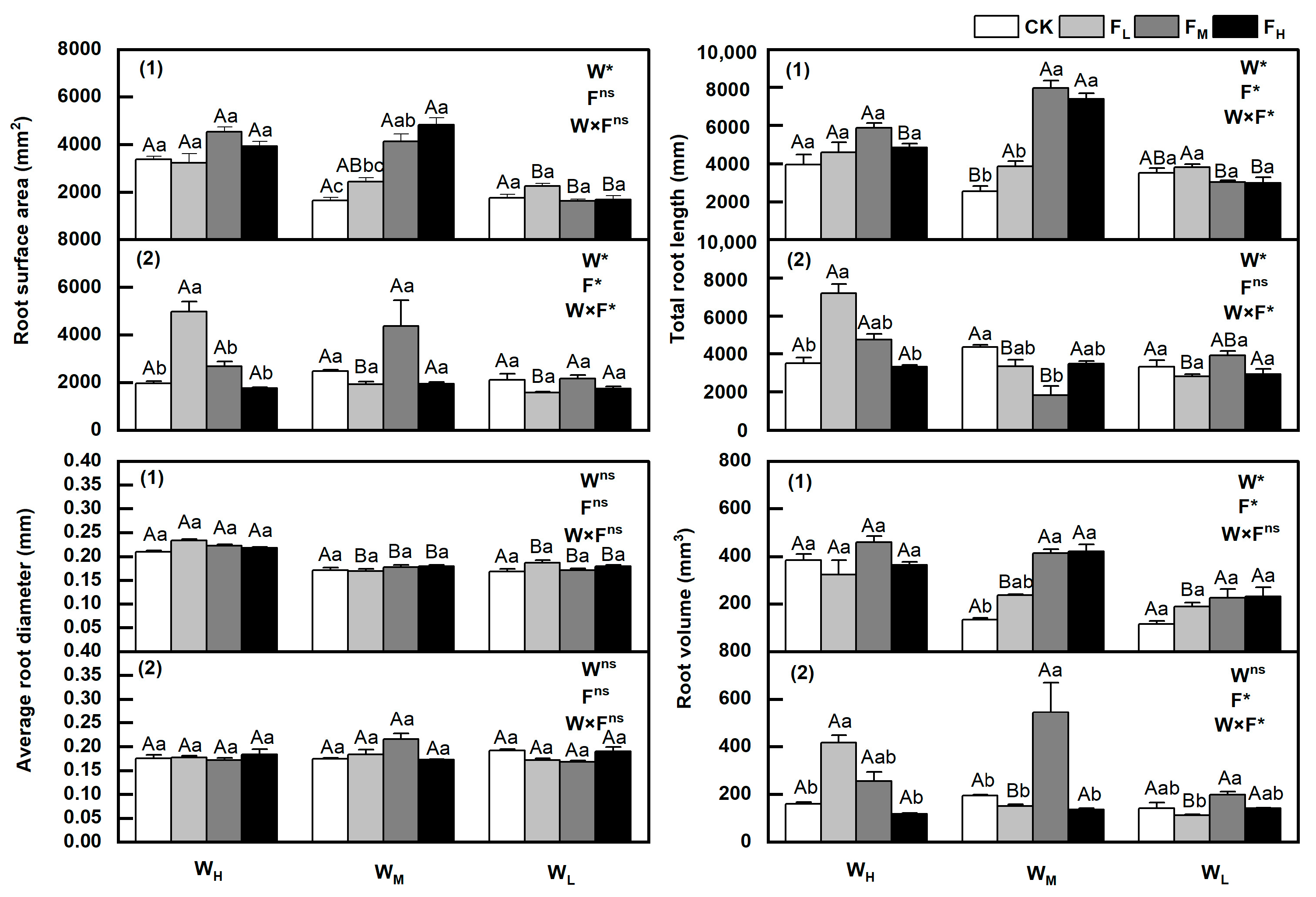
3.7. Changes in Root Characteristics
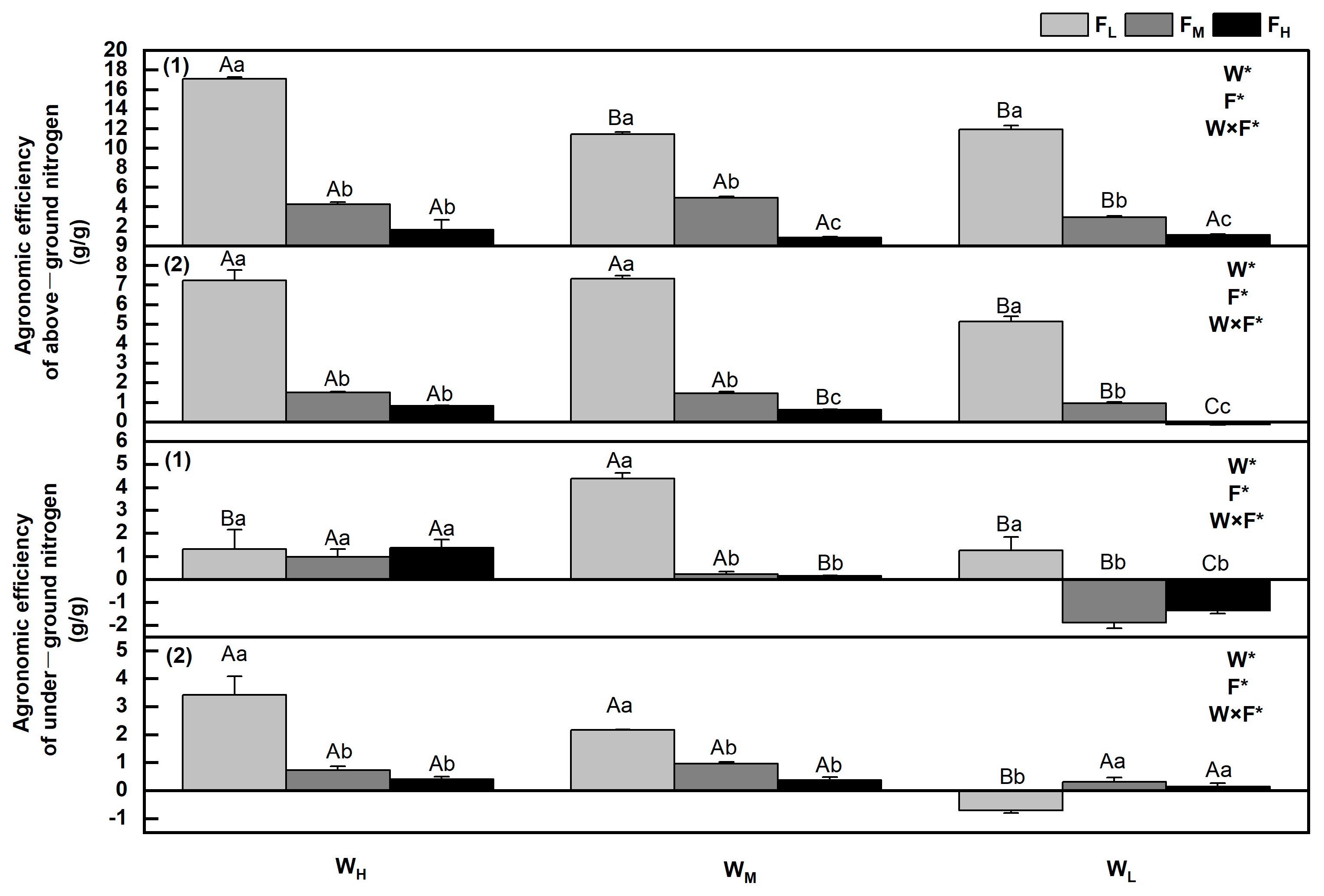
3.8. Changes in Agronomic Efficiency of Underground Nitrogen
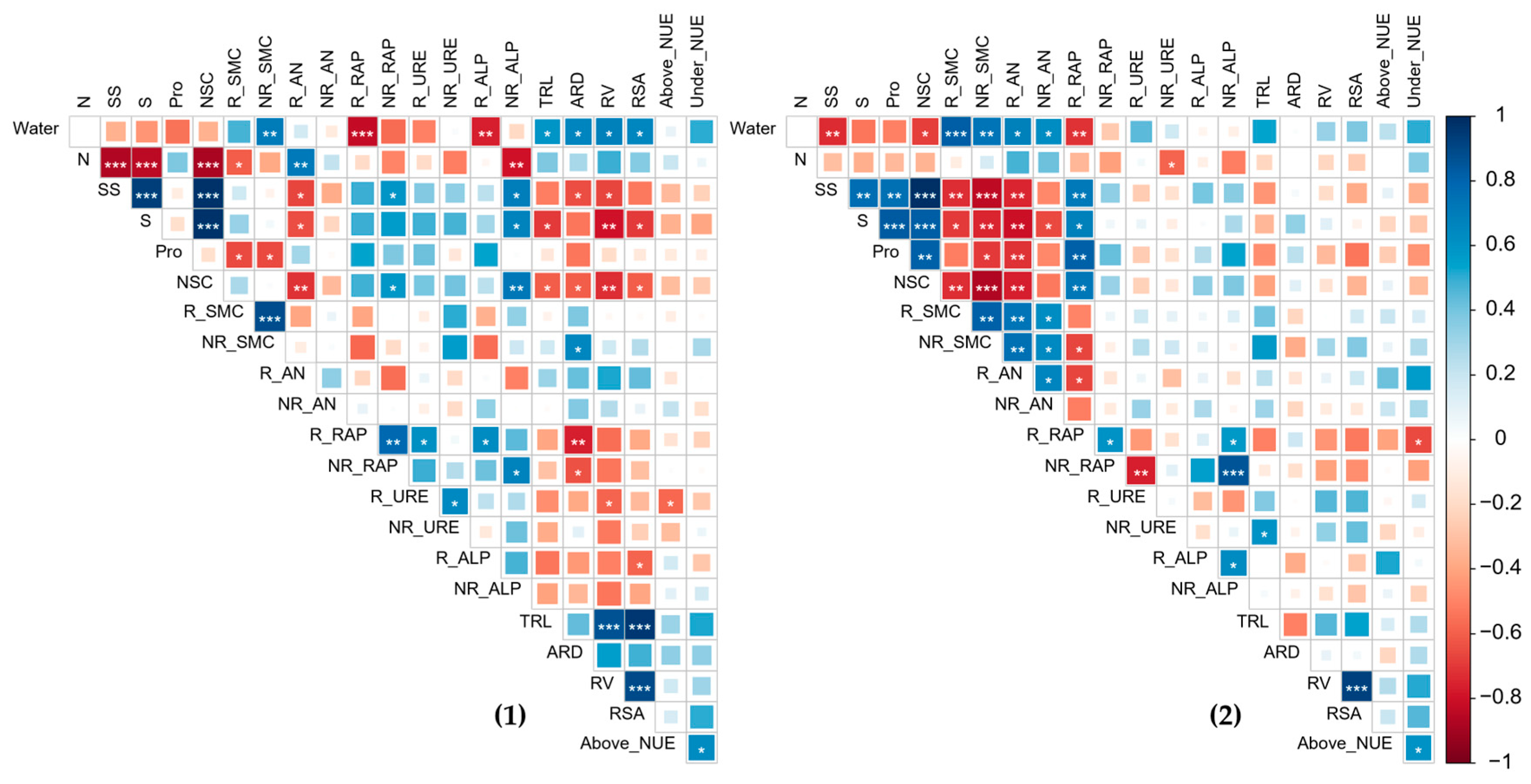
3.9. Nutrient Supply–Demand Relationship and Its Correlation with Physiological Resistance
4. Discussion
4.1. The Effects of Different Water and Fertiliser Conditions on the Physiological Regulation of Two Plant Species
4.2. Impacts of Different Water and Fertiliser Conditions on Nitrogen and Phosphorus Release
4.3. Effects of Different Water and Fertiliser Conditions on the Root Characteristics of Tested Plants
4.4. Impacts of Different Water and Fertiliser Conditions on Nitrogen Fertiliser Utilisation Efficiency in the Tested Plants
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, G.L.; Ren, G.H.; Dong, Q.M.; Shi, J.J.; Wang, Y.L. Above- and belowground responses along a degradation gradient in an alpine grassland on the Qinghai–Tibetan Plateau. CLEAN–Soil Air Water 2014, 42, 319–323. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, Z.H.; Malhi, S.; Li, S.Q.; Gao, Y.J.; Tian, X.H. Effects of nutrient and water management on crop production and nutrient and water use efficiency in dryland areas of China. Adv. Agron. 2009, 102, 223–265. [Google Scholar]
- Fang, X.M.; Li, Y.S.; Nie, J.; Wang, C.; Huang, K.H.; Zhang, Y.K.; Zhang, Y.L.; She, H.Z.; Liu, X.B.; Ruan, R.W.; et al. Effects of nitrogen fertiliser and planting density on leaf photosynthetic characteristics, agronomic traits, and grain yield in common buckwheat (Fagopyrum esculentum M.). Field Crops Res. 2018, 219, 160–168. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashin, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms, and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, Y.; Su, X.Y. The physiological responses of white clover to different leaf types under drought stress are associated with antioxidative enzyme protection and osmotic adjustment. Acta Prataculturae Sin. 2013, 22, 257–263. (In Chinese) [Google Scholar]
- Xiao, L.; Liu, G.B.; Li, P.; Xue, S. Responses of photosynthesis and nonstructural carbohydrates in Bothriochloa ischaemum to doubled CO2 concentration and drought stress. J. Plant Nutr. Fertil. 2017, 23, 389–397. (In Chinese) [Google Scholar]
- Wang, X.; Luo, W.T.; Yu, Q.; Yan, C.F.; Xu, Z.W.; Li, M.H.; Jiang, Y. Effects of nutrient addition on nitrogen, phosphorus, and nonstructural carbohydrate concentrations in the leaves of dominant plant species in a semiarid steppe. Chin. J. Ecol. 2014, 33, 1795–1802. (In Chinese) [Google Scholar]
- Cong, B.M.; Zhang, Y.X.; Wang, X.G.; Zhu, A.M.; Tian, Y.L.; Zhang, Q.X. Effects of nitrogen application on unstructured carbon and nitrogen in the leaves of Leymus chinensis in sandy land. Grassl. Turf. 2019, 39, 50–55. (In Chinese) [Google Scholar]
- Monreal, C.M.; Bergström, D.W. Soil enzymatic factors expressing the influence of land use, tillage system, and soil texture on soil biochemical quality. Can. J. Soil Sci. 2000, 80, 419–428. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, S.L.; Liu, Y. Responses to N and P fertilisation in a young Eucalyptus dunnii plantation: Microbial properties, enzyme activities, and dissolved organic matter. Appl. Soil. Ecol. 2008, 40, 484–490. [Google Scholar] [CrossRef]
- Wang, B.S.; Wang, Y.; Sun, Y.; Yu, L.R.; Lou, Y.S.; Fan, X.R.; Ren, L.X.; Xu, G.H. Watermelon responds to organic fertilizer by enhancing root-associated acid phosphatase activity to improve organic phosphorus utilization. J. Plant Physiol. 2022, 279, 153838. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Treseder, K.K. Soil extracellular enzyme activities correspond with abiotic factors more than fungal community composition. Biogeochemistry 2014, 117, 23–37. [Google Scholar] [CrossRef]
- Cregger, M.A.; Mcdowell, N.G.; Pangle, R.E.; Pockman, W.T.; Classen, A.T. Impact of precipitation change on nitrogen cycling in a semi-arid ecosystem. Funct. Ecol. 2015, 28, 1534–1544. [Google Scholar] [CrossRef]
- Li, C.H.; Jia, Z.J.; Tang, L.S.; Wu, Y.C.; Li, Y. Effect of model of fertilization on microbial abundance and enzyme activity in oasis farmland soil. Acta Pedol. Sin. 2012, 49, 567–574. (In Chinese) [Google Scholar]
- Sun, Y.N.; Li, Q.; Li, Y.K. The effect of nitrogen and phosphorus applications on soil enzyme activities in Qinghai-Tibetan alpine meadows. Acta Prataculturae Sin. 2016, 25, 18–26. (In Chinese) [Google Scholar]
- Zhang, G.N.; Chen, Z.H.; Zhang, A.M.; Chen, L.J.; Wu, Z.J. Nitrogen and phosphorus related hydrolytic enzyme activities influenced by N deposition under semi-arid grassland soil. Adv. Mater. Res. 2013, 726–731, 3847–3854. [Google Scholar] [CrossRef]
- Ludlow, M.M.; Muchow, R.C. A critical evaluation of traits for improving crop yields in water-limited environments. Adv. Agron. 1990, 43, 107–153. [Google Scholar]
- Rasse, D.P. Nitrogen deposition and atmospheric CO2 interactions on fine root dynamics in temperate forests: A theoretical model analysis. Glob. Chang. Biol. 2002, 8, 486–503. [Google Scholar] [CrossRef]
- Kuang, X.X.; Jiao, J.J. Review on climate change on the Tibetan Plateau during the last half century. J. Geophys. Res. Atmos. 2016, 121, 3979–4007. [Google Scholar] [CrossRef]
- Pornaro, C.; Dal Maso, M.; Macolino, S. Drought resistance and recovery of kentucky bluegrass (Poa Pratensis L.) cultivars under different nitrogen fertilisation rates. Agronomy 2021, 11, 1128. [Google Scholar] [CrossRef]
- Yang, D.; Ni, R.; Yang, S.; Yang, S.A.; Pu, Y.A.; Qian, M.; Yang, Y.Q.; Yang, Y.P. Functional Characterization of the Stipa purpurea P5CS gene under drought stress conditions. Int. J. Mol. Sci. 2021, 22, 9599. [Google Scholar] [CrossRef] [PubMed]
- Albaigés, J. Handbook of environmental analysis: Chemical pollutants in air, water, soil, and solid wastes. Int. J. Environ. Anal. Chem. 2017, 97, 1420. [Google Scholar] [CrossRef]
- Zhang, X. Crop Physiology Research Method; Chinese Agriculture Press: Beijing, China, 1992; pp. 201–202. [Google Scholar]
- Li, M.H.; Cherubini, P.; Dobbertin, M.; Arend, M.; Xiao, W.F.; Rigling, A. Responses of leaf nitrogen and mobile carbohydrates in different Quercus species/provenances to moderate climate changes. Plant Biol. 2013, 15, 177–184. [Google Scholar] [CrossRef]
- Li, W.; Hartmann, H.; Adams, H.D.; Zhang, H.; Jin, C.; Zhao, C.; Guan, D.; Wang, A.; Yuan, F.; Wu, J. The sweet side of global change–dynamic responses of non-structural carbohydrates to drought, elevated CO2 and nitrogen fertilization in tree species. Tree Physiol. 2018, 38, 1706–1723. [Google Scholar] [CrossRef]
- Zhang, W.T.; Li, S.; Li, Y.; Bai, Y.M.; Ma, J. Effects of nitrogen addition and precipitation change on non-structural carbohydrates in Reaumuria soongorica seedlings. Chin. J. Ecol. 2020, 39, 803–811. (In Chinese) [Google Scholar]
- Qian, Y.S.; Zhu, J.M.; Wu, J.B.; Zhang, X.Q.; Chai, M.L. Effects of fertilizing on manilagrass growth and physiological characteristics. Acta Partaculturae Sin. 2012, 21, 234–241. (In Chinese) [Google Scholar]
- Turner, L.B.; Humphreys, M.O.; Cairns, A.J.; Pollock, C.J. Carbon assimilation and partitioning into non-structural carbohydrate in contrasting varieties of Lolium perenne. Plant Physiol. 2002, 159, 257–263. [Google Scholar] [CrossRef]
- Cui, G.; Li, B.; He, W.H.; Yin, X.J.; Liu, S.Y.; Lian, L.; Zhang, Y.L.; Liang, W.X.; Zhang, P. Physiological analysis of the effect of altitudinal gradients on Leymus secalinus on the Qinghai-Tibetan Plateau. PLoS ONE 2018, 13, e0202881. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Zhou, X.H.; Nie, Y.Y.; Bai, S.H.; Zhou, L.Y.; Shao, J.J.; Cheng, W.S.; Wang, J.W.; Hu, F.Q.; Fu, Y.L. Droughtinduced changes in root biomass largely result from altered root morphological traits: Evidence from a synthesis of global field trials. Plant Cell Environ. 2018, 41, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Cofield, G.N.; Ruuska, S.A.; Aoki, N.; Lewis, D.C. Starch storage in the stems of wheat plants: Localization and temporal changes. Ann. Bot. 2009, 103, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Z.; Yan, C.; Luo, W.; Wang, R.; Han, X.; Li, M.H. Responses and sensitivity of N, P and mobile carbohydrates of dominant species to increased water, N and P availability in semi-arid grasslands in northern China. J. Plant Ecol. 2017, 10, 486–496. [Google Scholar] [CrossRef]
- Wang, Y.; Lambers, H. Root-released organic anions in response to low phosphorus availability: Recent progress, challenges and future perspectives. Plant Soil 2020, 447, 135–156. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, Y.; Ding, C.X.; Liu, Y.; Wu, K.X.; Jiang, F.Z.; Su, D.R. Water addition promotes vegetation recovery of degraded alpine meadows by regulating soil enzyme activity and nutrients in the Qinghai-Tibetan Plateau. Ecol. Eng. 2020, 158, 106047. [Google Scholar] [CrossRef]
- Song, F.B.; Han, X.Y.; Zhu, X.C.; Herbert, S.J. Response to water stress of soil enzymes and root exudates from drought and non-drought tolerant corn hybrids at different growth stages. Can. J. Soil Sci. 2012, 92, 501–507. [Google Scholar] [CrossRef]
- Liu, W.X.; Allson, S.D.; Xia, J.Y.; Liu, L.L.; Wan, S.Q. Precipitation regime drives warming responses of microbial biomass and activity in temperate steppe soils. Biol. Fertil. Soils 2016, 52, 469–477. [Google Scholar] [CrossRef]
- Chen, Z.K.; Khan, A.; Shi, X.J.; Hao, X.Z.; Tan, D.K.Y.; Luo, H.H. Water-nutrient management enhances root morpho-physiological functioning, phosphorus absorption, transportation and utilization of cotton in arid region. Ind. Crops Prod. 2020, 143, 111975. [Google Scholar] [CrossRef]
- Brueck, H. Effects of nitrogen supply on water-use efficiency of higher plants. J. Plant Nutr. Soil Sci. 2008, 171, 210–219. [Google Scholar] [CrossRef]
- Hoad, S.P.; Russell, G.; Lucas, M.E.; Bingham, I.J. The management of wheat, barley and oat root systems. Adv. Agron. 2001, 74, 193–246. [Google Scholar]
- Xue, Q.; Zhu, Z.; Musick, J.T.; Stewart, B.A.; Dusek, D.A. Root growth and water uptake in winter wheat under deficit irrigation. Plant Soil 2003, 257, 151–161. [Google Scholar] [CrossRef]
- Wang, H.; Yamauchi, A. Growth and function of roots under abiotic stress in soil. In Plant–Environment Interactions, 3rd ed.; Huang, B., Ed.; CRC Press: New York, NY, USA, 2006; pp. 271–320. [Google Scholar]
- Li, X.; Zeng, R.; Liao, H. Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant Biol. 2016, 58, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.N.; Sun, X.R.; Hou, S.; Chen, G.H.; Zhang, H.; Han, Y.X.; Zhou, J.; Wang, X.T.; Zhang, Z.X. N Addition Mitigates Water Stress via Different Photosynthesis and Water Traits for Three Native Plant Species in the Qinghai–Tibet Plateau. Agriculture 2022, 12, 1873. [Google Scholar] [CrossRef]
- Wang, D.J.; Zhou, H.K.; Yao, B.Q.; Wang, W.Y.; Dong, S.K.; Shang, Z.H.; She, Y.D.; Ma, L.; Huang, X.T.; Zhang, Z.H.; et al. Effects of nutrient addition on degraded alpine grasslands of the Qinghai-Tibetan Plateau: A meta-analysis. Agric. Ecosyst. Environ. 2020, 301, 106970. [Google Scholar] [CrossRef]
- Wilkins, P.W.; Allen, D.K.; Mytton, L.R. Differences in the nitrogen use efficiency of perennial ryegrass varieties under simulated rotational grazing and their effects on nitrogen recovery and herbage nitrogen content. Grass Forage Sci. 2000, 55, 69–76. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, C.; Zhao, N.; Sun, X.; Hou, S.; Wang, P. Physiological Nitrogen Uptake and Utilisation Responses in Two Native Plants from the Qinghai-Tibet Plateau under Different Water and Fertiliser Conditions. Agronomy 2024, 14, 440. https://doi.org/10.3390/agronomy14030440
Wang X, Zhang C, Zhao N, Sun X, Hou S, Wang P. Physiological Nitrogen Uptake and Utilisation Responses in Two Native Plants from the Qinghai-Tibet Plateau under Different Water and Fertiliser Conditions. Agronomy. 2024; 14(3):440. https://doi.org/10.3390/agronomy14030440
Chicago/Turabian StyleWang, Xiangtao, Chao Zhang, Ningning Zhao, Xingrong Sun, Shuai Hou, and Puchang Wang. 2024. "Physiological Nitrogen Uptake and Utilisation Responses in Two Native Plants from the Qinghai-Tibet Plateau under Different Water and Fertiliser Conditions" Agronomy 14, no. 3: 440. https://doi.org/10.3390/agronomy14030440



