The Application of Biochar Enhances Soil Organic Carbon and Rice Yields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.2.1. Experimental Design
2.2.2. Experimental Materials
2.2.3. Sample Collection
2.3. Sample Determination
2.3.1. Rice Yield
2.3.2. SOC and HS Composition
2.3.3. Extraction, Fractionation, and Purification of Humus Fractions
2.3.4. Structural Characterization of Soil HA
2.4. Statistical Analysis
3. Results
3.1. SOC and HS Composition
3.2. Elemental Composition of HA
3.3. Infrared Spectra of HA
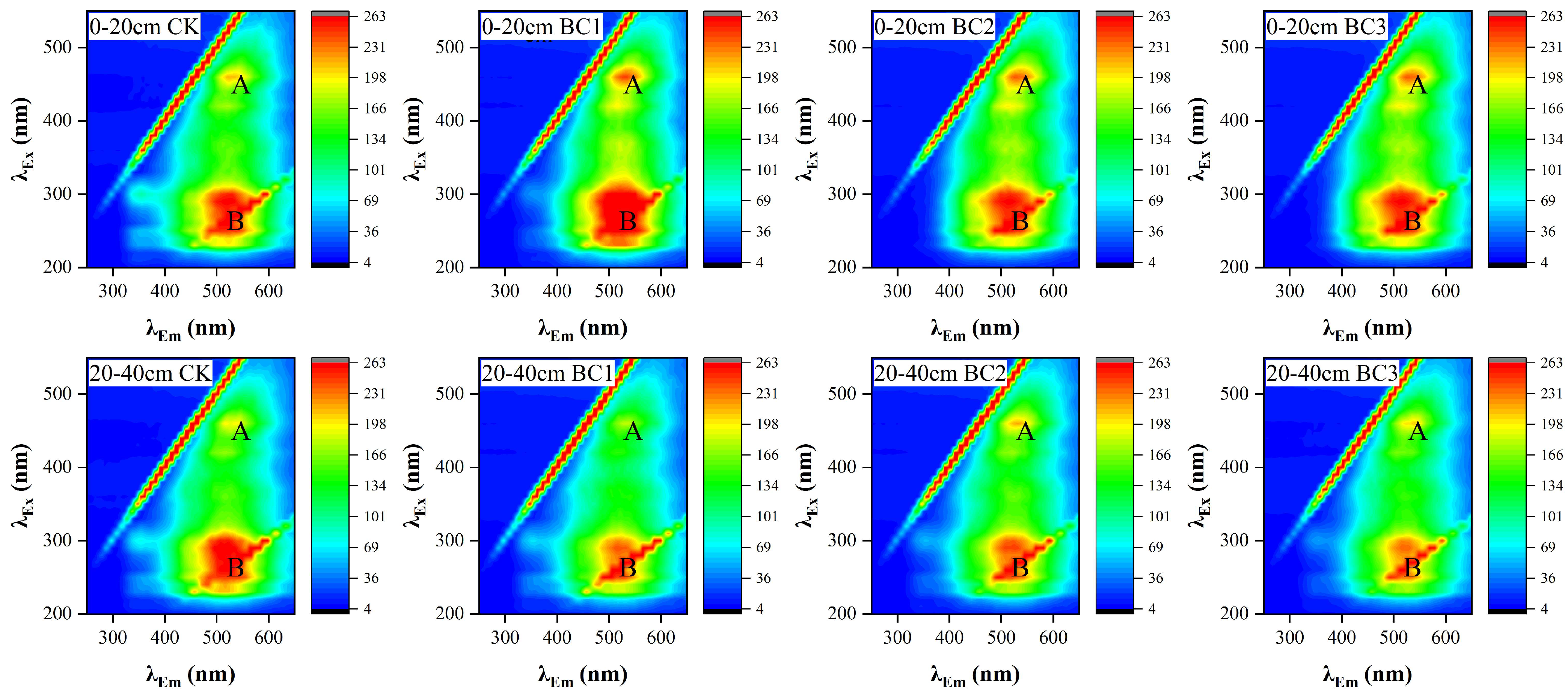
3.4. HA’s Fluorescence Spectra
3.5. Rice Yield
4. Discussion
4.1. Effect of the Application of Biomass Charcoal on SOC and HS Composition
4.2. Effect of Biochar’s Application on HA’s Structure
4.3. Effect of the Application of Biomass Charcoal on Yield and Component Factors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, X.; Chen, F.; Ma, T.; Hu, Y. Soil Quality Characteristics as Affected by Continuous Rice Cultivation and Changes in Cropping Systems in South China. Agriculture 2020, 10, 443. [Google Scholar] [CrossRef]
- Cui, Y.; Meng, J.; Wang, Q.; Zhang, W.; Cheng, X.; Chen, W. Effects of Straw and Biochar Addition on Soil Nitrogen, Carbon, and Super Rice Yield in Cold Waterlogged Paddy Soils of North China. J. Integr. Agric. 2017, 16, 1064–1074. [Google Scholar] [CrossRef]
- Nardi, S.; Morari, F.; Berti, A.; Tosoni, M.; Giardini, L. Soil Organic Matter Properties after 40 Years of Different Use of Organic and Mineral Fertilisers. Eur. J. Agron. 2004, 21, 357–367. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Yang, Y.; Chen, Y.; Jeyakumar, P.; Shao, Q.; Zhou, Y.; Ma, M.; Zhu, R.; Qian, Q.; et al. Effect of Biofertilizer and Wheat Straw Biochar Application on Nitrous Oxide Emission and Ammonia Volatilization from Paddy Soil. Environ. Pollut. 2021, 275, 116640. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jiang, Z.; Sun, X.; Ding, J.; Xu, J. Effects of Biochar Amendment on CO2 Emissions from Paddy Fields under Water-Saving Irrigation. IJERPH 2018, 15, 2580. [Google Scholar] [CrossRef] [PubMed]
- Devêvre, O.C.; Horwáth, W.R. Decomposition of Rice Straw and Microbial Carbon Use Efficiency under Different Soil Temperatures and Moistures. Soil Biol. Biochem. 2000, 32, 1773–1785. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-Mediated Changes in Soil Quality and Plant Growth in a Three Year Field Trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Pérez, M. The Role of Biochar Porosity and Surface Functionality in Augmenting Hydrologic Properties of a Sandy Soil. Sci. Total Environ. 2017, 574, 139–147. [Google Scholar] [CrossRef]
- Xie, Z.; Xu, Y.; Liu, G.; Liu, Q.; Zhu, J.; Tu, C.; Amonette, J.E.; Cadisch, G.; Yong, J.W.H.; Hu, S. Impact of Biochar Application on Nitrogen Nutrition of Rice, Greenhouse-Gas Emissions and Soil Organic Carbon Dynamics in Two Paddy Soils of China. Plant Soil 2013, 370, 527–540. [Google Scholar] [CrossRef]
- Mohan, D.; Abhishek, K.; Sarswat, A.; Patel, M.; Singh, P.; Pittman, C.U. Biochar Production and Applications in Soil Fertility and Carbon Sequestration—A Sustainable Solution to Crop-Residue Burning in India. RSC Adv. 2018, 8, 508–520. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Jiao, X.; Li, H.; An, Y.; Liu, K. Effects of Straw Returning Combine with Biochar on Water Quality under Flooded Condition. Water 2020, 12, 1633. [Google Scholar] [CrossRef]
- Yin, X.; Chen, J.; Cao, F.; Tao, Z.; Huang, M. Short-Term Application of Biochar Improves Post-Heading Crop Growth but Reduces Pre-Heading Biomass Translocation in Rice. Plant Prod. Sci. 2020, 23, 522–528. [Google Scholar] [CrossRef]
- Ndzelu, B.S.; Dou, S.; Zhang, X. Changes in Soil Humus Composition and Humic Acid Structural Characteristics under Different Corn Straw Returning Modes. Soil Res. 2020, 58, 452. [Google Scholar] [CrossRef]
- Piccolo, A.; Spaccini, R.; Nieder, R.; Richter, J. Sequestration of a Biologically Labile Organic Carbon in Soils by Humified Organic Matter. Clim. Chang. 2004, 67, 329–343. [Google Scholar] [CrossRef]
- Loke, P.F.; Kotzé, E.; Du Preez, C.C.; Twigge, L. Long-Term Effects of Wheat Production Management Practices on Some Carbon Fractions of a Semiarid Plinthustalfs. Soil Res. 2018, 56, 601. [Google Scholar] [CrossRef]
- Tamura, M.; Suseela, V.; Simpson, M.; Powell, B.; Tharayil, N. Plant Litter Chemistry Alters the Content and Composition of Organic Carbon Associated with Soil Mineral and Aggregate Fractions in Invaded Ecosystems. Glob. Chang. Biol. 2017, 23, 4002–4018. [Google Scholar] [CrossRef] [PubMed]
- De Mastro, F.; Cocozza, C.; Traversa, A.; Savy, D.; Abdelrahman, H.M.; Brunetti, G. Influence of Crop Rotation, Tillage and Fertilization on Chemical and Spectroscopic Characteristics of Humic Acids. PLoS ONE 2019, 14, e0219099. [Google Scholar] [CrossRef]
- Qualls, R.G. Biodegradability of Humic Substances and Other Fractions of Decomposing Leaf Litter. Soil Sci. Soc. Am. J. 2004, 68, 1705–1712. [Google Scholar] [CrossRef]
- Spaccini, R.; Mbagwu, J.S.C.; Conte, P.; Piccolo, A. Changes of Humic Substances Characteristics from Forested to Cultivated Soils in Ethiopia. Geoderma 2006, 132, 9–19. [Google Scholar] [CrossRef]
- Liu, X.; Dou, S.; Zheng, S. Effects of Corn Straw and Biochar Returning to Fields Every Other Year on the Structure of Soil Humic Acid. Sustainability 2022, 14, 15946. [Google Scholar] [CrossRef]
- Ma, R.; Dou, S.; Zhang, Y.; Wu, D.; Ndzelu, B.S.; Xie, S.; YaLiHong, D. Different Soil Particle Size Changes the 15N Retention in Soil and 15N Utilization by Maize. Sci. Total Environ. 2022, 843, 157133. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.R.; Gregorich, E.G. (Eds.) Soil Sampling and Methods of Analysis, 2nd ed.; Canadian Society of Soil Science; CRC Press: Pinawa, MB, Canada; Boca Raton, FL, USA, 2008; ISBN 978-0-8493-3586-0. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; Agronomy Monographs; Wiley: Hoboken, NJ, USA, 1983; pp. 539–579. ISBN 978-0-89118-977-0. [Google Scholar]
- Mi, W.; Sun, Y.; Gao, Q.; Liu, M.; Wu, L. Changes in Humus Carbon Fractions in Paddy Soil given Different Organic Amendments and Mineral Fertilizers. Soil Tillage Res. 2019, 195, 104421. [Google Scholar] [CrossRef]
- Zhang, G.; Dou, S.; Meng, F.; Yin, X.; Zhou, X. Transformation of Biochar into Extracted Humic Substances under Short-Term Laboratory Incubation Conditions: Evidence from Stable Carbon Isotopes. Soil Tillage Res. 2022, 215, 105189. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of Soil Organic Matter as an Ecosystem Property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Li, L.; Zheng, J.; Qu, J.; Zheng, J.; Zhang, X.; Pan, G. Consistent Increase in Abundance and Diversity but Variable Change in Community Composition of Bacteria in Topsoil of Rice Paddy under Short Term Biochar Treatment across Three Sites from South China. Appl. Soil Ecol. 2015, 91, 68–79. [Google Scholar] [CrossRef]
- Gao, J.; Shi, Z.; Wu, H.; Lv, J. Fluorescent Characteristics of Dissolved Organic Matter Released from Biochar and Paddy Soil Incorporated with Biochar. RSC Adv. 2020, 10, 5785–5793. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Dippold, M.; Gao, Y.; Blagodatskaya, E.; Kuzyakov, Y. Biochar Affects Soil Organic Matter Cycling and Microbial Functions but Does Not Alter Microbial Community Structure in a Paddy Soil. Sci. Total Environ. 2016, 556, 89–97. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Bogomolova, I.; Glaser, B. Biochar Stability in Soil: Decomposition during Eight Years and Transformation as Assessed by Compound-Specific 14C Analysis. Soil Biol. Biochem. 2014, 70, 229–236. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and Negative Carbon Mineralization Priming Effects among a Variety of Biochar-Amended Soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Ingelmo, F.; Molina, M.J.; Soriano, M.D.; Gallardo, A.; Lapeña, L. Influence of Organic Matter Transformations on the Bioavailability of Heavy Metals in a Sludge Based Compost. J. Environ. Manag. 2012, 95, S104–S109. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, I.A.; Tsutsuki, K.; Navarrete, R.A. Humus Composition and the Structural Characteristics of Humic Substances in Soils under Different Land Uses in Leyte, Philippines. Soil Sci. Plant Nutr. 2010, 56, 289–296. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar Amendment Improves Crop Production in Problem Soils: A Review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef]
- Simonetti, G.; Francioso, O.; Nardi, S.; Berti, A.; Brugnoli, E.; Francesco Morari, E.L. Characterization of Humic Carbon in Soil Aggregates in a Long-Term Experiment with Manure and Mineral Fertilization. Soil Sci. Soc. Am. J. 2012, 76, 880–890. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Y.; Liu, J.; Yuan, J.; Liang, Y.; Ren, J.; Cai, H. Effects of Maize Straw and Its Biochar Application on Organic and Humic Carbon in Water-Stable Aggregates of a Mollisol in Northeast China: A Five-Year Field Experiment. Soil Tillage Res. 2019, 190, 1–9. [Google Scholar] [CrossRef]
- Zhao, S.; Ta, N.; Li, Z.; Yang, Y.; Zhang, X.; Liu, D.; Zhang, A.; Wang, X. Varying Pyrolysis Temperature Impacts Application Effects of Biochar on Soil Labile Organic Carbon and Humic Fractions. Appl. Soil Ecol. 2018, 123, 484–493. [Google Scholar] [CrossRef]
- Tan, Z.; Lin, C.S.K.; Ji, X.; Rainey, T.J. Returning Biochar to Fields: A Review. Appl. Soil Ecol. 2017, 116, 1–11. [Google Scholar] [CrossRef]
- Rivero, C.; Chirenje, T.; Ma, L.Q.; Martinez, G. Influence of Compost on Soil Organic Matter Quality under Tropical Conditions. Geoderma 2004, 123, 355–361. [Google Scholar] [CrossRef]
- Zhang, Y.; Dou, S.; Hamza, B.; Ye, S.; Zhang, D. Mechanisms of Three Fungal Types on Humic-Like Substances Formation during Solid-State Fermentation of Corn Straw. Int. J. Agric. Biol. 2020, 23, 970–976. [Google Scholar]
- Zhang, X.; Dou, S.; Ndzelu, B.S.; Zhang, Y.; Liu, X. Accumulation of Straw-Derived Carbon and Changes in Soil Humic Acid Structural Characteristics during Corn Straw Decomposition. Can. J. Soil. Sci. 2021, 101, 452–465. [Google Scholar] [CrossRef]
- Masciandaro, G.; Ceccanti, B. Assessing Soil Quality in Different Agro-Ecosystems through Biochemical and Chemico-Structural Properties of Humic Substances. Soil Tillage Res. 1999, 51, 129–137. [Google Scholar] [CrossRef]
- Guimarães, D.V.; Gonzaga, M.I.S.; Da Silva, T.O.; Da Silva, T.L.; Da Silva Dias, N.; Matias, M.I.S. Soil Organic Matter Pools and Carbon Fractions in Soil under Different Land Uses. Soil Tillage Res. 2013, 126, 177–182. [Google Scholar] [CrossRef]
- Zhang, X.; Dou, S.; Ndzelu, B.S.; Guan, X.W.; Zhang, B.Y.; Bai, Y. Effects of Different Corn Straw Amendments on Humus Composition and Structural Characteristics of Humic Acid in Black Soil. Commun. Soil Sci. Plant Anal. 2020, 51, 107–117. [Google Scholar] [CrossRef]
- Zheng, S.; Dou, S.; Duan, H.M. Effects of straw enrichment and deep incorporation on humus composition and humic acid structure of black soil profile in northeast China. Appl. Ecol. Env. Res. 2022, 20, 1051–1063. [Google Scholar] [CrossRef]
- Piccolo, A.; Mbagwu, J.S.C. Role of Hydrophobic Components of Soil Organic Matter in Soil Aggregate Stability. Soil Sci. Soc. Am. J. 1999, 63, 1801–1810. [Google Scholar] [CrossRef]
- Maryganova, V.; Szajdak, L.W.; Tychinskaya, L. Hydrophobic and Hydrophilic Properties of Humic Acids from Soils under Shelterbelts of Different Ages. Chem. Ecol. 2010, 26, 25–33. [Google Scholar] [CrossRef]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Evaluation of Humic Substances during Co-Composting of Food Waste, Sawdust and Chinese Medicinal Herbal Residues. Bioresour. Technol. 2014, 168, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zech, W.; Ziegler, F.; Kögel-Knabner, I.; Haumaier, L. Humic Substances Distribution and Transformation in Forest Soils. Sci. Total Environ. 1992, 117–118, 155–174. [Google Scholar] [CrossRef]
- Filip, Z.; Tesařová, M. Microbial Degradation and Transformation of Humic Acids from Permanent Meadow and Forest Soils. Int. Biodeterior. Biodegrad. 2004, 54, 225–231. [Google Scholar] [CrossRef]
- Senesi, N.; D’Orazio, V.; Ricca, G. Humic Acids in the First Generation of EUROSOILS. Geoderma 2003, 116, 325–344. [Google Scholar] [CrossRef]
- Jindo, K.; Sánchez-Monedero, M.A.; Matsumoto, K.; Sonoki, T. The Efficiency of a Low Dose of Biochar in Enhancing the Aromaticity of Humic-Like Substance Extracted from Poultry Manure Compost. Agronomy 2019, 9, 248. [Google Scholar] [CrossRef]
- Bayer, C.; Martin-Neto, L.; Mielniczuk, J.; Ceretta, C.A. Effect of No-till Cropping Systems on Soil Organic Matter in a Sandy Clay Loam Acrisol from Southern Brazil Monitored by Electron Spin Resonance and Nuclear Magnetic Resonance. Soil Tillage Res. 2000, 53, 95–104. [Google Scholar] [CrossRef]
- Yang, Z. Reducing Capacities and Redox Potentials of Humic Substances Extracted from Sewage Sludge. Chemosphere 2016, 144, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Fan, L.; Jiang, L.; Yang, S.; Zou, Y.; Uphoff, N. Continuous Applications of Biochar to Rice: Effects on Grain Yield and Yield Attributes. J. Integr. Agric. 2019, 18, 563–570. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Jiao, X.; Li, H.; Hu, T.; Jiang, H.; Mahmoud, A. Effects of Biochar on Water Quality and Rice Productivity under Straw Returning Condition in a Rice-Wheat Rotation Region. Sci. Total Environ. 2022, 819, 152063. [Google Scholar] [CrossRef] [PubMed]
- Tanazawa, Y.; Tomotsune, M.; Suzuki, T.; Koizumi, H.; Yoshitake, S. Photosynthetic Response of Young Oaks to Biochar Amendment in Field Conditions over 3 Years. J. For. Res. 2021, 26, 116–126. [Google Scholar] [CrossRef]
- Driesen, E.; Van Den Ende, W.; De Proft, M.; Saeys, W. Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Bera, T.; Bhaduri, D.; Sarkar, B.; Mandal, S.; Wade, P.; Kumari, S.; Biswas, S.; Menon, M.; Pathak, H.; et al. A Review on Biochar Modulated Soil Condition Improvements and Nutrient Dynamics Concerning Crop Yields: Pathways to Climate Change Mitigation and Global Food Security. Chemosphere 2019, 227, 345–365. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Y.; Zhang, W.; Sui, Y.; Jin, D.; Xin, W.; Yi, J.; He, D. Biochar Prepared at Different Pyrolysis Temperatures Affects Urea-Nitrogen Immobilization and N 2 O Emissions in Paddy Fields. PeerJ 2019, 7, e7027. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Chen, M.; Liu, J.; Ding, X. A Sustainable Option: Biochar Addition Can Improve Soil Phosphorus Retention and Rice Yield in a Saline–Alkaline Soil. Environ. Technol. Innov. 2021, 24, 102070. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Yang, Y.; Xia, X.; Li, F.; Yang, Z.; Xing, B. Biochar’s Stability and Effect on the Content, Composition and Turnover of Soil Organic Carbon. Geoderma 2020, 364, 114184. [Google Scholar] [CrossRef]


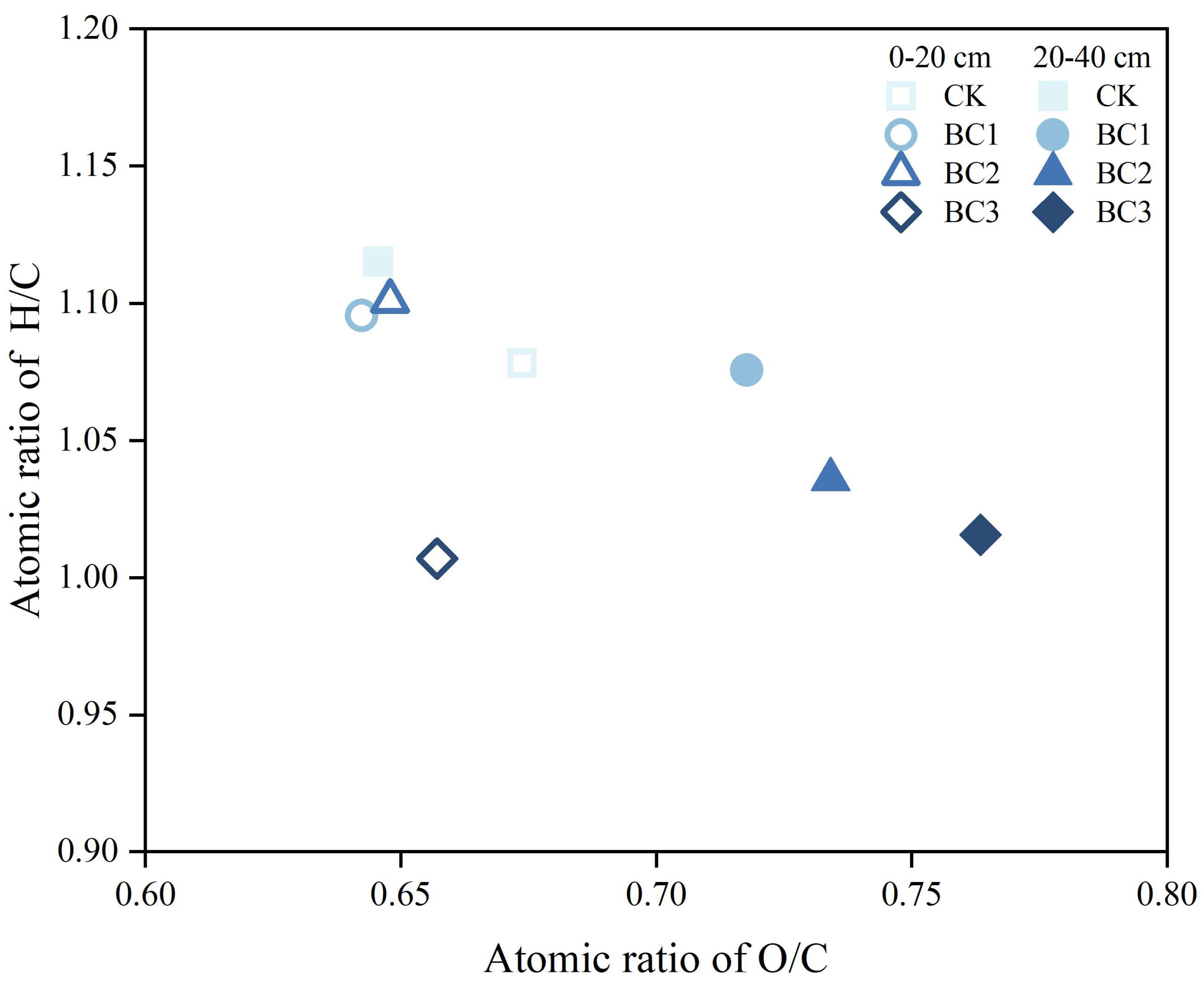


| Depth (cm) | Treatment | 2920 cm−1 | 2850 cm−1 | 1720 cm−1 | 1620 cm−1 | I2920/I1720 | I2920/I1620 |
|---|---|---|---|---|---|---|---|
| 0–20 | CK | 2.68 ± 0.17 a | 1.00 ± 0.09 a | 10.74 ± 0.55 a | 12.02 ± 1.79 a | 0.25 ± 0.01 a | 0.223 ± 0.03 a |
| BC1 | 2.07 ± 1.02 a | 1.07 ± 0.16 a | 10.26 ± 1.00 a | 12.63 ± 1.04 a | 0.21 ± 0.11 a | 0.17 ± 0.09 a | |
| BC2 | 2.67 ± 0.20 a | 1.02 ± 0.15 a | 11.13 ± 0.63 a | 13.14 ± 1.13 a | 0.24 ± 0.03 a | 0.20 ± 0.03 a | |
| BC3 | 2.61 ± 0.24 a | 1.09 ± 0.13 a | 10.41 ± 0.33 a | 12.45 ± 1.21 a | 0.25 ± 0.02 a | 0.21 ± 0.04 a | |
| 20–40 | CK | 2.70 ± 0.08 b | 1.03 ± 0.02 b | 10.05 ± 0.46 b | 13.01 ± 1.87 d | 0.27 ± 0.02 ab | 0.21 ± 0.04 a |
| BC1 | 2.83 ± 0.11 b | 1.01 ± 0.06 b | 11.22 ± 0.76 a | 16.64 ± 0.81 c | 0.25 ± 0.02 b | 0.17 ± 0.01 ab | |
| BC2 | 2.72 ± 0.48 b | 1.22 ± 0.08 a | 10.30 ± 0.35 ab | 20.70 ± 0.79 b | 0.27 ± 0.05 ab | 0.13 ± 0.03 b | |
| BC3 | 3.37 ± 0.06 a | 1.12 ± 0.13 ab | 10.44 ± 0.33 ab | 24.05 ± 0.36 a | 0.32 ± 0.01 a | 0.14 ± 0.01 b |
| Depth (cm) | Treatment | Peak A | Peak B | ||
|---|---|---|---|---|---|
| Ex/Em | FI (a.u.) | Ex/Em | FI (a.u.) | ||
| 0–20 | CK | 460/525 | 243.42 ± 5.92 a | 280/530 | 242.03 ± 17.47 a |
| BC1 | 460/525 | 235.36 ± 5.70 a | 280/525 | 253.95 ± 4.66 a | |
| BC2 | 460/529 | 217.53 ± 5.43 b | 280/522 | 253.56 ± 8.57 a | |
| BC3 | 460/525 | 212.60 ± 2.82 b | 280/516 | 239.64 ± 11.44 a | |
| 20–40 | CK | 460/530 | 243.29 ± 2.15 a | 280/526 | 248.26 ± 16.21 a |
| BC1 | 460/522 | 202.48 ± 3.59 b | 280/516 | 220.18 ± 2.42 b | |
| BC2 | 460/523 | 195.59 ± 4.09 c | 280/530 | 209.89 ± 3.40 bc | |
| BC3 | 460/524 | 152.84 ± 5.52 d | 280/528 | 200.90 ± 4.73 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Dou, S.; Guo, D.; Zhao, H. The Application of Biochar Enhances Soil Organic Carbon and Rice Yields. Agronomy 2024, 14, 455. https://doi.org/10.3390/agronomy14030455
Yang C, Dou S, Guo D, Zhao H. The Application of Biochar Enhances Soil Organic Carbon and Rice Yields. Agronomy. 2024; 14(3):455. https://doi.org/10.3390/agronomy14030455
Chicago/Turabian StyleYang, Chuang, Sen Dou, Dan Guo, and Hangjin Zhao. 2024. "The Application of Biochar Enhances Soil Organic Carbon and Rice Yields" Agronomy 14, no. 3: 455. https://doi.org/10.3390/agronomy14030455






