A Review of the Influence of Genotype, Environment, and Food Processing on the Bioactive Compound Profile of Red Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Review Structure
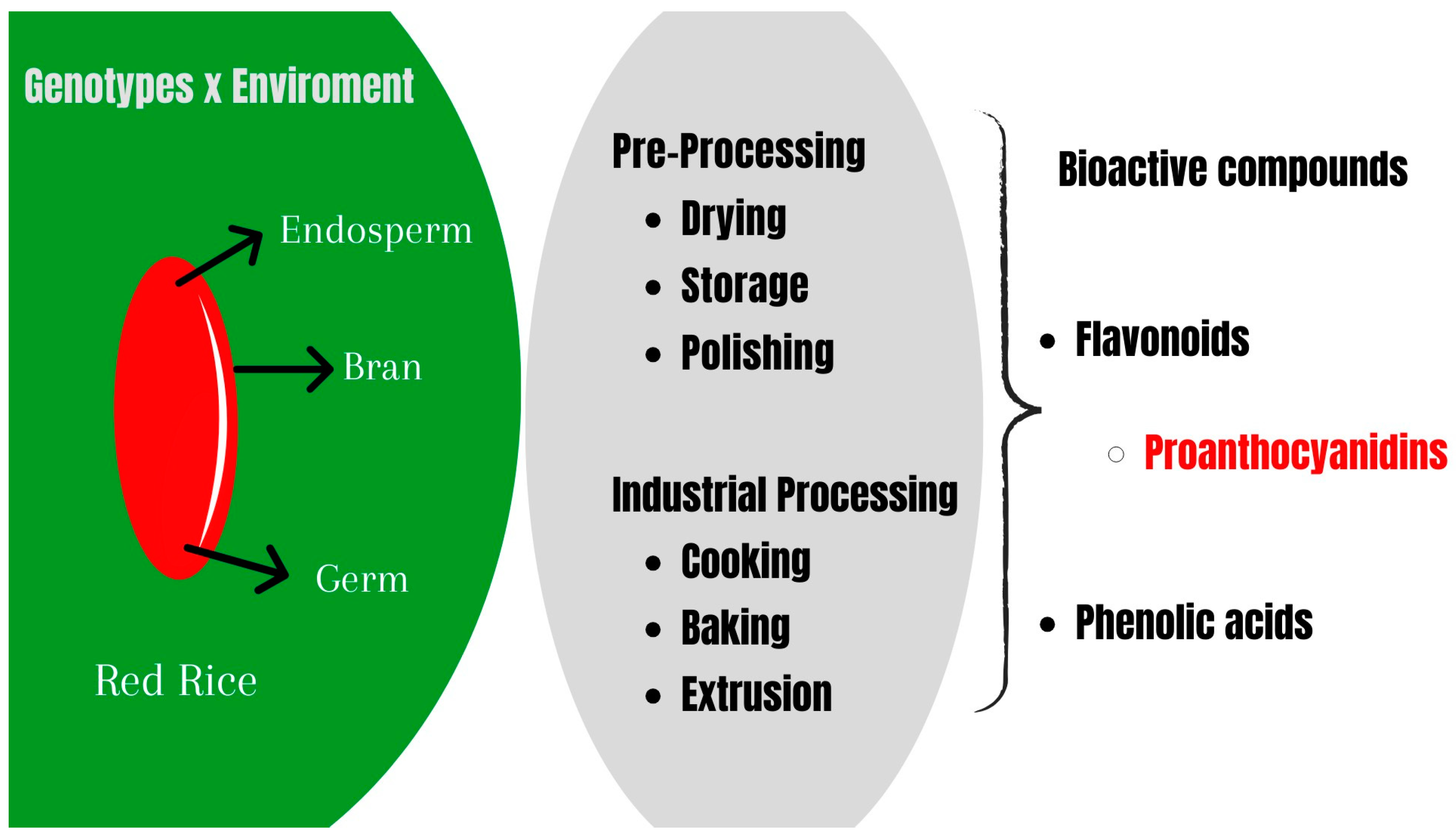
3. The Role of Different Red Rice Genotypes and Growth Environments in the Phytochemical Profile
4. Effect of Industrial Pre-Processing on the Bioactive Compounds in Red Rice
4.1. Drying and Storage
4.2. Polishing
5. Bioactive Compounds in Red Rice Products
5.1. Cooked Products
5.2. Bakery Products
5.3. Extruded Products
5.4. Germinated Red Rice
6. Health Benefits of Red Rice Products
7. Conclusions and Final Considerations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations List
References
- Lang, G.H.; Rockenbach, B.A.; Ferreira, C.D.; Oliveira, M. Delayed interval drying of red rice: Effects on cooking properties, in vitro starch digestibility and phenolic content. J. Product Stored. 2020, 87, 101613. [Google Scholar] [CrossRef]
- Wickert, E.; Schiocchet, M.A.; Noldin, J.A.; Raimondi, J.V.; Andrade, A.; Scheuermann, K.K.; Marschalek, R.; Martins, G.N.; Hickel, E.; Eberhardt, D.S.; et al. Exploring variability: New Brazilian varieties SCS119 Rubi and SCS120 Onix for specialty rice markets. Open J. Genet. 2014, 4, 157–165. [Google Scholar] [CrossRef]
- EMBRAPA. 2021. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/209710/1/Doc-262-Os-arrozes-vermelhos-AINFO-1.pdf (accessed on 20 February 2024).
- Chen, X.; Yang, Y.; Yang, X.; Zhu, G.; Lu, X.; Jia, J.; Diao, B.; Yu, S.; Ali, A.; Zhang, H.; et al. Investigation of flavonoid components and their associated antioxidant capacity in different pigmented rice varieties. Food Res. Int. 2022, 161, 111726. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.P.; Hoffmann, J.F.; Ferreira, C.D.; Diehl, G.W.; Rossi, R.C.; Ziegle, V. Effect of germination on the nutritional and bioactive properties of red rice grains and their application in the production of cupcakes. Int. J. Gastron. Food Sci. 2021, 25, 100379. [Google Scholar] [CrossRef]
- Finocchiaro, F.; Ferrari, B.; Gianinetti, A. A biodiversity study of flavonoid content in rice caryopsis showing the simultaneous accumulation of anthocyanins and proanthocyanidins in a black grain genotype. J. Cereal Sci. 2010, 51, 28–34. [Google Scholar] [CrossRef]
- Meza, S.L.R.; Massaretto, I.L.; Sinnecker, P.; Schmiele, M.; Chang, K.; Noldin, J.A.; Marquez, U.M.L. Impact of thermoplastic extrusion process on chemical, nutritional, technological and sensory properties of gluten-free breakfast cereals from pigmented rice. Int. J. Food Sci. Technol. 2021, 56, 3218–3226. [Google Scholar] [CrossRef]
- Singh, S.P.; Vanlalsanga, S.K.; Mehta, Y.; Singh, T. New insight into the pigmented rice of northeast India revealed highantioxidant and mineral compositions for better human health. Heliyon 2022, 8, 10464. [Google Scholar] [CrossRef]
- Mwiinga, B.; Sibiya, J.; Kondwakwenda, A.; Musvosvi, C.; Chigeza, G. Genotype x environment interaction analysis of soybean (Glycine max (L.) Merrill) grain yield across production environments in Southern Africa. Field Crops Res. 2020, 256, 107922. [Google Scholar] [CrossRef]
- Ziegler, V.; Ferreira, C.D.; Hoffmann, J.F.; Chaves, F.C.; Vanier, N.L.; de Oliveira, M.; Elias, M.C. Cooking quality properties and free and bound phenolic content of brown, black and red rice grains stored at different temperatures for six months. Food Chem. 2018, 242, 427–434. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Lang, G.H.; Lindemann, I.S.; Timm, N.S.; Hoffmann, J.F.; Ziegler, V.; Oliveira, M. Postharvest UV-C irradiation for mold control and mycotoxin reduction in brown, black and red rice during long-term storage. Food Chem. 2021, 339, 127810. [Google Scholar] [CrossRef]
- Shao, Y.; Hu, Z.; Yu, Y.; Mou, R.; Zhu, Z.; Beta, T. Phenolic acids, anthocyanins, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chem. 2018, 239, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Sumczynski, D.; Kotásková, E.; Družbíková, H.; Mlček, J. Determination of contents and antioxidant activity of free and bound phenolics compounds and in vitro digestibility of commercial black and red rice (Oryza sativa L.) varieties. Food Chem. 2016, 211, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Meera, K.; Smita, M.; Haripriya, S.; Sem, S. Varietal influence on the antioxidant properties and glycemic index of pigmented and unpigmented rice. J. Cereal Sci. 2018, 87, 202–208. [Google Scholar] [CrossRef]
- Susmitha, T.; Baran, T.; Bagchib, B.; Deb, S.; Biswas, T.; Adak, T.; Banerjee, H.; Srikumar, A. Color, texture and nutritional properties evaluation of Pigmented Rice-Based Fermented Steam Cooked Foods Idli. Food Chem. Adv. 2022, 1, 100021. [Google Scholar] [CrossRef]
- Ramos, A.H.; Timm, N.S.; Rockenbach, B.A.; Ferreira, C.D.; Hoffmann, J.F.; Oliveira, M. Red rice drying and storage: Effects on technological properties and phenolic compounds of the raw and cooked grains. J. Cereal Sci. 2022, 103, 103405. [Google Scholar] [CrossRef]
- Waewkum, P.; Singthong, J. Functional properties and bioactive compounds of pigmented brown rice flour. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100289. [Google Scholar] [CrossRef]
- Devi, L.M.; Badwaik, L.S. Variety difference in physico-chemical, cooking, textural, pasting and phytochemical properties of pigmented rice. Food Chem. Adv. 2022, 1, 100059. [Google Scholar] [CrossRef]
- Nayeem, S.; Sundararajan, S.; Ashok, A.K.; Abusaliya, A.; Ramalingam, S. Effects of cooking on the phytochemical and antioxidant properties of rare varieties of pigmented and unpigmented Indian rice. Biocatal. Agric. Biotechnol. 2021, 32, 101928. [Google Scholar] [CrossRef]
- Lang, G.H.; Kringela, D.H.; Acunha, T.S.; Ferreira, C.D.; Dias, A.R.G.; Zavareze, E.R.; Oliveira, M. Cake of brown, black and red rice: Influence of transglutaminase on technological properties, in vitro starch digestibility and phenolic compounds. Food Chem. 2020, 318, 126480. [Google Scholar] [CrossRef]
- Fan, M.; Yan, Y.; Waleed, A.A.; Qian, H.; Li, Y.; Rao, Z.; Wang, L. Germination-induced changes in anthocyanins and proanthocyanidins: A pathway to boost bioactive compounds in red rice. Food Chem. 2024, 433, 137283. [Google Scholar] [CrossRef]
- Samyor, D.; Das, A.B.; Deka, S.C. Pigmented rice a potential source of bioactive compounds: A review. Int. J. Food Sci. Tecnol. 2017, 52, 1073–1081. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Cao, C.; Shen, T.; Zhong, L.; He, H.; Chen, X. Comprehensive metabolomic and proteomic analysis in biochemical metabolic pathways of rice spikes under drought and submergence stress. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2019, 1867, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Cañizares, L.C.C.; Timm, N.S.; Lang, G.H.; Gaioso, C.A.; Ferreira, C.D.; Oliveira, M. Effects of using wind exhausters on the quality and cost of soybean storage on a real scale. J. Stored Prod. Res. 2021, 93, 101834. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, X.; Zhang, M.; Blanchard, C. Phenolics, flavonoids, proanthocyanidins and antioxidant activity of brown rice with different pericarp colors after storage. J. Stored Prod. Res. 2014, 59, 120–125. [Google Scholar] [CrossRef]
- Sheng, K.; Zheng, H.; Shui, S.; Yan, L.; Liu, C.; Zheng, L. Comparison of postharvest UV-B and UV-C treatments in table grape: Changes in phenolic compounds and their transcription of biosynthetic genes during storage. Postharvest Biol. Technol. 2018, 138, 74–81. [Google Scholar] [CrossRef]
- Wu, X.; Guan, W.; Yan, R.; Lei, J.; Xu, L.; Wang, Z. Postharvest Biology and Technology Effects of UV-C on antioxidant activity, total phenolics and main phenolic compounds of the melanin biosynthesis pathway in different tissues of button mushroom. Postharvest Biol. Technol. 2016, 118, 51–58. [Google Scholar] [CrossRef]
- Reddy, C.K.; Kimi, L.; Haripriya, S.; Kang, N. Effects of Polishing on Proximate Composition, Physico-Chemical Characteristics, Mineral Composition and Antioxidant Properties of Pigmented Rice. Rice Sci. 2017, 24, 241–252. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Ragaee, S.; Seetharaman, K.; Abdel-Aal, E.S.M. The impact of milling and thermal processing on phenolic compounds in cereal grains. Crit. Rev. Food Sci. Nutr. 2014, 54, 837–849. [Google Scholar] [CrossRef]
- Nascimento, L.A.; Abhilasha, A.; Singh, J.; Elias, A.C.; Colussi, R. Rice Germination and Its Impact on Technological and Nutritional Properties: A Review. Rice Sci. 2022, 29, 201–215. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Analysis of proximate composition, mineral content and fatty acids of aromatic and non-aromatic Indian rice. Rice Sci. 2017, 24, 21–31. [Google Scholar] [CrossRef]
- Lee, J.; Min, S.G.; Hong, S.W.; Kim, J.H.; Kim, G.; Yun, Y. Anti-obesity effects of kimchi with red yeast rice in 3T3-L1 adipocytes and high-fat diet-induced obese mice. J. Funct. Foods 2023, 106, 105594. [Google Scholar] [CrossRef]
- Xiong, Z.; Cao, X.; Wen, Q.; Chen, Z.; Cheng, Z.; Huang, X.; Zhang, Y.; Long, C.; Zhang, Y.; Huang, Z. An overview of the bioactivity of monacolin K/lovastatin. Food Chem. Toxicol. 2019, 131, 110585. [Google Scholar] [CrossRef]
- NCCIH. Effects of Black Rice Anthocyanin Enrichment on Bread Digestibility and Glycemic Index Current Developments in Nutrition. In Red Yeast Rice: What You Need to Know; NCCIH: Bethesda, MD, USA, 2021. [Google Scholar]
- Huang, Y.; Li, P.; Li, Z.; Zhu, D.; Fan, Y.; Wang, X.; Wang, S. Red yeast rice dietary intervention reduces oxidative stress-related inflammation and improves intestinal microbiota. Food Funct. 2022, 13, 6583–6595. [Google Scholar] [CrossRef]
- Rahmani, P.; Melekoglu, E.; Tavakoli, S.; Malekpour Alamdari, N.; Rohani, P.; Sohouli, M.H. Impact of red yeast rice supplementation on lipid profile: A systematic review and meta-analysis of randomized controlled trials. Expert Rev. Clin. Pharmacol. 2023, 16, 73–81. [Google Scholar] [CrossRef]
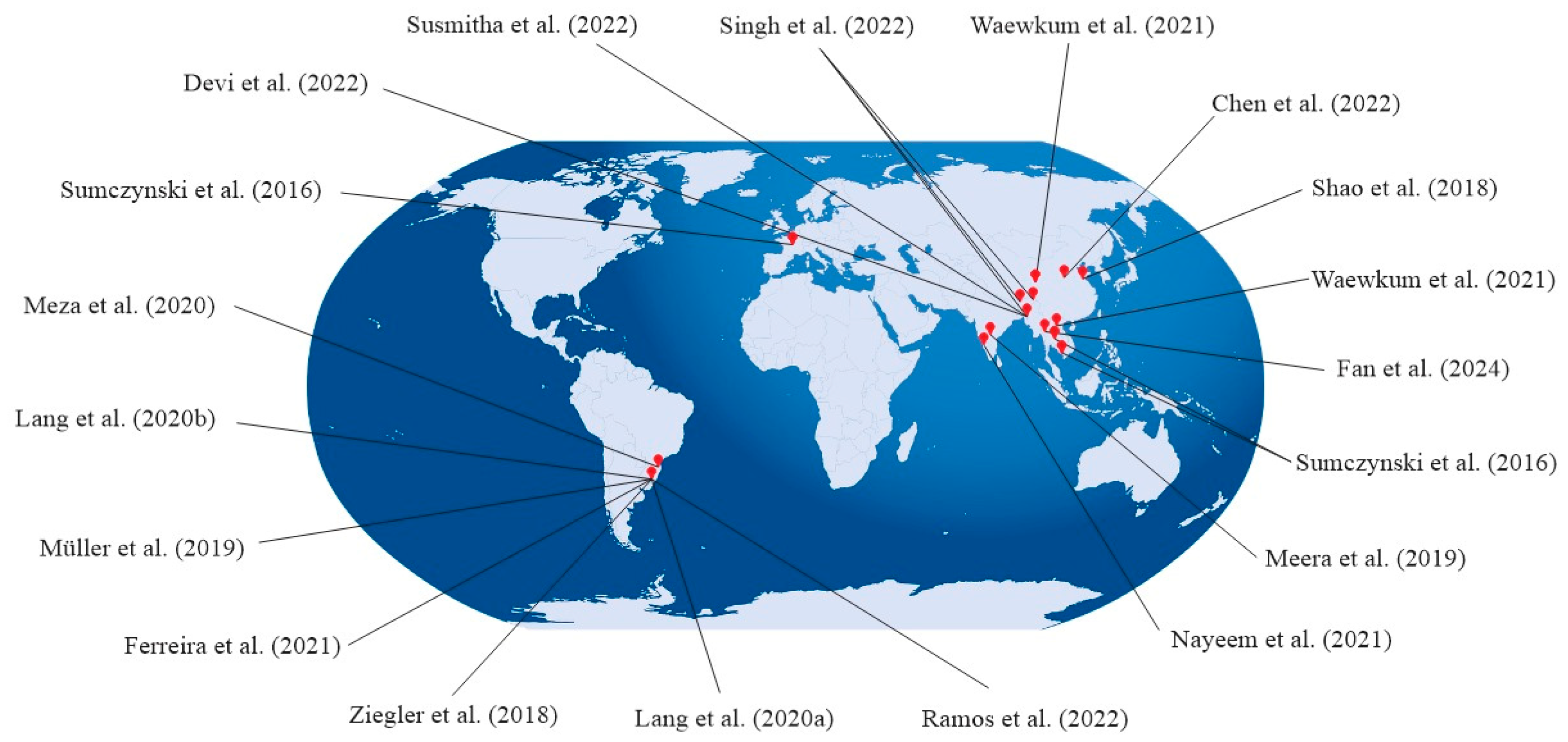
| Variables | Condition | Total Free Phenolics | Total Flavonoids | Total Proanthocyanidins | Antioxidant Activity | Individual Free Phenolics (Range) | References |
|---|---|---|---|---|---|---|---|
| Delayed drying and stored at 12 months | 0–6 days | 6.59–5.19 mg GAE g−1 | - | 3.13–2.17 mg CE g−1 | - | - | [1] |
| Genotypes | Hongnuo Bianduhongmi Hongcaomi, Hongxiangmi Yueyahongmi, Jingganghongmi Sikoutianyoujihongmi | 1.45 a 2.69 mg GAE g−1 | 0.94 a 1.31 mg RE g−1 | - | 80.60 a 113.45 μmol Trolox g−1 (ORAC assay) | Catechin: 3.26–6.13 µg g−1 Epicatechin: 1.11–1.43 µg g−1 Ferulic acid: 0.10–1.03 µg g−1 Naringin: 1.31–2.58 µg g−1 Protocatechuic acid: 1.45–9.79 µg g−1 Quercetin: 1.75–2.60 µg g−1 | [4] |
| Rice cake sprouted at different times | 8–40 h | 0.67–1.13 mg GAE g−1 | 0.46–1.14 mg CE g−1 | - | 7.77–10.70 μmol TE g−1 (DPPH assay) | Caffeic acid: 0.54–0.78 μg g−1 Catechin: 1.16–2.06 μg g−1 Coumaric acid: 0.42–0.86 μg g−1 Ferulic acid: 4.52–5.86 μg g−1 Myricetin: 0.32–0.69 μg g−1 Quercetin: 0.55–4.19 μg g−1 Rutin: 3.19–7.39 μg g−1 | [5] |
| Snacks | - | 100.21 mg GAE g−1 | 0.99 mg CE g−1 | 0.83 mg CE g−1 | 3.1 mol TE g−1 (DPPH assay) 24.4 mol TE g−1 (ORAC assay) | - | [7] |
| Genotypes | Lumre Tsulu tsuk Aamda Tasung Kawnglawn Fazu Menil mibabaret | 4.87–9.00 mg GAE g−1 | 0.33–2.86 mg QE 100 g−1 | - | 52.23–97.69% (DPPH assay) | - | [8] |
| Stored at 6 months | 16–40 °C | 11.3–14.6 mg GAE g−1 | - | 0.981–0.987 mg CE g−1 | 0.8–3.5 mg TE g−1 | Catechin: 2.3–4.6 µg g−1 Ferulic acid: 3.5–6.9 µg g−1 p-Coumaric acid: 0.2–0.9 µg g−1 | [10] |
| UV-C and stored for 6 months | 0–3 h | - | - | - | - | Caffeic acid: 2.3–2.7 µg g−1 Coumaric acid: 5.4–6.0 µg g−1 Ferulic acid: 4.4–5.5 µg g−1 Gallic acid: 0.0–1.1 µg g−1 Vanillic acid: 2.7–5.5 µg g−1 | [11] |
| Genotypes | Jinggangshanhongmi Xiangwanxian 12 Qianxiuhong Changhong No. 2 Hongmi 2 | - | 1.62–3.83 mg CE g−1 | - | - | Ferulic acid: 1.32–2.59 µg g−1 Isoferulic acid: 0.27–0.82 µg g−1 p-coumaric acid: 1.76–1.56 µg g−1 | [12] |
| Location | France Cambodia Thailand | 0.0076–0.0119 mg RE g−1 | 0.0822–0.2057 mg RE g−1 | - | 0.00159–0.00066 mmol TE g−1 (DPPH assay) | Caffeic acid: 19.5–25.3 µg g−1 Catechin: 4.2–5.7 µg g−1 Cinnamic acid: 0.1–1.1 µg g−1 Ferulic acid: 2.7–5.3 µg g−1 Gallic acid: 10.2–10.3 µg g−1 m-Coumaric acid: 0.2–0.3 µg g−1 Protocatechuic acid: 20.4–30.2 µg g−1 Quercetin: 2.3–3.4 µg g−1 Rutin: 1.3–3.3 µg g−1 Syringic acid: 1.6–2.9 µg g−1 trans-p-coumaric acid: 0.5–3.7 µg g−1 Vanillic acid: 0.5–1.2 µg g−1 | [13] |
| Idli | - | 0.19 mg GAE g−1 | 99.10 mg QE g−1 | - | 0.11 mg FA g−1 (DPPH assay) | - | [15] |
| Drying temperature and stored for 12 months | 40–100 °C | - | - | - | - | Caffeic acid: 2.39–2.50 µg g−1 Catechin: 4.47–4.57 µg g−1 Epicatechin: 2.62–2.76 µg g−1 Ferulic acid: 3.78–3.81 µg g−1 Luteolin: 1.86–1.89 µg g−1 p-coumaric acid: 1.69–1.73 µg g−1 | [16] |
| Drying temperature cooked | 40–100 °C | - | - | - | - | Caffeic acid: 2.44–3.30 µg g−1 Catechin: 3.02–4.08 µg g−1 Chlorogenic acid: 4.36–8.58 µg g−1 Epicatechin: 4.75–4.85 µg g−1 Ferulic acid: 3.09–3.44 µg g−1 Luteolin: 1.70–2.57 µg g−1 p-coumaric acid: 2.05–2.07 µg g−1 | [16] |
| Flour | - | 11.029 mg GAE g−1 | 60.422 mg QE g−1 | - | 2.538 mg TEAC g −1 (DPPH assay) | - | [17] |
| Bran | - | 10.62 mg GAE g−1 | 11.22 mg QE g−1 | - | 90.93% (DPPH assay) | - | [18] |
| Cooking | - | 220–319 mg GAE g−1 | 1000–1200 mg QE g−1 | - | - | - | [19] |
| Pie with transglutaminase | - | 432.8 mg GAE g−1 | - | - | - | Caftaric acid: 0.51–0.52 µg g−1 Catechin: 7.05–8.93 µg g−1 Hydroxybenzoic acid: 9.82–11.58 µg g−1 p-coumaric acid: 1.72–2.05 µg g−1 | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.A.; Cañizares, L.d.C.C.; Meza, S.L.R.; Peres, B.B.; Jappe, S.N.; Timm, N.d.S.; Oliveira, M.d.; Coradi, P.C. A Review of the Influence of Genotype, Environment, and Food Processing on the Bioactive Compound Profile of Red Rice (Oryza sativa L.). Agronomy 2024, 14, 616. https://doi.org/10.3390/agronomy14030616
Rodrigues LA, Cañizares LdCC, Meza SLR, Peres BB, Jappe SN, Timm NdS, Oliveira Md, Coradi PC. A Review of the Influence of Genotype, Environment, and Food Processing on the Bioactive Compound Profile of Red Rice (Oryza sativa L.). Agronomy. 2024; 14(3):616. https://doi.org/10.3390/agronomy14030616
Chicago/Turabian StyleRodrigues, Larissa Alves, Lázaro da Costa Corrêa Cañizares, Silvia Leticia Rivero Meza, Betina Bueno Peres, Silvia Naiane Jappe, Newiton da Silva Timm, Maurício de Oliveira, and Paulo Carteri Coradi. 2024. "A Review of the Influence of Genotype, Environment, and Food Processing on the Bioactive Compound Profile of Red Rice (Oryza sativa L.)" Agronomy 14, no. 3: 616. https://doi.org/10.3390/agronomy14030616





