Evaluation of Maize Hybrids for Resistance to Ear Rot Caused by Dominant Fusarium Species in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolation and Species Identification
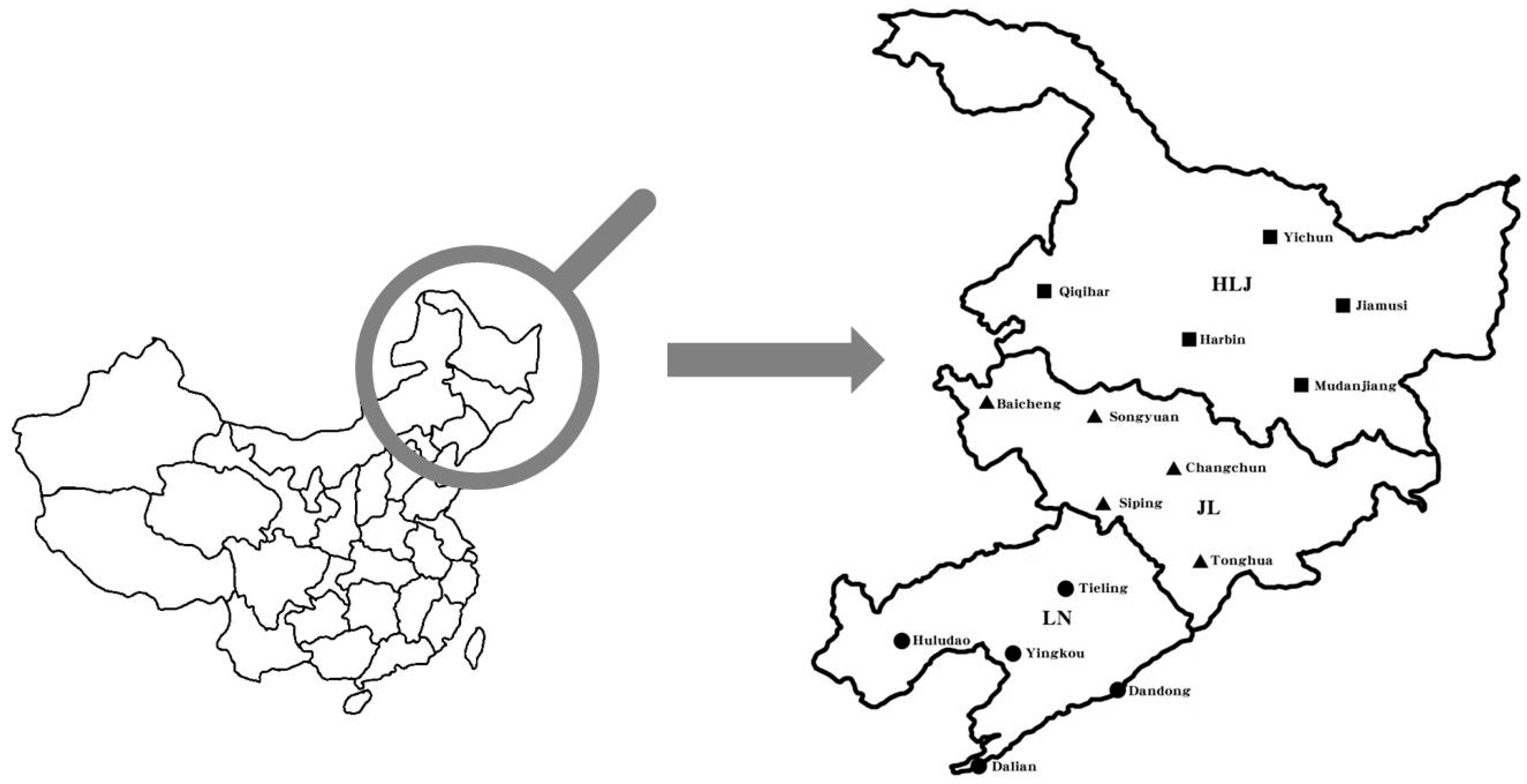
2.2. Field Experiment Design and Inoculum Preparation
2.3. Inoculation and Evaluation
3. Results
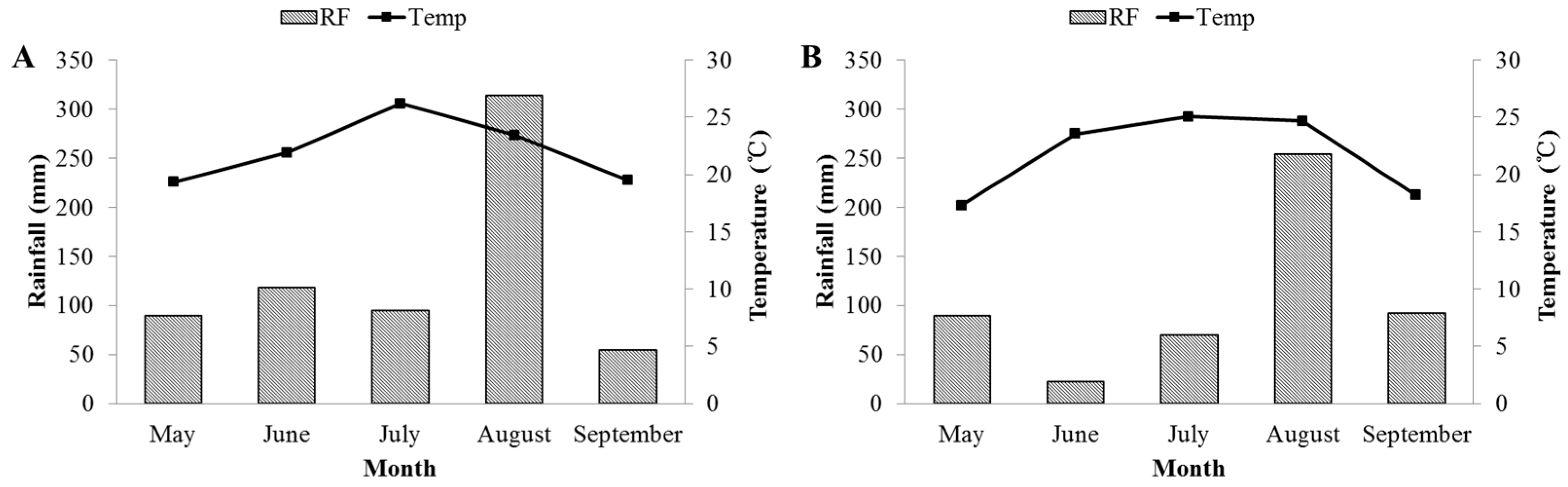
3.1. Weather Conditions
3.2. Occurrence Frequency and Distribution of Different Species of Fusarium
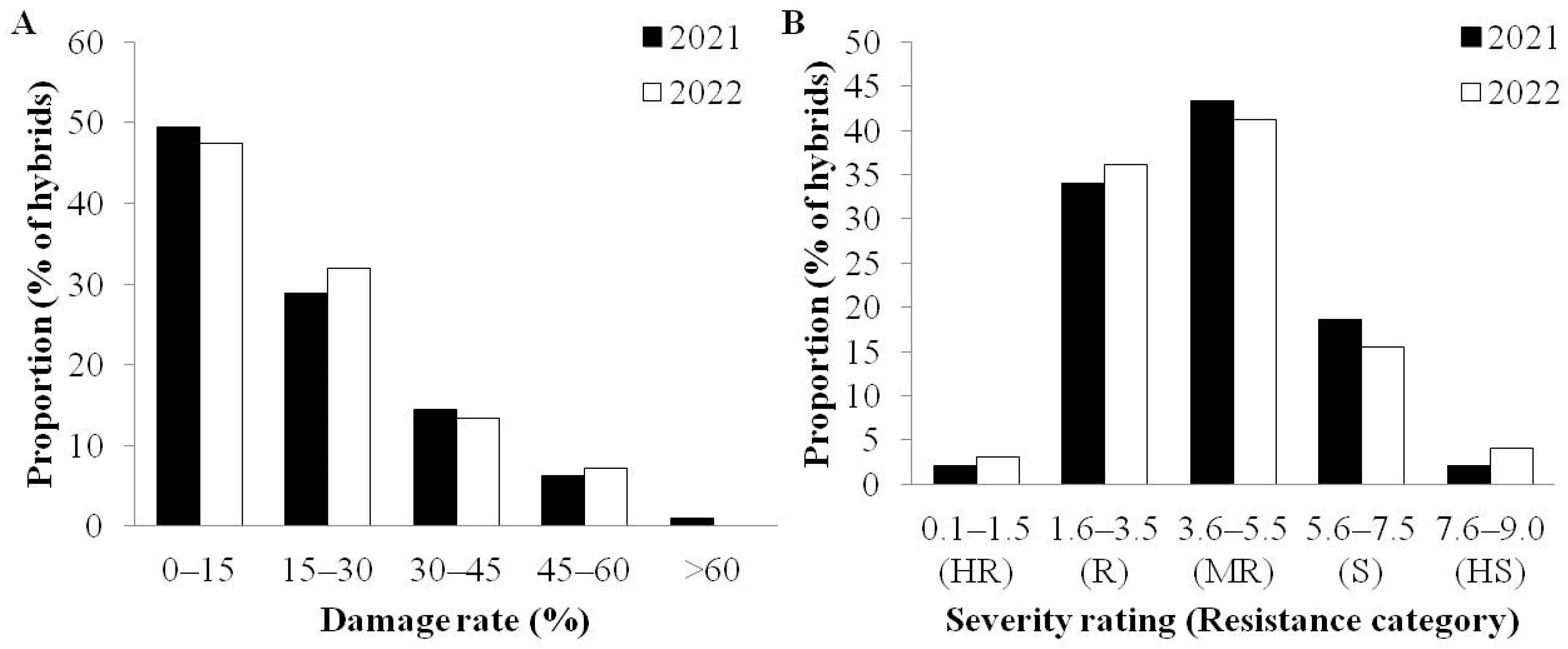
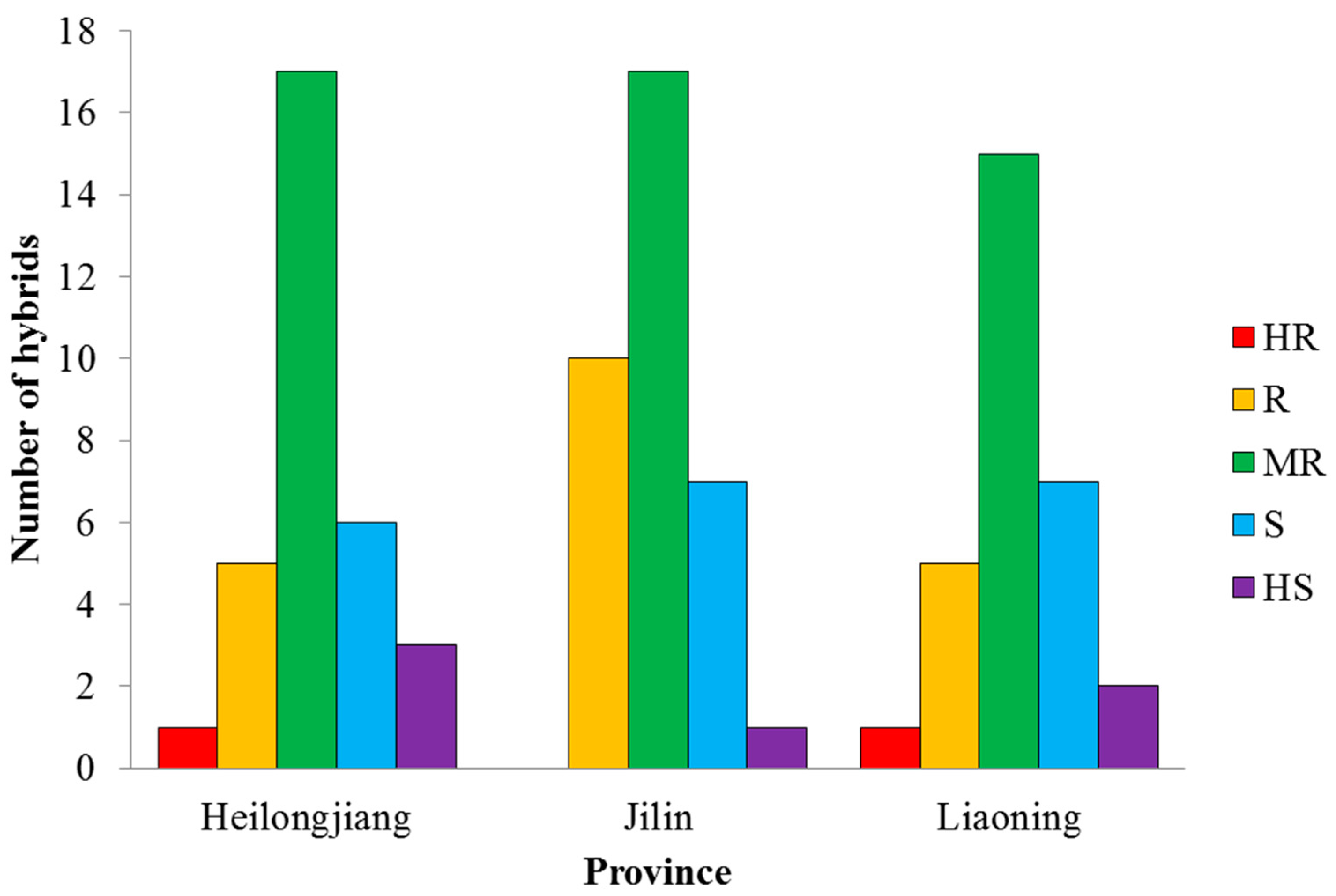
3.3. Evaluation of Maize Hybrids
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, L.N.; Qu, Q.; Cao, Z.Y.; Guo, Z.Y.; Jia, H.; Liu, N.; Wang, Y.H.; Dong, J.G. The relationship analysis on corn stalk rot and ear rot according to Fusarium species and fumonisin contamination in kernels. Toxins 2019, 11, 320. [Google Scholar] [CrossRef]
- Logrieco, A.; Mulè, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Lemmens, M.; Reid, L.M. Breeding for resistance to ear rots caused by Fusarium spp. in maize—A review. Plant Breed. 2012, 131, 1–19. [Google Scholar] [CrossRef]
- Vigier, B.; Reid, L.M.; Seifert, K.A.; Stewart, D.W.; Halminton, R.I. Distribution and prediction of Fusarium species associated with maize ear rot in Ontario. Can. J. Plant Pathol. 1997, 19, 60–65. [Google Scholar] [CrossRef]
- Munkvold, G.P. Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur. J. Plant Pathol. 2003, 109, 705–713. [Google Scholar] [CrossRef]
- Reddy, K.; Salleh, B.; Saad, B.; Abbas, H.; Abel, C.; Shier, W. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Sundheim, L.; Tsehaye, H. Fumonisin in Zambia and neighboring countries in a changing climate. Adv. Environ. Res. 2015, 39, 69–84. [Google Scholar]
- Wu, F. Measuring the economic impacts of Fusarium toxins in animal feeds. Anim. Feed Sci. Technol. 2007, 137, 363–374. [Google Scholar] [CrossRef]
- Ammar, M.; Merfat, A.; Walid, N.; Paul, H.V.; Mohammad, H. Morphological and molecular characterization of Fusarium isolated from maize in Syria. J. Phytopathol. 2013, 161, 452–458. [Google Scholar]
- Borah, S.N.; Goswami, D.; Sarma, H.K.; Cameotra, S.S.; Deka, S. Rhamnolipid biosurfactant against Fusarium verticillioides to control stalk and ear rot disease of maize. Front. Microbiol. 2016, 7, 1505. [Google Scholar] [CrossRef]
- Leslie, J.F.; Anderson, L.L.; Bowden, R.L.; Lee, Y.W. Inter- and intra-specific genetic variationin Fusarium. Int. J. Food Mircobiol. 2007, 119, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Presello, D.A.; Iglesiasm, J.; Botta, G.; Reid, L.M.; Lori, G.A.; Eyhérabide, G.H. Stability of maize resistance to the ear rots caused by Fusarium graminearum and F. verticillioides in Argentinian and Canadian environments. Euphytica 2006, 147, 403–407. [Google Scholar] [CrossRef]
- Stewart, D.W.; Reid, L.M.; Nicol, R.W.; Schaafsma, A.W. A mathematical simulation of growth of Fusarium in maize ears after artificial inoculation. Phytopathology 2002, 92, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Aguín, O.; Cao, A.; Pintos, C.; Santiago, R.; Mansilla, P.; Butrón, A. Occurrence of Fusarium species in maize kernels grown in northwestern Spain. Plant Pathol. 2014, 63, 946–951. [Google Scholar] [CrossRef]
- Bottalico, A. Fusarium diseases of cereals: Species complex and related mycotoxins profiles, in Europe. J. Plant Pathol. 1998, 80, 85–103. [Google Scholar]
- Boutigny, A.L.; Ward, T.J.; Ballois, N.; Iancu, G.; Ioos, R. Diversity of the Fusarium graminearum species complex on French cereals. Eur. J. Plant Pathol. 2014, 138, 133–148. [Google Scholar] [CrossRef]
- Covarelli, L.; Stifano, S.; Beccari, G.; Raggi, L.; Lattanzio, V.M.T.; Albertini, E. Characterization of Fusarium verticillioides strains isolated from maize in Italy: Fumonis in production, pathogenicity and genetic variability. Food Microbiol. 2012, 31, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Dorn, B.; Forrer, H.R.; Schurch, S.; Vogelgsang, S. Fusarium species complex on maize in Switzerland: Occurrence, prevalence, impact and mycotoxins in commercial hybrids under natural infection. Eur. J. Plant Pathol. 2009, 125, 51–61. [Google Scholar] [CrossRef]
- Lew, H.; Adler, A.; Edinger, W. Moniliformin and the European corn borer (Ostrinia nubilalis). Mycotoxin Res. 1991, 7A, 71–76. [Google Scholar] [CrossRef]
- Scauflaire, J.; Mahieu, O.; Louvieaux, J.; Foucart, G.; Renard, F.; Munaut, F. Biodiversity of Fusarium species in ears and stalks of maize plants in Belgium. Eur. J. Plant Pathol. 2011, 131, 59–66. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Manandhar, G.; Plattner, R.D.; Margos, C.M.; Shrestha, K.; McCormick, S.P. Occurrence of Fusarium species and mycotoxins in Nepalese maize and wheat and the effect of traditional processing methods on mycotoxin levels. J. Agric. Food Chem. 2000, 48, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, A.W.; Limay-rios, V.; Tamburic-illincic, L. Mycotoxins and Fusarium species associated with maize ear rot in Ontario, Canada. Cereal Res. Commun. 2008, 36, 525–527. [Google Scholar]
- Sliva, J.J.; Viaro, H.P.; Ferranti, L.S.; Oliveira, A.L.M.; Ferreira, J.M.; Ruas, C.F.; Ono, E.Y.S.; Fungaro, M.H.P. Genetic structure of Fusarium verticillioides populations and occurrence of fumonisins in maize grown in Southern Brazil. Crop Prot. 2017, 99, 160–167. [Google Scholar] [CrossRef]
- Small, I.M.; Flett, B.C.; Marasas, W.F.O.; McLeod, A.; Stander, M.A.; Viljoen, A. Resistance in maize inbred lines to Fusarium verticillioides and fumonisin accumulation in South Africa. Plant Dis. 2012, 96, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Tsehaye, H.; Brurberg, M.B.; Sundheim, L.; Assefa, D.; Tronsmo, A.; Tronsmo, A.M. Natural occurrence of Fusarium species and fumonisin on maize grains in Ethiopia. Eur. J. Plant Pathol. 2017, 147, 141–155. [Google Scholar] [CrossRef]
- Duan, C.X.; Qin, Z.H.; Yang, Z.H.; Li, W.X.; Sun, S.L.; Zhu, Z.D.; Wang, X.M. Identification of pathogenic Fusarium spp. causing maize ear rot and potential mycotoxin production in China. Toxins 2016, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.W.; Xu, J.; Jiang, Y.; Hu, L.; van der Lee, T.; Waalwijk, C.; Zhang, W.M.; Xu, X.D. Survey for toxigenic Fusarium species on maize kernels in China. World Mycotoxin J. 2020, 13, 213–223. [Google Scholar] [CrossRef]
- Qiu, J.B.; Xu, J.H.; Dong, F.; Yin, X.C.; Shi, J.R. Isolation and characterization of Fusarium verticillioides from maize in eastern China. Eur. J. Plant Pathol. 2015, 142, 791–800. [Google Scholar] [CrossRef]
- Sun, H.; Li, P.; Guo, N.; Shi, J.; Zhang, J.Q.; Zhang, H.J. Identification and biological characteristics of Fusarium temperatum causing maize ear rot. J. Maize Sci. 2020, 28, 177–183. [Google Scholar]
- Du, Q.; Duan, C.X.; Li, S.C.; Tang, Z.L.; Luo, J.Y. First report of maize ear rot caused by Fusarium concentricum in China. Plant Dis. 2020, 104, 1539–1540. [Google Scholar] [CrossRef]
- Duan, C.X.; Du, Q.; Wang, B.B. First report of maize ear rot caused by Fusarium sacchari in China. Plant Dis. 2019, 103, 2674. [Google Scholar] [CrossRef]
- Shang, G.F.; Yu, H.; Yang, J.; Zeng, Z.; Hu, Z.Q. First report of Fusarium miscanthi causing ear rot on maize in China. Plant Dis. 2021, 105, 1565. [Google Scholar] [CrossRef]
- Wang, B.B.; Guo, C.; Sun, S.L.; Zhu, Z.D.; Duan, C.X. First report of maize ear rot caused by Fusarium sporotrichioides in China. Plant Dis. 2020, 104, 567–568. [Google Scholar] [CrossRef]
- Munkvold, G.P. Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 2003, 41, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Presello, D.A.; Reid, L.M.; Butler, G.; Mather, D.E. Pedigree selection for Gibberella ear rot resistance in maize populations. Euphytica 2005, 143, 1–8. [Google Scholar] [CrossRef]
- Afolabi, C.G.; Ojiambo, P.S.; Ekpo, E.J.A.; Menkir, A.; Bandyopadhyay, R. Evaluation of maize inbred lines for resistance to Fusarium ear rot and fumonisin accumulation in grain in tropical Africa. Plant Dis. 2007, 91, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.J.; Maragos, C.A.; Pataky, J.K.; White, D.G. Sources of resistance to fumonisin accumulation in grain and Fusarium ear and kernel rot of corn. Phytopathology 2004, 94, 251–260. [Google Scholar] [CrossRef]
- Kleinschmidt, C.E.; Clements, M.J.; Maragos, C.M.; Pataky, J.K.; White, D.G. Evaluation of food-grade dent corn hybrids for severity of Fusarium ear rot and fumonisin accumulation in grain. Plant Dis. 2005, 89, 291–297. [Google Scholar] [CrossRef]
- Löffler, M.; Kessel, B.; Ouzunova, M.; Miedaner, T. Population parameters for resistance to Fusarium graminearum and Fusarium verticillioides ear rot among large sets of early, mid-late and late maturing European maize (Zea mays L.) inbred lines. Theor. Appl. Genet. 2010, 120, 1053–1062. [Google Scholar] [CrossRef]
- Santiago, R.; Cao, A.; Malvar, R.A.; Reid, L.M.; Butrón, A. Assessment of corn resistance to fumonisin accumulation in a broad collection of inbred lines. Field Crops Res. 2013, 149, 193–202. [Google Scholar] [CrossRef]
- Czembor, E.; Stepien, L.; Waskiewicz, A. Effect of environmental factors on Fusarium species and associated mycotoxins in maize grain grown in Poland. PLoS ONE 2015, 10, e0133644. [Google Scholar] [CrossRef] [PubMed]
- Doohan, F.M.; Brennan, J.; Cooke, B.M. Influence of climatic factors on Fusarium species pathogenic to cereals. Eur. J. Plant Pathol. 2003, 109, 755–768. [Google Scholar] [CrossRef]
- Duan, C.X.; Wang, X.M.; Wu, X.F.; Yang, Z.H.; Song, F.J.; Zhao, L.P.; Sun, S.L.; Zhu, Z.D. Analysis of maize accessions resistance to Pythium stalk rot and Fusarium ear rot. J. Plant Genet. Resour. 2015, 16, 947–954. [Google Scholar]
- Xu, J.; Jiang, Y.; Qin, P.W.; Liu, K.J.; Hu, L.; Sun, H.J.; Xu, X.D. Test for ear rot resistance against Fusarium verticillioides and Fusarium graminearum in imported maize germplasm. J. Plant Genet. Resour. 2019, 20, 20–25. [Google Scholar]
- Yang, J.W.; Wang, J.J.; Zhao, B.P.; Li, Y.N.; Jia, X.; Wang, F.R. Identification and evaluation of resistance of maize hybrids to Fusarium graminearum ear rot. J. Hebei Agric. Sci. 2020, 24, 47–49. [Google Scholar]
- Zhao, J.R.; Wang, R.H. Factors promoting the steady increase of American maize production and their enlightenments for China. J. Maize Sci. 2009, 17, 156–159. [Google Scholar]
- Bluhm, B.H.; Flaherty, J.E.; Cousin, M.A.; Woloshuk, C.P. Multiplex polymerase chain reaction assay for the differential detection of trichothecene- and fumonisin-producing species of Fusarium in cornmeal. J. Food Prot. 2002, 65, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Fox, R.T.V.; Culham, A. Development of a PCR-based assay for rapid and reliable identification of pathogenic Fusariam. FEMS Microbiol. Lett. 2003, 218, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Mulè, G.; Susca, A.; Stea, G.; Moretti, A. A species-specific PCR assay based on the calmodulin partial gene for identification of Fusarium verticillioides, F. proliferatum and F. subglutinans. Eur. J. Plant Pathol. 2004, 110, 495–502. [Google Scholar] [CrossRef]
- Nicholson, P.; Simpson, D.R.; Weston, G.; Rezanoor, H.N.; Joyce, D. Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol. Mol. Plant P. 1998, 53, 17–37. [Google Scholar] [CrossRef]
- O’Donnell, K.; Nirenberg, H.I.; Aoki, T.; Cigelnik, E. A multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 2000, 41, 61–78. [Google Scholar] [CrossRef]
- Li, X.Y.; Ma, Z.J.; Gai, X.T.; Wang, H.D.; Yao, Y.; Sun, Y.Q.; Gao, Z.G. Identification of Fusarium species causing maize ear rot in Northeast China and the diversity of F. verticillioides. J. Shenyang Agric. Univ. 2018, 49, 136–142. [Google Scholar]
- Fallahi, M.; Saremi, H.; Javan-Nikkhah, M.; Somma, S.; Haidukowski, M.; Legrieco, A.F.; Moretti, A. Isolation, molecular identification and mycotoxin profile of Fusarium species isolated from maize kernels in Iran. Toxins 2019, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.C.; Liu, Y.X.; Jiang, Y.; Li, R.J.; Pang, M.H.; Liu, Y.C.; Dong, J.G. Fusarium species identification and fumonisin production in maize kernels from Shandong Province, China, from 2012 to 2014. Food Addit. Contam. B 2016, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Jiang, D.; Xu, L.K.; Zhang, S.Q.; Ji, P.S.; Pan, H.Y.; Jiang, B.W.; Shen, Z.B. Evaluation of diversity and resistance of maize varieties to Fusarium spp. causing ear rot in maize under conditions of natural infection. Czech J. Genet. Plant Breed. 2019, 55, 131–137. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, X.D.; Lv, G.Z. Fusarium species and its isolation frequency from rot ears of maize in Northeast China. J. Fungal Res. 2011, 9, 9–24, 36. [Google Scholar]
- Xiao, S.Q.; Xu, J.N.; Yan, L.B.; Sui, Y.H.; Xue, C.S.; Chen, J. Identification and distribution of Fusarium species causing maize ear rot in Liaoning Province. J. Plant Prot. 2017, 44, 803–808. [Google Scholar]
- Arias, S.L.; Theumer, M.G.; Mary, V.S.; Rubinstein, H.R. Fumonisins: Probable role as effectors in the complex interaction of susceptible and resistant maize hybrids and Fusarium verticillioides. J. Agric. Food Chem. 2012, 60, 5667–5675. [Google Scholar] [CrossRef]
- Blandino, M.; Reyneri, A.; Vanara, F.; Tamietti, G.; Pietri, A. Influence of agricultural practices on Fusarium infection, fumonisin and deoxynivalenol contamination of maize kernels. World Mycotoxin J. 2009, 2, 409–418. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kuang, Y.; Splivallo, R.; Chatterjee, P.; Karlovsky, P. Interactions among filamentous fungi Aspergillus niger, Fusarium verticillioides and Clonostachys rosea: Fungal biomass, diversity of secreted metabolites and fumonisin production. BMC Microbiol. 2016, 6, 83. [Google Scholar] [CrossRef]
- Reid, L.M.; Spaner, D.; Mather, D.E.; Bolton, A.T.; Hamilton, R.I. Resistance of maize hybrids and inbreds following silk inoculation with three isolates of Fusarium graminearum. Plant Dis. 1993, 77, 1248–1251. [Google Scholar] [CrossRef]
- Wang, L.J.; Xu, X.D.; Liu, Z.H.; Dong, H.Y.; Jiang, Y.; Zhang, M.H. Inoculation technique and screening maize germplasm resistance to Fusarium ear rot. J. Plant Genet. Resour. 2007, 8, 145–148. [Google Scholar]
- Cao, A.; Santiago, R.; Ramos, A.J.; Souto, X.C.; Aguín, O.; Malvar, R.A.; Butrón, A. Critical environmental and genotypic factors for Fusarium verticillioides infection, fungal growth and fumonisin contamination in maize grown in Northwestern Spain. Int. J. Food Microbiol. 2014, 177, 63–71. [Google Scholar] [CrossRef]
- Chen, J.F.; Ding, J.Q.; Li, H.M.; Li, Z.M.; Sun, X.D.; Li, J.J.; Wang, R.X.; Dai, X.D.; Dong, H.F.; Song, W.B.; et al. Detection and verification of quantitative trait loci for resistance to Fusarium ear rot in maize. Mol. Breed. 2012, 30, 1649–1656. [Google Scholar] [CrossRef]
- Kebede, A.Z.; Johnston, A.; Schneiderman, D.; Bosnich, W.; Harris, L.J. Transcriptome profiling of two maize inbreds with distinct responses to Gibberella ear rot disease to identify candidate resistance genes. BMC Genom. 2018, 19, 131–143. [Google Scholar] [CrossRef]
- Czembor, E.; Ochodzki, R. Resistance of flint and dent maize forms for colonization by Fusarium spp. and mycotoxins contamination. Maydica 2009, 54, 263–267. [Google Scholar]
- Guo, C.; Guo, M.K.; Wei, H.Y.; Guo, J.G. Identification of resistance of maize germplasm resources to ear rot. Acta Agric. Jiangxi 2015, 27, 50–52. [Google Scholar]
- Duan, C.X.; Cui, L.N.; Xia, Y.S.; Dong, H.Y.; Yang, Z.H.; Hu, Q.Y.; Sun, S.L.; Li, X.; Zhu, Z.D.; Wang, X.M. Precise characterization and analysis of maize germplasm resources for resistance to Fusarium ear rot and Gibberella ear rot. Acta Agron. Sin. 2022, 48, 2155–2167. [Google Scholar]
- Nerbass, F.R.; Casa, R.T.; Kuhnem, P.R.; Vieira, J.A.L.; Valente, J.B. Field evaluation of maize for Gibberella ear rot resistance using silk channel and kernel inoculation with Fusarium meridionale. Trop. Plant Pathol. 2015, 40, 388–393. [Google Scholar] [CrossRef]
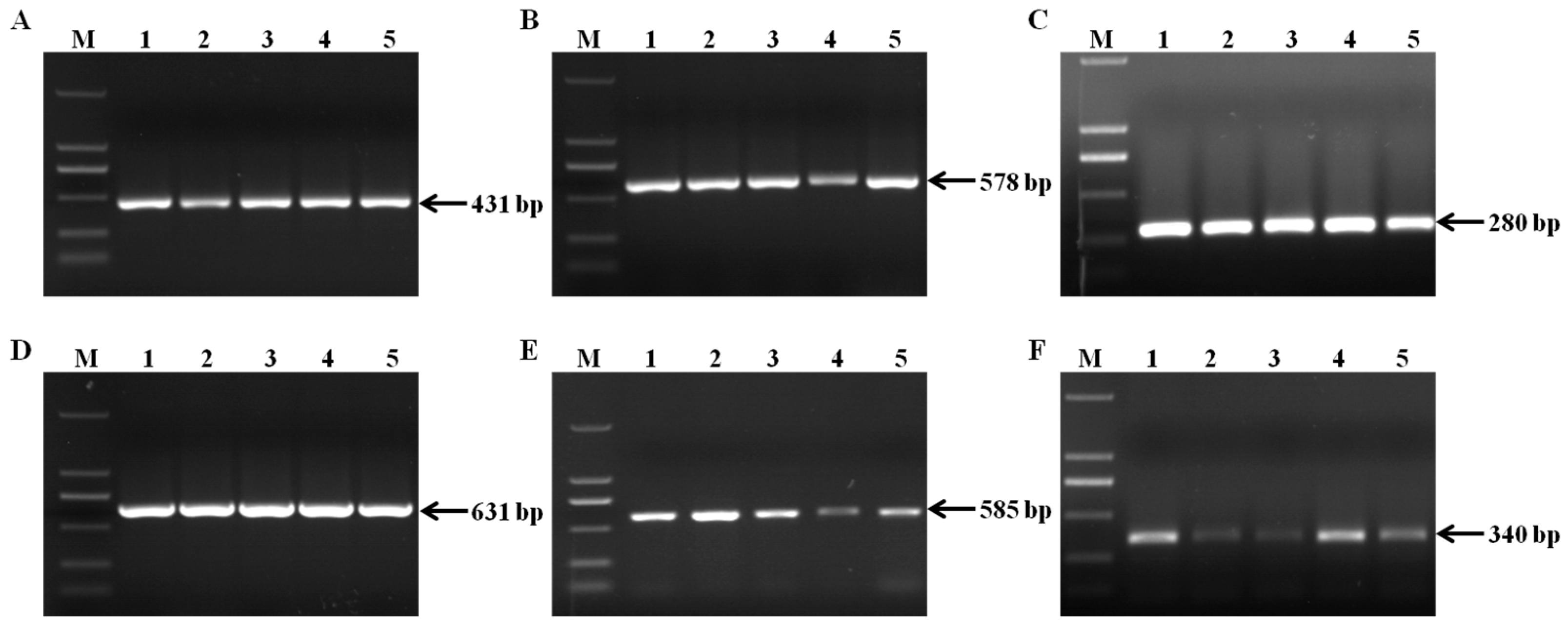

| Species | Primer | Sequences (5′-3′) | Annealing Temperature (°C) | Product Size (bp) | Reference |
|---|---|---|---|---|---|
| Fusarium spp. | ITSF | AACTCCCAAACCCCTGTGAACATA | 58 | 431 | [47] |
| ITSR | TTTAACGGCGTGGCCGC | ||||
| F. oxysporum | FoF1 | ACATACCACTTGTTGCCTCG | 58 | 340 | [48] |
| FoR1 | CGCCAATCAATTTGAGGAACG | ||||
| F. verticillioides | VER1 | CTTCCTGCGATGTTTCTCC | 56 | 578 | [49] |
| VER2 | AATTGGCCATTGGTATTATATATCTA | ||||
| F. proliferatum | PRO1 | CTTTCCGCCAAGTTTCTTC | 56 | 585 | [49] |
| PRO2 | TGTCAGTAACTCGACGTTGTTG | ||||
| F. subglutinans | SUB1 | CTGTCGCTAACCTCTTTATCCA | 56 | 631 | [49] |
| SUB2 | CAGTATGGACGTTGGTATTATATCTAA | ||||
| F. culmorum | Fc01F | ATGGTGAACTCGTCCTGGC | 59 | 570 | [50] |
| Fc01R | CCCTTCTTACGCCAATCTCG | ||||
| F. graminearum species complex | Fg16NF | ACAGATGACAAGATTCAGGCACA | 57 | 280 | [50] |
| Fg16NR | TTCTTTGACATCTGTTCAACCCA |
| Province Name | Site Name | No. of Species | No. of Isolates a | All Species b | |||||
|---|---|---|---|---|---|---|---|---|---|
| Fv | Fs | Fp | Fo | Fb | Fg | ||||
| Heilongjiang | Yichun | 4 | 9 | 7 | 8 | … | 8 | … | 32 |
| Jiamusi | 5 | 13 | 6 | 5 | 1 | 10 | … | 35 | |
| Qiqihar | 4 | 6 | … | … | 2 | 4 | 4 | 16 | |
| Harbin | 3 | 9 | … | … | … | 7 | 6 | 22 | |
| Mudanjiang | 4 | 11 | 3 | … | 3 | 10 | … | 27 | |
| All sites c | 6 | 48 | 16 | 13 | 6 | 39 | 10 | 132 | |
| Per site (%) | - | 36.4 | 12.1 | 9.8 | 4.5 | 29.5 | 7.6 | - | |
| Jilin | Baicheng | 4 | 11 | 9 | … | 3 | 2 | … | 25 |
| Songyuan | 4 | 7 | 5 | 9 | … | 3 | … | 24 | |
| Changchun | 3 | 9 | 6 | 7 | … | … | … | 22 | |
| Siping | 2 | 14 | … | … | … | 5 | … | 19 | |
| Tonghua | 3 | 9 | … | … | 2 | 4 | … | 15 | |
| All sites c | 5 | 50 | 20 | 16 | 5 | 14 | … | 105 | |
| Per site (%) | - | 47.6 | 19.0 | 15.2 | 4.8 | 13.3 | … | - | |
| Liaoning | Tieling | 3 | 13 | … | … | 3 | 9 | … | 25 |
| Huludao | 4 | 11 | 6 | … | … | 8 | 6 | 31 | |
| Yingkou | 5 | 15 | 2 | 7 | 1 | … | 3 | 28 | |
| Dandong | 4 | 10 | … | … | 2 | 6 | 2 | 20 | |
| Dalian | 2 | 14 | … | … | … | 11 | … | 25 | |
| All sites c | 6 | 63 | 8 | 7 | 6 | 34 | 11 | 129 | |
| Per site (%) | - | 48.8 | 6.2 | 5.4 | 4.7 | 26.4 | 8.5 | - | |
| All provinces d | 6 | 161 | 44 | 36 | 17 | 87 | 21 | 366 | |
| Per province (%) | - | 44.0 | 12.0 | 9.8 | 4.6 | 23.8 | 5.7 | - | |
| No. | Hybrid Name | Province a | 2021 | 2022 | FRC e | No. | Hybrid Name | Province a | 2021 | 2022 | FRC e | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DR b (%) | SR c | RC d | DR b (%) | SR c | RC d | DR b (%) | SR c | RC d | DR b (%) | SR c | RC d | ||||||||
| 1 | Damin3307 | HLJ | 41.73 | 6.40 | S | 40.04 | 6.20 | S | S | 52 | Jinongda598 | JL | 46.17 | 7.07 | S | 49.17 | 7.47 | S | S |
| 2 | Demeiya1 | HLJ | 11.54 | 3.67 | MR | 6.26 | 3.07 | R | MR | 53 | Jinongyu1881 | JL | 5.30 | 2.80 | R | 4.34 | 2.47 | R | R |
| 3 | Demeiya3 | HLJ | 6.03 | 3.07 | R | 4.61 | 2.47 | R | R | 54 | Jinongyu719 | JL | 14.73 | 4.33 | MR | 13.08 | 4.00 | MR | MR |
| 4 | Dika517 | HLJ | 53.54 | 7.60 | HS | 38.75 | 6.53 | S | HS | 55 | Laike818 | JL | 7.72 | 3.40 | R | 7.10 | 3.07 | R | R |
| 5 | Dongnong254 | HLJ | 21.67 | 4.87 | MR | 23.68 | 5.20 | MR | MR | 56 | Liaoke38 | JL | 26.31 | 5.00 | MR | 22.32 | 5.07 | MR | MR |
| 6 | Dongnong259 | HLJ | 5.11 | 3.13 | R | 18.13 | 4.53 | MR | MR | 57 | Limin33 | JL | 14.63 | 3.73 | MR | 18.64 | 4.33 | MR | MR |
| 7 | Dunyu213 | HLJ | 15.23 | 4.00 | MR | 8.39 | 3.13 | R | MR | 58 | Nonghua101 | JL | 8.47 | 3.33 | R | 14.46 | 4.00 | MR | MR |
| 8 | Fuer116 | HLJ | 16.24 | 4.00 | MR | 33.21 | 6.07 | S | S | 59 | Pingan169 | JL | 11.75 | 3.60 | MR | 11.11 | 3.47 | R | MR |
| 9 | Hetian4 | HLJ | 18.86 | 4.47 | MR | 13.80 | 4.00 | MR | MR | 60 | Tianyu108 | JL | 7.24 | 3.07 | R | 16.24 | 3.73 | MR | MR |
| 10 | Heyu27 | HLJ | 19.08 | 4.27 | MR | 38.06 | 5.47 | MR | MR | 61 | Xiangyu998 | JL | 32.34 | 5.67 | S | 53.36 | 7.87 | HS | HS |
| 11 | Huamei2 | HLJ | 4.94 | 2.73 | R | 3.62 | 2.27 | R | R | 62 | Xiongyu581 | JL | 6.32 | 3.07 | R | 12.37 | 3.53 | MR | MR |
| 12 | Huanong887 | HLJ | 1.07 | 1.20 | HR | 0.54 | 1.00 | HR | HR | 63 | Yinghe165 | JL | 30.25 | 5.40 | MR | 22.93 | 4.53 | MR | MR |
| 13 | Jingnongke728 | HLJ | 55.91 | 7.07 | S | 56.42 | 7.53 | HS | HS | 64 | Youdi519 | JL | 6.87 | 3.20 | R | 6.43 | 3.00 | R | R |
| 14 | Jinongda935 | HLJ | 17.04 | 4.20 | MR | 10.38 | 3.20 | R | MR | 65 | Youdi599 | JL | 39.12 | 5.73 | S | 44.15 | 6.47 | S | S |
| 15 | Jiudan318 | HLJ | 64.07 | 8.20 | HS | 55.55 | 7.60 | HS | HS | 66 | Youdi919 | JL | 55.93 | 7.00 | S | 48.97 | 6.60 | S | S |
| 16 | Keyu16 | HLJ | 14.65 | 3.93 | MR | 8.26 | 3.13 | R | MR | 67 | Zeyu517 | JL | 25.95 | 5.07 | MR | 17.90 | 4.47 | MR | MR |
| 17 | Longdan86 | HLJ | 16.03 | 4.47 | MR | 22.03 | 5.20 | MR | MR | 68 | Danyu402 | LN | 22.43 | 5.07 | MR | 15.61 | 4.13 | MR | MR |
| 18 | Longfuyu9 | HLJ | 8.42 | 2.80 | R | 3.47 | 2.27 | R | R | 69 | Danyu405 | LN | 31.75 | 6.07 | S | 24.08 | 5.20 | MR | S |
| 19 | Longken10 | HLJ | 11.74 | 3.73 | MR | 29.79 | 5.53 | S | S | 70 | Dongdan118 | LN | 30.16 | 5.33 | MR | 22.15 | 4.73 | MR | MR |
| 20 | Longyu10 | HLJ | 12.40 | 3.87 | MR | 11.08 | 3.13 | R | MR | 71 | Dongdan1501 | LN | 6.19 | 3.00 | R | 9.13 | 3.47 | R | R |
| 21 | Longyu828 | HLJ | 3.85 | 2.47 | R | 3.45 | 2.33 | R | R | 72 | Dongdan60 | LN | 10.16 | 3.27 | R | 7.54 | 2.53 | R | R |
| 22 | Lvdan2 | HLJ | 23.54 | 4.73 | MR | 34.54 | 6.00 | S | S | 73 | Dongdan6531 | LN | 27.73 | 5.67 | S | 21.88 | 4.73 | MR | S |
| 23 | Nendan18 | HLJ | 35.67 | 6.07 | S | 29.97 | 5.27 | MR | S | 74 | Dongdan70 | LN | 36.71 | 6.13 | S | 40.79 | 6.67 | S | S |
| 24 | Ruifuer1 | HLJ | 17.69 | 4.13 | MR | 11.33 | 3.67 | MR | MR | 75 | Dongtiannuo100 | LN | 10.72 | 3.07 | R | 15.70 | 3.67 | MR | MR |
| 25 | Suiyu23 | HLJ | 5.72 | 3.00 | R | 18.75 | 4.13 | MR | MR | 76 | Hongkai49 | LN | 1.63 | 1.47 | HR | 0.93 | 1.13 | HR | HR |
| 26 | Xianyu335 | HLJ | 54.02 | 7.13 | S | 43.32 | 6.20 | S | S | 77 | Hongshuo1798 | LN | 13.84 | 4.07 | MR | 7.64 | 3.20 | R | MR |
| 27 | Xianyu696 | HLJ | 11.81 | 3.20 | R | 8.24 | 2.73 | R | R | 78 | Hongshuo899 | LN | 3.62 | 1.80 | R | 1.36 | 1.40 | HR | R |
| 28 | Xianzhengda408 | HLJ | 4.84 | 2.47 | R | 18.09 | 4.07 | MR | MR | 79 | Jiaduoxing939 | LN | 11.80 | 3.13 | R | 10.28 | 2.80 | R | R |
| 29 | Xinkeyu1 | HLJ | 6.21 | 2.73 | R | 22.21 | 4.60 | MR | MR | 80 | Jinshi566 | LN | 20.55 | 4.53 | MR | 14.57 | 4.27 | MR | MR |
| 30 | Yinongyu10 | HLJ | 17.60 | 4.27 | MR | 13.17 | 3.20 | R | MR | 81 | Jinyuan15 | LN | 50.43 | 7.33 | S | 59.97 | 8.07 | HS | HS |
| 31 | Zhitai3 | HLJ | 15.64 | 4.27 | MR | 11.07 | 3.47 | R | MR | 82 | Lianda288 | LN | 13.57 | 4.20 | MR | 9.34 | 3.13 | R | MR |
| 32 | Zhongdan909 | HLJ | 8.85 | 3.33 | R | 16.82 | 4.27 | MR | MR | 83 | Liangyu88 | LN | 32.83 | 6.20 | S | 26.05 | 5.40 | MR | S |
| 33 | Changdan551 | JL | 24.67 | 5.07 | MR | 17.68 | 4.40 | MR | MR | 84 | Liangyu911 | LN | 10.38 | 3.87 | MR | 7.13 | 2.87 | R | MR |
| 34 | Deyu919 | JL | 5.85 | 3.00 | R | 5.34 | 2.53 | R | R | 85 | Liangyu99 | LN | 20.23 | 4.67 | MR | 25.25 | 5.20 | MR | MR |
| 35 | Dika159 | JL | 8.69 | 3.33 | R | 14.63 | 4.00 | MR | MR | 86 | Liaodan565 | LN | 7.34 | 3.47 | R | 13.37 | 3.93 | MR | MR |
| 36 | Dika516 | JL | 28.33 | 5.07 | MR | 21.37 | 4.47 | MR | MR | 87 | Liaohe308 | LN | 43.79 | 6.73 | S | 43.72 | 6.80 | S | S |
| 37 | Fulai77 | JL | 20.48 | 4.47 | MR | 11.56 | 3.33 | R | MR | 88 | Shenhai49 | LN | 31.81 | 5.80 | S | 48.84 | 7.73 | HS | HS |
| 38 | Fulai818 | JL | 22.72 | 4.53 | MR | 33.73 | 5.73 | S | S | 89 | ShennongT100 | LN | 2.73 | 1.67 | R | 7.76 | 2.47 | R | R |
| 39 | Fumin105 | JL | 43.07 | 6.60 | S | 38.04 | 6.20 | S | S | 90 | Shennuo18 | LN | 27.84 | 5.33 | MR | 14.21 | 3.40 | R | MR |
| 40 | Fumin108 | JL | 23.61 | 5.27 | MR | 27.82 | 5.53 | S | S | 91 | Shenyu35 | LN | 26.27 | 5.13 | MR | 33.20 | 5.73 | S | S |
| 41 | Heyu301 | JL | 25.60 | 4.93 | MR | 26.67 | 5.20 | MR | MR | 92 | Tieyan120 | LN | 12.10 | 3.53 | MR | 6.38 | 2.80 | R | MR |
| 42 | Heyu9 | JL | 9.07 | 3.07 | R | 15.03 | 3.53 | MR | MR | 93 | Tieyan358 | LN | 13.32 | 3.73 | MR | 19.33 | 4.47 | MR | MR |
| 43 | Huadan398 | JL | 10.32 | 3.20 | R | 9.75 | 3.00 | R | R | 94 | Tieyan38 | LN | 9.75 | 3.67 | MR | 14.74 | 4.27 | MR | MR |
| 44 | Jidan1402 | JL | 7.13 | 2.53 | R | 1.76 | 1.40 | HR | R | 95 | Tieyan58 | LN | 35.68 | 5.80 | S | 33.46 | 5.40 | MR | S |
| 45 | Jidan551 | JL | 8.75 | 3.33 | R | 8.29 | 3.13 | R | R | 96 | Xindan336 | LN | 17.91 | 4.07 | MR | 23.99 | 4.67 | MR | MR |
| 46 | Jidan558 | JL | 9.11 | 3.47 | R | 8.64 | 3.27 | R | R | 97 | Zhengdan958 | LN | 9.82 | 3.20 | R | 15.82 | 3.93 | MR | MR |
| 47 | Jidan56 | JL | 5.32 | 2.80 | R | 6.73 | 3.20 | R | R | B73 | Control | 66.17 | 8.27 | HS | 69.13 | 8.40 | HS | HS | |
| 48 | Jidan96 | JL | 24.85 | 5.47 | MR | 11.34 | 2.80 | R | MR | X178 | Control | 4.82 | 2.73 | R | 4.48 | 2.53 | R | R | |
| 49 | Jingke968 | JL | 6.54 | 2.93 | R | 5.78 | 2.87 | R | R | Mean | … | 19.86 | 4.32 | … | 20.22 | 4.29 | … | … | |
| 50 | Jinkai7 | JL | 30.72 | 5.53 | S | 28.58 | 5.13 | MR | S | LSD(p=0.05) f | … | 2.98 | 0.30 | … | 3.01 | 0.32 | … | … | |
| 51 | Jinongda585 | JL | 27.76 | 5.40 | MR | 21.76 | 4.73 | MR | MR | CV (%) | … | 75.27 | 34.87 | … | 74.57 | 37.74 | … | … | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Wang, J.; Wen, S.; Ren, J.; Hui, H.; Huang, Y.; Yang, J.; Zhao, B.; Liu, B.; Gao, Z. Evaluation of Maize Hybrids for Resistance to Ear Rot Caused by Dominant Fusarium Species in Northeast China. Agronomy 2024, 14, 855. https://doi.org/10.3390/agronomy14040855
Ma Z, Wang J, Wen S, Ren J, Hui H, Huang Y, Yang J, Zhao B, Liu B, Gao Z. Evaluation of Maize Hybrids for Resistance to Ear Rot Caused by Dominant Fusarium Species in Northeast China. Agronomy. 2024; 14(4):855. https://doi.org/10.3390/agronomy14040855
Chicago/Turabian StyleMa, Zhoujie, Jianjun Wang, Shenghui Wen, Jiankai Ren, Hongyan Hui, Yufei Huang, Junwei Yang, Bianping Zhao, Bo Liu, and Zenggui Gao. 2024. "Evaluation of Maize Hybrids for Resistance to Ear Rot Caused by Dominant Fusarium Species in Northeast China" Agronomy 14, no. 4: 855. https://doi.org/10.3390/agronomy14040855





