Characterization and Mineralization Rates of Low Temperature Peanut Hull and Pine Chip Biochars
Abstract
:1. Introduction
- Peanut hull and PC biochar will mineralize at different rates that will correlate to an aliphatic and volatile matter concentration in the biochar.
- Aging in soil will decrease aliphatic and volatile matter as well as C and H concentrations in biochars, and will increase oxygen-containing functionalities on the surface of biochars.
- Biochar will affect the N status of the soil by either immobilizing mineralized soil N or mineralizing biochar N depending on the C to N ratio of the biochar.
2. Materials and Methods
2.1. Biochar Production
2.2. Soil
2.3. Mineralization Studies
2.4. Thermogravimetric Analysis
2.5. Laboratory Analysis
2.6. Statistical Analysis

3. Results
3.1. Feedstock and Biochar Analysis
| C:N | C | N | P | K | S | Ca | Mg | |
|---|---|---|---|---|---|---|---|---|
| ----------------------------------------mg kg−1--------------------------------------- | ||||||||
| Biochar | ||||||||
| PH | 35 C | 729,000 B | 21,000 A | 2180 | 19,000 | 852 | 5410 | 2720 |
| PC | 214 B | 785,000 A | 3,710 C | 577 | 2,820 | 350 | 4030 | 1230 |
| Feedstock | ||||||||
| PH | 38 C | 504,000 C | 14,000 B | 893 | 7,510 | 900 | 1560 | 790 |
| PC | 259 A | 503,000 C | 1,870 C | 181 | 877 | 200 | 930 | 296 |
| ASTM D5142 | TGA | |||||||
|---|---|---|---|---|---|---|---|---|
| Moisture | Volatiles | Fixed C | Ash | Moisture | Aliphatic | Volatile | Ash | |
| --------------------------g kg−1----------------------- | ---------------------------g kg−1---------------------- | |||||||
| PH biochar | 81 | 252 | 598 | 69 | 56 | 62 | 219 | 102 |
| PC biochar | 74 | 278 | 630 | 18 | 37 | 54 | 235 | 38 |
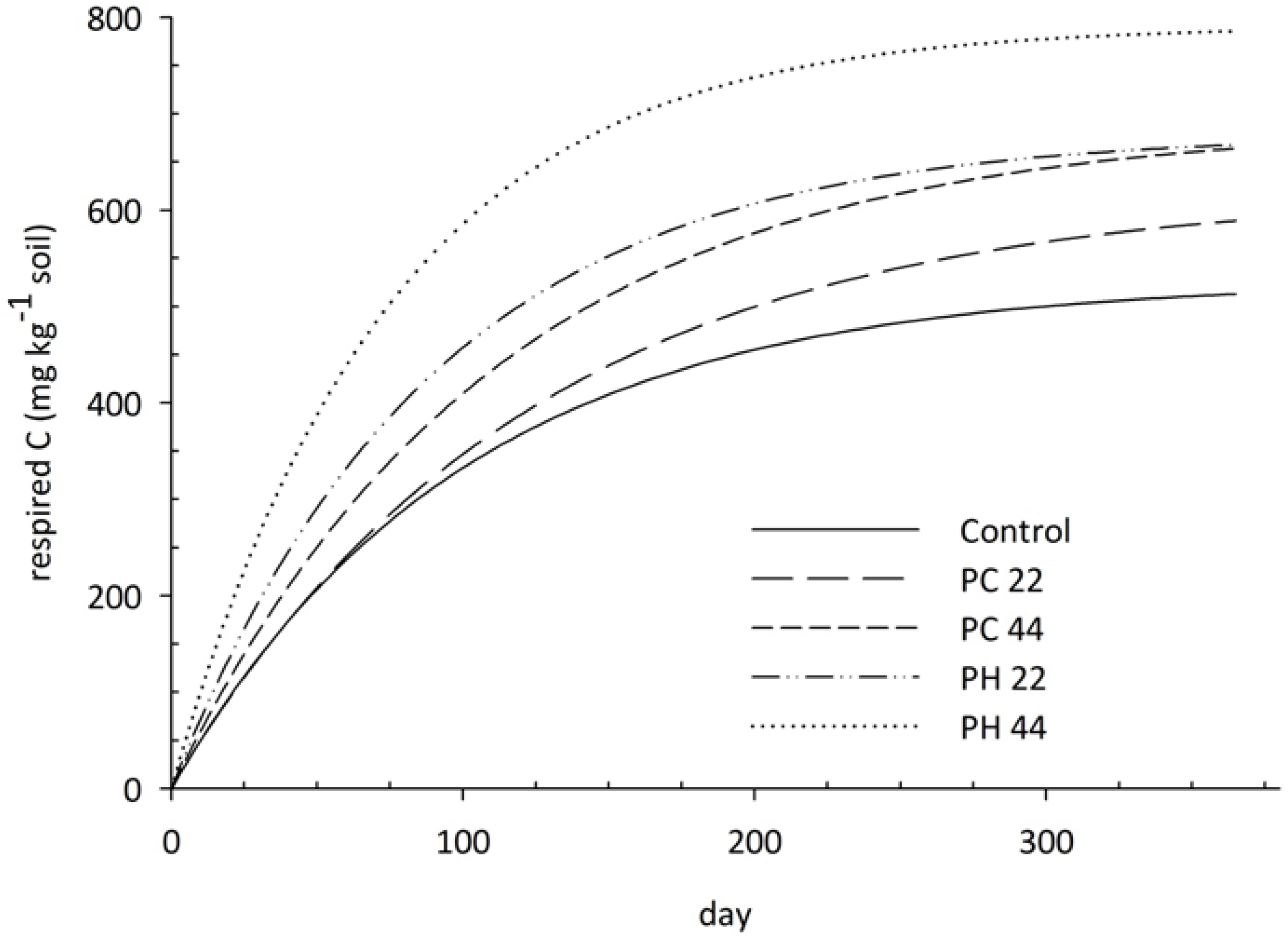
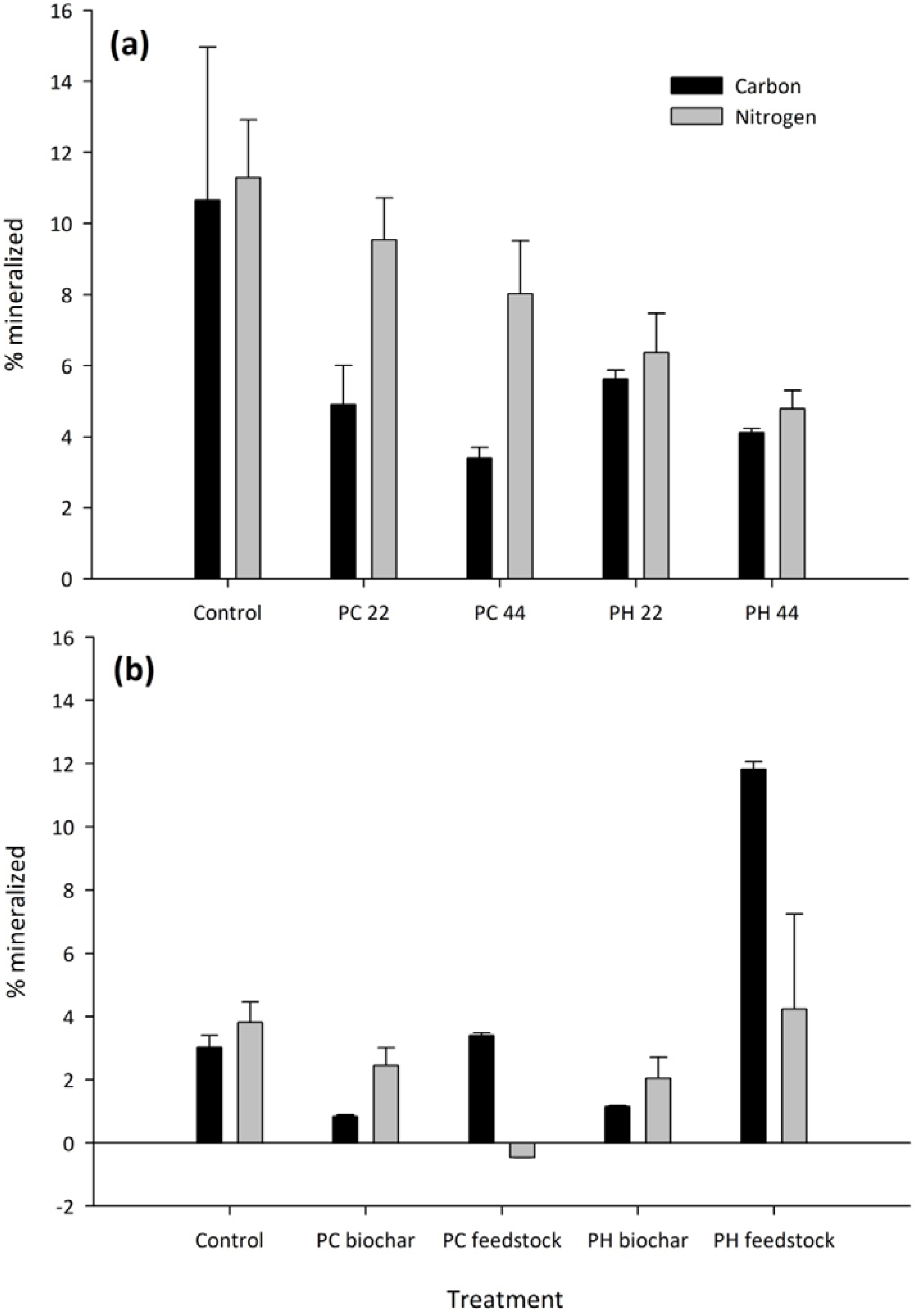
3.2. Carbon and Nitrogen Mineralization
| C respiration | N mineralization | ||
|---|---|---|---|
| Rmax | k | ||
| mg C kg−1 soil | day−1 | mg kg−1 soil | |
| Effect means | |||
| Feedstock | |||
| PH biochar | 735 | 0.0127 | 30.2 |
| PC biochar | 620 | 0.0086 | 26.5 |
| Application Rate | |||
| 22 Mg ha−1 | 650 | 0.0097 | 28.2 |
| 44 Mg ha−1 | 740 | 0.0112 | 28.7 |
| Pr > F | Pr > F | ||
| Feedstock | 0.0642 | 0.0007 | 0.1157 |
| Application rate | 0.0411 | 0.0637 | 0.7789 |
| Interaction | 0.5875 | 0.3551 | 0.3620 |
| C respiration | N mineralization | |
|---|---|---|
| cum. resp. | ||
| mg C kg−1 soil | mg kg−1 soil | |
| Treatment means | ||
| PH feedstock | 2277A | 26.8A |
| PH biochar | 222C | 13.5AB |
| PC feedstock | 692B | −1.4B |
| PC biochar | 171C | 7.9AB |
| Effect means | ||
| Feedstock | ||
| PH | 1250 | 20.2 |
| PC | 432 | 3.3 |
| Pr > F | Pr > F | |
| Feedstock | <0.0001 | 0.0065 |
| State | <0.0001 | 0.6941 |
| Interaction | <0.0001 | 0.0431 |
3.3. Ultimate Analysis and Thermal Decomposition of Fresh and Aged Biochars
| Combustion | TGA | |||||||
|---|---|---|---|---|---|---|---|---|
| C:N | C | N | H | Moisture | Aliphatic | Volatile | Ash | |
| g kg−1 char | g kg−1 char | |||||||
| Treatment means | ||||||||
| PH fresh | 35C | 730B | 21A | 34.6A | 56A | 62C | 219B | 102B |
| PH aged | 39C | 663D | 17B | 31.9B | 58A | 77A | 241A | 155A |
| PC fresh | 216A | 795A | 3.8C | 35.7A | 37C | 54D | 235AB | 38C |
| PC aged | 155B | 697C | 4.6C | 31.9B | 45B | 68B | 252A | 122B |
| Effect means | ||||||||
| Feedstock | ||||||||
| PH | 37 | 697 | 19 | 33 | 57 | 70 | 230 | 128 |
| PC | 185 | 746 | 4 | 34 | 41 | 61 | 243 | 80 |
| Condition | ||||||||
| Fresh | 126 | 763 | 12 | 35 | 47 | 58 | 227 | 70 |
| Aged | 97 | 680 | 11 | 32 | 51 | 73 | 247 | 139 |
| Pr > F | Pr > F | |||||||
| Feedstock | <0.0001 | <0.0001 | <0.0001 | 0.2165 | <0.0001 | <0.0001 | 0.0473 | <0.0001 |
| Condition | <0.0001 | <0.0001 | 0.0002 | <0.0001 | 0.0715 | <0.0001 | 0.0065 | <0.0001 |
| Interaction | <0.0001 | 0.0011 | <0.0001 | 0.4039 | 0.2670 | 0.9380 | 0.7248 | 0.0235 |
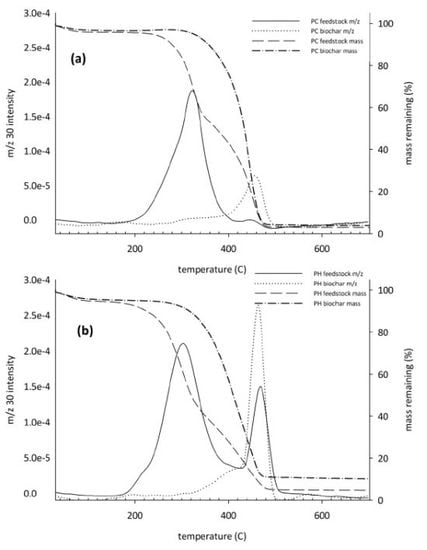
3.4. Controlled Combustion Analysis
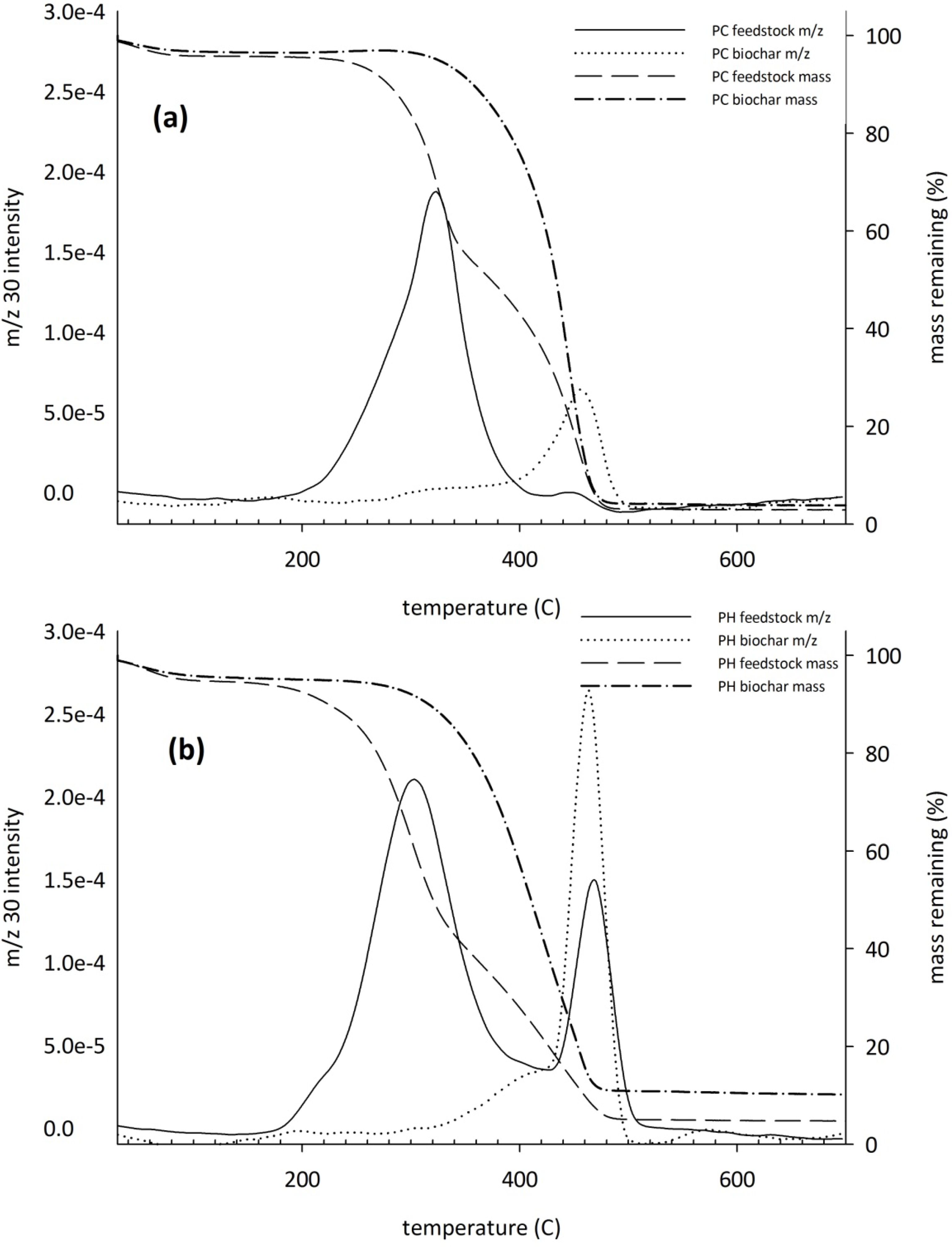
3.5. Fourier Transform Infrared Analysis
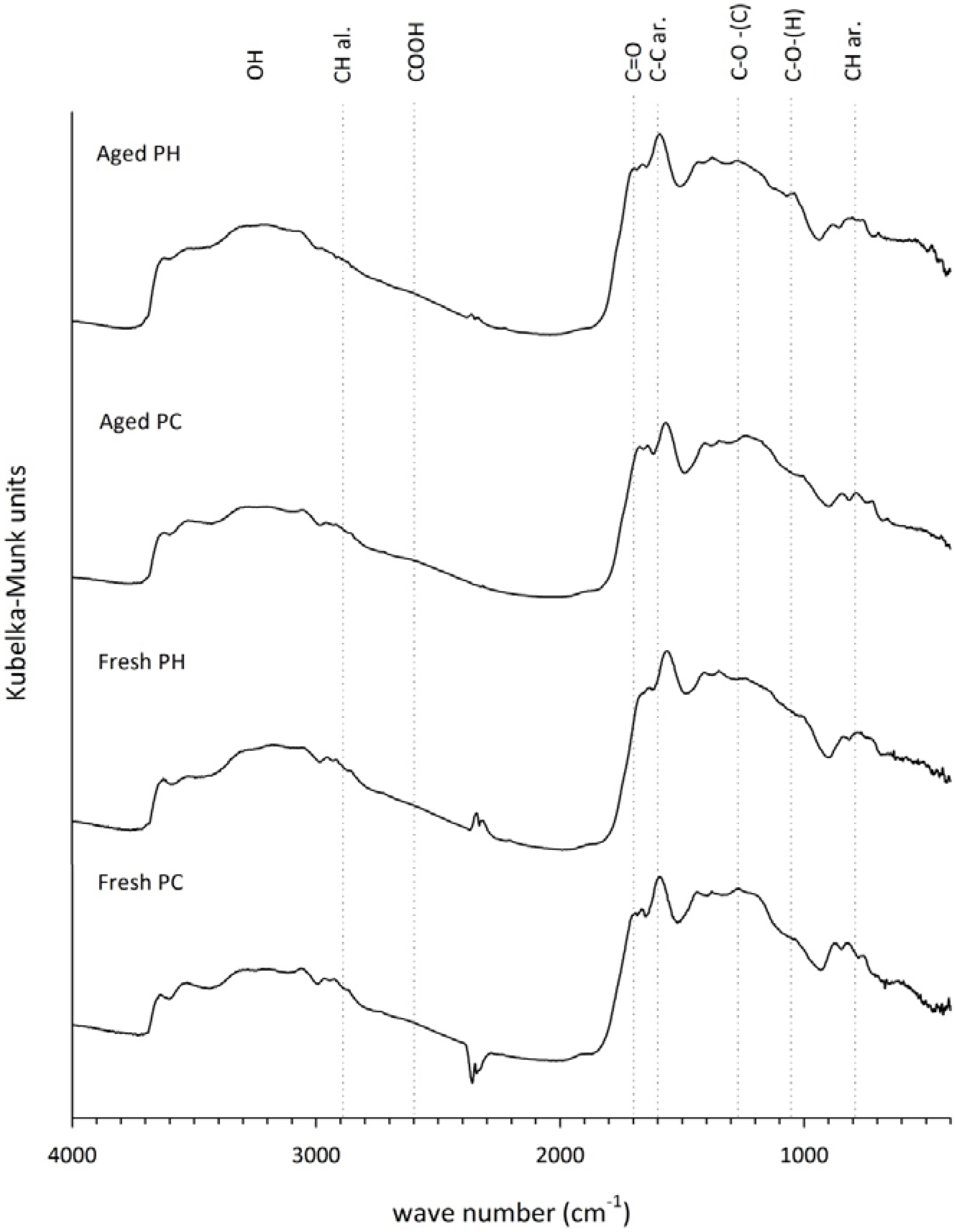
4. Discussion
5. Conclusions
References
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Gaunt, J.L.; Lehmann, J. Energy balance and emissions associated with biochar sequestration and pyrolysis bioenergy production. Environ. Sci. Technol. 2008, 42, 4152–4158. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Sohi, S.; Thies, J.E.; Skjemstad, J.O.; Luizão, F.J.; Engelhard, M.H.; Neves, E.G.; Wirick, S. Stability of biomass-derived black carbon in soils. Geochimica Et Cosmochimica Acta 2008, 72, 6069–6078. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological anthrosol and a ferralsol of the central amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Steiner, C.; Glaser, B.; Teixeira, W.G.; Lehmann, J.; Blum, W.E.H.; Zech, W. Nitrogen retention and plant uptake on a highly weathered central amazonian ferralsol amended with compost and charcoal. J. Plant Nutr. Soil Sci. Zeitschrift Fur Pflanzenernahrung Und Bodenkunde 2008, 171, 893–899. [Google Scholar] [CrossRef]
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod. Biorefin. 2009, 3, 547–562. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar]
- Antal, M.J.; Gronli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Guerrero, M.; Ruiz, M.P.; Millera, A.; Alzueta, M.U.; Bilbao, R. Characterization of biomass chars formed under different devolatilization conditions: Differences between rice husk and eucalyptus. Energy Fuels 2008, 22, 1275–1284. [Google Scholar] [CrossRef]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustain. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Bird, M.I.; Ascough, P.L.; Young, I.M.; Wood, C.V.; Scottc, A.C. X-ray microtomographic imaging of charcoal. J. Archaeol. Sci. 2008, 35, 2698–2706. [Google Scholar] [CrossRef]
- Keech, O.; Carcaillet, C.; Nilsson, M.C. Adsorption of allelopathic compounds by wood-derived charcoal: The role of wood porosity. Plant Soil 2005, 272, 291–300. [Google Scholar] [CrossRef]
- Wardle, D.A.; Nilsson, M.C.; Zackrisson, O. Fire-derived charcoal causes loss of forest humus. Science 2008, 320, 629. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Bruun, S.; Jensen, E.S.; Jensen, L.S. Microbial mineralization and assimilation of black carbon: Dependency on degree of thermal alteration. Org. Geochem. 2008, 39, 839–845. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.Q.; Bogomolova, I.; Xu, X.L. Black carbon decomposition and incorporation into soil microbial biomass estimated by c-14 labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef]
- Smith, J.L.; Collins, H.P.; Bailey, V.L. The effect of young biochar on soil respiration. Soil Biol. Biochem. 2010, 42, 2345–2347. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Hilscher, A.; Heister, K.; Siewert, C.; Knicker, H. Mineralisation and structural changes during the initial phase of microbial degradation of pyrogenic plant residues in soil. Org. Geochem. 2009, 40, 332–342. [Google Scholar] [CrossRef]
- Deenik, J.L.; McClellan, T.; Uehara, G.; Antal, M.J.; Campbell, S. Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci. Soc. Am. J. 2010, 74, 1259–1270. [Google Scholar] [CrossRef]
- Jones, D.L.; Murphy, D.V.; Khalid, M.; Ahmad, W.; Edwards-Jones, G.; DeLuca, T.H. Short-term biochar-induced increase in soil co2 release is both biotically and abiotically mediated. Soil Biol. Biochem. 2011, 43, 1723–1731. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Liang, B.Q.; Lehmann, J.; Sohi, S.P.; Thies, J.E.; O'Neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; Luizao, F.J. Black carbon affects the cycling of non-black carbon in soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Pietikainen, J.; Kiikkila, O.; Fritze, H. Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos 2000, 89, 231–242. [Google Scholar]
- Zackrisson, O.; Nilsson, M.C.; Wardle, D.A. Key ecological function of charcoal from wildfire in the boreal forest. Oikos 1996, 77, 10–19. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Speir, R.A.; Harris, K.; Das, K.C.; Lee, R.D.; Morris, L.A.; Fisher, D.S. Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron. J. 2010, 102, 623–633. [Google Scholar] [CrossRef]
- Chan, K.Y.; Zwieten, L.V.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Tagoe, S.O.; Horiuchi, T.; Matsui, T. Effects of carbonized and dried chicken manures on the growth, yield, and n content of soybean. Plant Soil 2008, 306, 211–220. [Google Scholar] [CrossRef]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J.; Holben, W.E. Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef]
- Spokas, K.A.; Baker, J.M.; Reicosky, D.C. Ethylene: Potential key for biochar amendment impacts. Plant Soil 2010, 333, 443–452. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar adsorbed ammonia is bioavailable. Plant Soil 2012, 350, 57–69. [Google Scholar] [CrossRef]
- Schomberg, H.H.; Gaskin, J.W.; Harris, K.; Das, K.C.; Novak, J.M.; Busscher, W.J.; Watts, D.W.; Woodroof, R.H.; Lima, I.M.; Ahmedna, M.; et al. Influence of biochar on nitrogen fractions in a coastal plain soil. J. Environ. Qual. 2012, 41, 1087–1095. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Laird, D.A.; Ahmedna, M.A.; Niandou, M.A.S. Short-term CO2 mineralization after additions of biochar and switchgrass to a typic kandiudult. Geoderma 2010, 154, 281–288. [Google Scholar] [CrossRef]
- Lyons, G.; Kilpatrick, M.; Sharma, H.S.S.; Noble, R.; Dobrovin-Pennington, A.; Hobbs, P.; Andrews, F.; Carmichael, E. Characterization of recycled mushroom compost leachate by chemical analysis and thermogravimetry-mass spectrometry. J. Agric. Food Chem. 2008, 56, 6488–6497. [Google Scholar] [CrossRef]
- USEPA, Methods for Determination of Metals in Environmental Smples, Supplement 1; Environment Monitoring Systems Laboratory, Office of Research and Development: Cincinnati, OH, USA, 1994.
- Deenik, J.L.; Diarra, A.; Uehara, G.; Campbell, S.; Sumiyoshi, Y.; Antal, M.J. Charcoal ash and volatile matter effects on soil properties and plant growth in an acid ultisol. Soil Sci. 2011, 176, 336–345. [Google Scholar] [CrossRef]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci. Soc. Am. J. 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Kara, O.; Bolat, I. Short-term effects of wildfire on microbial biomass and abundance in black pine plantation soils in turkey. Ecol. Indic. 2009, 9, 1151–1155. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Aust. J. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lehmann, J.; Engelhard, M.H. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochimica Et Cosmochimica Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lehmann, J. Ageing of black carbon along a temperature gradient. Chemosphere 2009, 75, 1021–1027. [Google Scholar] [CrossRef]
- Varhegyi, G.; Szabo, P.; Till, F.; Zelei, B.; Antal, M.J.; Dai, X.F. Tg, tg-ms, and ftir characterization of high-yield biomass charcoals. Energy Fuels 1998, 12, 969–974. [Google Scholar] [CrossRef]
- Stanczyk, K.; Dziembaj, R.; Piwowarska, Z.; Witkowski, S. Transformation of nitrogen structures in carbonization of model compounds determined by xps. Carbon 1995, 33, 1383–1392. [Google Scholar] [CrossRef]
- Schmiers, H.; Friebel, J.; Streubel, P.; Hesse, R.; Kopsel, R. Change of chemical bonding of nitrogen of polymeric n-heterocyclic compounds during pyrolysis. Carbon 1999, 37, 1965–1978. [Google Scholar] [CrossRef]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Harris, K.; Gaskin, J.; Cabrera, M.; Miller, W.; Das, K.C. Characterization and Mineralization Rates of Low Temperature Peanut Hull and Pine Chip Biochars. Agronomy 2013, 3, 294-312. https://doi.org/10.3390/agronomy3020294
Harris K, Gaskin J, Cabrera M, Miller W, Das KC. Characterization and Mineralization Rates of Low Temperature Peanut Hull and Pine Chip Biochars. Agronomy. 2013; 3(2):294-312. https://doi.org/10.3390/agronomy3020294
Chicago/Turabian StyleHarris, Keith, Julia Gaskin, Miguel Cabrera, William Miller, and K.C. Das. 2013. "Characterization and Mineralization Rates of Low Temperature Peanut Hull and Pine Chip Biochars" Agronomy 3, no. 2: 294-312. https://doi.org/10.3390/agronomy3020294
APA StyleHarris, K., Gaskin, J., Cabrera, M., Miller, W., & Das, K. C. (2013). Characterization and Mineralization Rates of Low Temperature Peanut Hull and Pine Chip Biochars. Agronomy, 3(2), 294-312. https://doi.org/10.3390/agronomy3020294