Evidence for Heterosis in Italian Ryegrass (Lolium multiflorum Lam.) Based on Inbreeding Depression in F2 Generation Offspring from Biparental Crosses
Abstract
:1. Introduction
2. Results
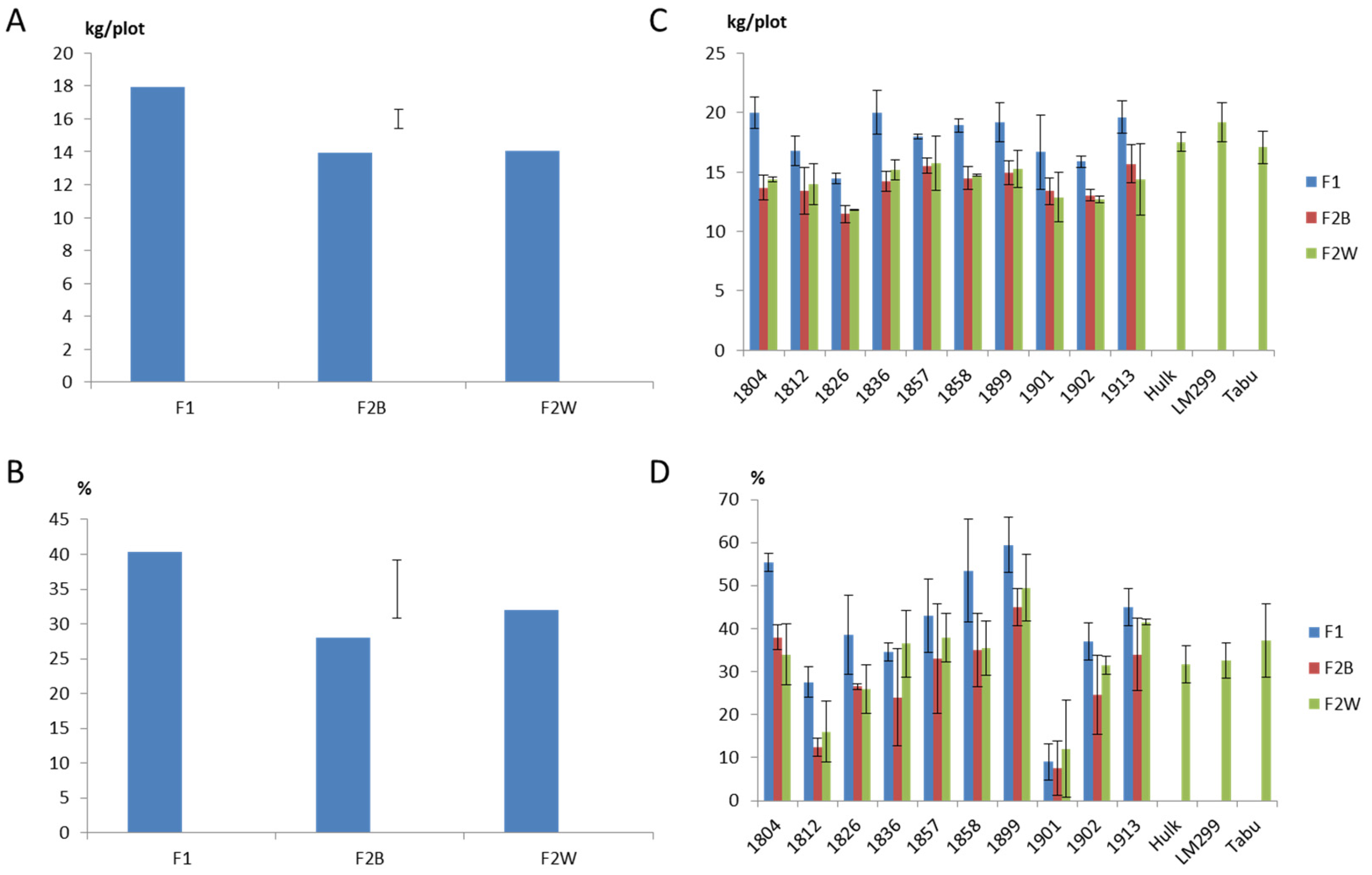
2.1. Field Evaluation of F1 Progeny Sets
2.2. Field Evaluation of F2 Progeny Sets
3. Discussion
3.1. Effectiveness of Phenotypic Selection from Spaced Plant Nursery
3.2. Heterosis and Inbreeding Depression
4. Materials and Methods
4.1. Selection of Individual Plants as Parents
4.2. Generation of F1 Full Sib Families and Field Test of Performance
4.3. Generation of F2 Families and Field Test of Performance
4.4. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stewart, A.; Hayes, R. Ryegrass breeding-balancing trait priorities. Irish J. Agric. Food Res. 2011, 50, 31–46. [Google Scholar]
- Chaves, B.; de Vliegher, A.; van Waes, J.; Carlier, L.; Marynissen, B. Change in agronomic performance of Lolium perenne and Lolium multiflorum varieties in the past 40 years based on date from Belgian VCU trials. Plant Breed. 2009, 128, 680–690. [Google Scholar] [CrossRef]
- Wilkins, P.W.; Lovatt, J.A. Gains in dry matter yield and herbage quality from breeding perennial ryegrass. Irish J. Agric. Food Res. 2011, 50, 23–30. [Google Scholar]
- Woodfield, D.R. Genetic improvement in New Zealand forage cultivars. In Proceedings of the New Zealand Grasslands Association, Hawkes Bay, New Zealand, 1999; Volume 61, pp. 3–7.
- Gout, M.; Jones, S. Estimates of the annual sales of proprietary cultivars in Australia, and the value of pastures to the livestock and cropping industries. In Presentation to Pastures Australia, Adelaide, Australia, 23–24 February 2006.
- Redfearn, D.D.; Venuto, B.C.; Pitman, W.D.; Blouin, D.C.; Alison, M.W. Multilocation annual ryegrass cultivar performance over a twelve-year period. Crop Sci. 2005, 45, 2388–2393. [Google Scholar] [CrossRef]
- Brummer, E.C. Capturing heterosis in forage crop cultivar development. Crop Sci. 1999, 39, 943–954. [Google Scholar] [CrossRef]
- Foster, C.A. A study of the theoretical expectation of F1 hybridity resulting from bulk interpopulation hybridization in herbage grasses. J. Agric. Sci. 1971, 76, 295–300. [Google Scholar] [CrossRef]
- Foster, C.A. Interpopulational and intervarietal hybridization in Lolium perenne breeding: Heterosis under non-competitive conditions. J. Agric. Sci. 1971, 76, 107–130. [Google Scholar] [CrossRef]
- Foster, C.A. Interpopulational and intervarietal hybridization in Lolium perenne: Heterosis under simulated-sward conditions. J. Agric. Sci. 1971, 76, 401–409. [Google Scholar] [CrossRef]
- Foster, C.A. Interpopulational and intervarietal F1 hybrids in Lolium perenne: Performance in field sward conditions. J. Agric. Sci. 1973, 80, 463–477. [Google Scholar] [CrossRef]
- Islam, M.S.; Studer, B.; Møller, I.M.; Asp, T. Genetics and biology of cytoplasmic male sterility and its applications in forage and turf grass breeding. Plant Breed. 2014, 133, 299–312. [Google Scholar] [CrossRef]
- England, F. The use of incompatibility for the production of F1 hybrids in forage grasses. Heredity 1974, 32, 183–188. [Google Scholar] [CrossRef]
- Posselt, U.K. Hybrid production in Lolium perenne based on incompatibility. Euphytica 1993, 71, 29–33. [Google Scholar] [CrossRef]
- Pembleton, L.W.; Shinozuka, H.; Wang, J.; Spangenberg, G.C.; Forster, J.W.; Cogan, N.O.I. Design of an F1 hybrid breeding strategy for ryegrasses based on selection of self-incompatibility locus-specific alleles. Front. Plant Sci. 2015, 6, 764. [Google Scholar] [CrossRef] [PubMed]
- Posselt, U.K. Identification of heterotic patterns in perennial ryegrass. In Sustainable Use of Genetic Diversity in Forage and Turf Breeding; Huyghe, C., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 569–572. [Google Scholar]
- Wang, J.; Pembleton, L.W.; Baillie, R.C.; Drayton, M.C.; Hand, M.L.; Bain, M.; Sawbridge, T.I.; Spangenberg, G.C.; Forster, J.W.; Cogan, N.O.I. Development and implementation of a multiplexed single nucleotide polymorphism genotyping tool for differentiation of ryegrass species and cultivars. Mol. Breed. 2014, 33, 435–451. [Google Scholar] [CrossRef]
- Wang, J.; Cogan, N.O.I.; Pembleton, L.W.; Forster, J.W. Variance, inter-trait correlation, heritability and trait-marker association of herbage yield, nutritive values, and morphological characteristics in Italian ryegrass (Lolium multiflorum Lam.). Crop Pasture Sci. 2015, 66, 973–984. [Google Scholar]
- Radojevic, I.; Simpson, R.J.; St. John, J.A.; Humphreys, M.O. Chemical composition and in vitro digestibility of lines of Lolium perenne selected for high concentrations of water-soluble carbohydrate. Aust. J. Agric. Res. 1994, 45, 901–912. [Google Scholar] [CrossRef]
- Vogel, K.P.; Pederson, J.F. Breeding systems for cross pollinated perennial grasses. In Plant Breeding Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Oxford, UK, 1993; Volume 11, pp. 251–274. [Google Scholar]
- Waldron, B.L.; Robins, J.G.; Peel, M.D.; Jensen, K.B. Predicted efficiency of spaced-plant selection to indirectly improve tall fescue sward yield and quality. Crop Sci. 2008, 48, 443–449. [Google Scholar] [CrossRef]
- Conaghan, P.; Casler, M.D. A theoretical and practical analysis of optimum breeding system for perennial ryegrass. Irish J. Agric. Food Res. 2011, 50, 47–63. [Google Scholar]
- Hayes, B.; Cogan, N.O.I.; Pembleton, L.; Goddard, M.; Wang, J.; Spangenberg, G.C.; Forster, J.W. Prospects for genomic selection in forage plant species. Plant Breed. 2013, 132, 133–143. [Google Scholar] [CrossRef]
- Casler, M.D.; Vogel, K.P. Accomplishments and impact from breeding for increased forage nutritional value. Crop Sci. 1999, 39, 12–20. [Google Scholar] [CrossRef]
- McGrath, D. A note on the influence of nitrogen application and time of cutting on water soluble carbohydrate production by Italian ryegrass. Irish J. Agric. Food Res. 1992, 31, 189–192. [Google Scholar]
- Bhatt, A. Studies on heterosis and inbreeding depression in forage sorghum [Sorghum bicolor (L.) Moench]. Agric. Sci. Digest 2008, 28, 258–261. [Google Scholar]
- Becker, H.C. Breeding synthetic varieties of crop plants. Plant Genet. Breed. Rev. 1988, 1, 31–54. [Google Scholar]
- Hayward, M.D.; Abdultah, I.B. Selection and stability of synthetic varieties of Lolium perenne 1. The selected character and its expression over generations of multiplication. Theor. Appl. Genet. 1985, 70, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Piano, E.; Annicchiarico, P.; Romani, M.; Pecetti, L. Effect of the number of parents and their combining ability on the performance of synthetic varieties in tall fescue. Aust. J. Agric. Res. 2007, 58, 1100–1105. [Google Scholar] [CrossRef]
- Casler, M.D.; Diaby, M.; Stendal, C. Heterosis and inbreeding depression for forage yield and fiber concentration in smooth bromegrass. Crop Sci. 2005, 45, 44–50. [Google Scholar] [CrossRef]
- ‘Tabu’ in Plant Varieties J. 2002, 15, 60.
- ‘Warrior’ in Plant Varieties J. 2003, 20, 162.
- East, E.M. Heterosis. Genetics 1936, 21, 375–397. [Google Scholar] [PubMed]
- Bernardo, R. Relationship between single-cross performance and molecular marker heterozygosity. Theor. Appl. Genet. 1992, 83, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Visscher, P.M.; Hill, W.G.; Wray, N.R. Heritability in the genomics era—Concepts and misconceptions. Nat. Rev. Genet. 2008, 9, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, P.W.; Lovatt, J.A. Chromosome doubling and top-crossing as a means of exploiting heterosis in perennial ryegrass. In Proceedings of Breeding and Seed Production for Conventional and Organic Agriculture, Proceeding of the 26th EUCAPRIA Fodder Crops and Amenity Grasses Section and the 16th Medicago spp. Group Joint Meeting, Perugia, Italy, 3–7 September 2006; pp. 52–55.
- Robins, J.; Bushman, B.; Escribano, S.; Jensen, K. Heterosis for protein, digestibility, fiber, and water soluble carbohydrates in nine sources of orchardgrass germplasm. Euphytica 2015, 204, 503–511. [Google Scholar] [CrossRef]
- Pembleton, L.W.; Wang, J.; Cogan, N.O.I.; Pryce, J.E.; Ye, G.; Bandaranayake, C.K.; Hand, M.L.; Baillie, R.C.; Drayton, M.C.; Lawless, K.; et al. Candidate gene-based association genetics analysis of herbage quality traits in perennial ryegrass (Lolium perenne L.). Crop Pasture Sci. 2013, 64, 244–253. [Google Scholar] [CrossRef]
- Payne, R.W.; Murray, D.A.; Harding, S.A.; Baird, D.B.; Soutar, D.M. GenStat for Windows Introduction; VSN International: Hemel Hempstead, UK, 2009. [Google Scholar]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Pearson Education Limited: Harlow, UK, 1996; pp. 160–181. [Google Scholar]
| b | S.E. | p-Value | b | S.E. | p-Value | ||
|---|---|---|---|---|---|---|---|
| WSC-1 | 0.47 | 0.222 | 0.037 | CP-1 | 0.42 | 0.184 | 0.026 |
| WSC-2 | 0.31 | 0.110 | 0.006 | CP-2 | 0.26 | 0.125 | 0.077 |
| WSC-3 | 0.29 | 0.094 | 0.003 | CP-3 | 0.28 | 0.111 | 0.012 |
| WSC-4 | 0.33 | 0.095 | <0.001 | CP-4 | 0.29 | 0.103 | 0.006 |
| WSC-5 | 0.33 | 0.102 | 0.002 | CP-5 | 0.03 | 0.116 | 0.817 |
| WSC-8 | 0.30 | 0.067 | <0.001 | CP-8 | 0.33 | 0.122 | 0.008 |
| Mean WSC | 0.32 | 0.078 | <0.001 | Mean CP | 0.21 | 0.076 | 0.008 |
| NDF-1 | 0.42 | 0.177 | 0.020 | ADF-1 | 0.19 | 0.163 | 0.251 |
| NDF-2 | 0.30 | 0.100 | 0.004 | ADF-2 | 0.25 | 0.108 | 0.022 |
| NDF-3 | 0.43 | 0.130 | 0.001 | ADF-3 | 0.34 | 0.122 | 0.006 |
| NDF-4 | 0.31 | 0.120 | 0.012 | ADF-4 | 0.26 | 0.126 | 0.044 |
| NDF-5 | 0.24 | 0.109 | 0.032 | ADF-5 | 0.18 | 0.152 | 0.242 |
| NDF-8 | 0.37 | 0.095 | <0.001 | ADF-8 | 0.21 | 0.128 | 0.110 |
| Mean NDF | 0.34 | 0.080 | <0.001 | Mean ADF | 0.23 | 0.071 | 0.002 |
| DMD-1 | 0.34 | 0.215 | 0.121 | ||||
| DMD-2 | 0.14 | 0.148 | 0.338 | ||||
| DMD-3 | 0.44 | 0.161 | 0.008 | ||||
| DMD-4 | 0.15 | 0.241 | 0.547 | ||||
| DMD-5 | 0.22 | 0.241 | 0.361 | ||||
| DMD-8 | 0.38 | 0.185 | 0.042 | ||||
| Mean DMD | 0.26 | 0.118 | 0.029 |
| Family | Parent 1 | Parent 2 |
|---|---|---|
| 1804 | LM299-96 | Tabu-37 |
| 1812 | Hulk-35 | LM299-85 |
| 1826 | Warrior-77 | Warrior-181 |
| 1836 | LM299-48 | Hulk-54 |
| 1857 | Tabu-47 | Tabu-60 |
| 1858 | Hulk-81 | Warrior-55 |
| 1899 | LM299-20 | LM414-55 |
| 1901 | Tabu-58 | Accelerate-89 |
| 1902 | LM299-61 | Warrior-26 |
| 1913 | LM299-17 | Accelerate-87 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Pembleton, L.W.; Cogan, N.O.I.; Forster, J.W. Evidence for Heterosis in Italian Ryegrass (Lolium multiflorum Lam.) Based on Inbreeding Depression in F2 Generation Offspring from Biparental Crosses. Agronomy 2016, 6, 49. https://doi.org/10.3390/agronomy6040049
Wang J, Pembleton LW, Cogan NOI, Forster JW. Evidence for Heterosis in Italian Ryegrass (Lolium multiflorum Lam.) Based on Inbreeding Depression in F2 Generation Offspring from Biparental Crosses. Agronomy. 2016; 6(4):49. https://doi.org/10.3390/agronomy6040049
Chicago/Turabian StyleWang, Junping, Luke W. Pembleton, Noel O. I. Cogan, and John W. Forster. 2016. "Evidence for Heterosis in Italian Ryegrass (Lolium multiflorum Lam.) Based on Inbreeding Depression in F2 Generation Offspring from Biparental Crosses" Agronomy 6, no. 4: 49. https://doi.org/10.3390/agronomy6040049
APA StyleWang, J., Pembleton, L. W., Cogan, N. O. I., & Forster, J. W. (2016). Evidence for Heterosis in Italian Ryegrass (Lolium multiflorum Lam.) Based on Inbreeding Depression in F2 Generation Offspring from Biparental Crosses. Agronomy, 6(4), 49. https://doi.org/10.3390/agronomy6040049






