Evaluating Agricultural Management Effects on Alachlor Availability: Tillage, Green Manure, and Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Characterization and Experimental Design
2.2. Chemical
2.3. Soil Amendments
2.4. Sorption–Desorption
2.4.1. Alachlor Sorption on Ridge Till Versus Chisel Plow Soils
2.4.2. Alachlor Sorption/Desorption on Amended Soil
2.5. Mineralization
2.6. Statistical Analysis
3. Results and Discussion
3.1. Alachlor Sorption in Tillage Systems
3.2. Alachlor Sorption/Desorption in Amended Soils
3.3. Alachlor Mineralization in Amended Soils
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chauhan, B.S.; Gill, G.S.; Preston, C. Tillage system effects on weed ecology, herbicide activity and persistence: A review. Aust. J. Exp. Agric. 2006, 46, 1557. [Google Scholar] [CrossRef]
- Hall, J.K.; Murray, M.R.; Hartwig, N.L. Herbicide leaching and distribution in tilled and untilled soil. J. Environ. Qual. 1989, 18, 439. [Google Scholar] [CrossRef]
- Rojas, R.; Morillo, J.; Usero, J.; Delgado-Moreno, L.; Gan, J. Enhancing soil sorption capacity of an agricultural soil by addition of three different organic wastes. Sci. Total Environ. 2013, 458, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Glotfelty, D.E. Effects of conservation tillage practices on pesticide volatilization and degradation. In Effects of Conservation Tillage on Groundwater Quality: Nitrates and Pesticides; Logan, T.L., Davidson, J.M., Baker, J.L., Overcash, M.R., Eds.; Lewis Publishers: Chelsea, MI, USA, 1987; pp. 169–177. [Google Scholar]
- Alletto, L.; Coquet, Y.; Benoit, P.; Heddadj, D.; Barriuso, E. Tillage management effects on pesticide fate in soils. A review. Agron. Sustain. Dev. 2010, 30, 367–400. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Fact Sheet for Alachlor; USEPA: Washington, DC, USA, 1998.
- Yen, P.Y.; Koskinen, W.C.; Schweizer, E.E. Dissipation of alachlor in four soils as influenced by degradation and sorption processes. Weed Sci. 1994, 42, 233–240. [Google Scholar]
- Helling, C.S.; Zhuang, W.; Gish, T.J.; Coffman, C.B.; Isensee, A.R.; Kearney, P.C.; Hoagland, D.R.; Woodward, M.D. Persistence and leaching of atrazine, alachlor, and cyanazine under no-tillage practices. Chemosphere 1988, 17, 175–187. [Google Scholar] [CrossRef]
- Sharp, D.B. Alachlor. In Herbicides: Chemistry, Degradation, and Mode of Action; Kearney, P., Kaufman, D.D., Eds.; Dekker: New York, NY, USA, 1988; pp. 301–333. [Google Scholar]
- Weber, J.B.; Wilkerson, G.G.; Reinhardt, C.F. Calculating pesticide sorption coefficients (Kd) using selected soil properties. Chemosphere 2004, 55, 157–166. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Decision of 18 December 2006 Concerning the Non-Inclusion of Alachlor in Annex I to Council Directive 91/414/EEC and the Withdrawal of Authorisations for Plant Protection Products Containing This Active Substance. Available online: http://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:32006D0966 (accessed on 23 September 2017).
- Thurman, E.M.; Goolsby, D.A.; Aga, D.S.; Pomes, M.L.; Meyer, M.T. Occurrence of alachlor and its sulfonated metabolite in rivers and reservoirs of the Midwestern United States: The importance of sulfonation in the transport of chloroacetanilide herbicides. Environ. Sci. Technol. 1996, 30, 569–574. [Google Scholar] [CrossRef]
- United States Environmental Proctection Agency (USEPA). Restricted Use Product Summary Report; USEPA: Washington, DC, USA, 2016.
- Kolpin, D.W.; Thurman, E.M.; Goolsby, D.A. Occurrence of selected pesticides and their metabolites in near-surface aquifers of the Midwestern United States. Environ. Sci. Technol. 1996, 30, 335–340. [Google Scholar] [CrossRef]
- Peter, C.J.; Weber, J.B. Adsorption, mobility, and efficacy of alachlor and metolachlor as influenced by soil properties. Weed Sci. 1985, 33, 874–881. [Google Scholar]
- Soil Science Society of America (SSSA). Glossary of Soil Science Terms; Soil Science Society of America: Madison, WI, USA, 1997. [Google Scholar]
- Triplett, G.B.; Van Doren, D.M. Nitrogen, Phosphorus, and potassium fertilization of non-tilled maize. Agron. J. 1969, 61, 637. [Google Scholar] [CrossRef]
- Bailey, G.W.; White, J.L. Review of adsorption and desorption of organic pesticides by soil colloids, with implications concerning pesticide bioactivity. J. Agric. Food Chem. 1964, 12, 324–332. [Google Scholar] [CrossRef]
- Clay, S.A.; Koskinen, W.C.; Carlson, P. Alachlor movement through intact soil columns taken from two tillage systems taken from two tillage systems. Weed Technol. 1991, 5, 485–489. [Google Scholar]
- Petersen, B.B.; Shea, P.J.; Wicks, G.A. Acetanilide activity and dissipation as influenced by formulation and wheat stubble. Weed Sci. 1988, 36, 243–249. [Google Scholar]
- Doran, J.W. Microbial changes associated with residue management with reduced tillage. Soil Sci. Soc. Am. J. 1980, 44, 518. [Google Scholar] [CrossRef]
- Shipitalo, M.; Dick, W.; Edwards, W. Conservation tillage and macropore factors that affect water movement and the fate of chemicals. Soil Tillage Res. 2000, 53, 167–183. [Google Scholar] [CrossRef]
- Guo, L.; Bicki, T.J.; Felsot, A.S.; Hinesly, T.D. Sorption and movement of alachlor in soil modified by carbon-rich wastes. J. Environ. Qual. 1993, 22, 186. [Google Scholar] [CrossRef]
- Graber, E.R.; Tsechansky, L.; Gerstl, Z.; Lew, B. High surface area biochar negatively impacts herbicide efficacy. Plant Soil 2011, 353, 95–106. [Google Scholar] [CrossRef]
- Locke, M.A.; Gaston, L.A.; Zablotowicz, R.M. Alachlor biotransformation and sorption in soil from two soybean tillage systems. J. Agric. Food Chem. 1996, 44, 1128–1134. [Google Scholar] [CrossRef]
- Locke, M.A. Sorption-desorption kinetics of alachlor in surface soil from two soybean Tillage Systems. J. Environ. Qual. 1992, 21, 558. [Google Scholar] [CrossRef]
- Guo, L.; Bicki, T.J.; Felsot, A.S.; Hinesly, T.D. Phytotoxicity of atrazine and alachlor in soil amended with sludge, manure and activated carbon. J. Environ. Sci. Health Part B 1991, 26, 513–527. [Google Scholar] [CrossRef]
- Hatfield, J. Ridge tillage for corn and soybean production: Environmental quality impacts. Soil Tillage Res. 1998, 48, 145–154. [Google Scholar] [CrossRef]
- Williams, A.; Davis, A.S.; Ewing, P.M.; Grandy, A.S.; Kane, D.A.; Koide, R.T.; Mortensen, D.A.; Smith, R.G.; Snapp, S.S.; Spokas, K.A.; et al. A comparison of soil hydrothermal properties in zonal and uniform tillage systems across the US Corn Belt. Geoderma 2016, 273, 12–19. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Mortensen, D.A.; Smith, R.G.; Snapp, S.S.; Spokas, K.A.; et al. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Giori, F.G.; Tornisielo, V.L.; Regitano, J.B. The role of sugarcane residues in the sorption and leaching of herbicides in two tropical soils. Water Air Soil Pollut. 2014, 225, 1935. [Google Scholar] [CrossRef]
- OECD (Economic Co-operation and Development). Guideline 106: Adsorption—Desorption Using a Batch Equilibrium Method; OECD Publishing: Paris, France, 2000. [Google Scholar]
- Bowman, B.T.; Sans, W.W. Partitioning behavior of insecticides in soil-water systems: II. Desorption hysteresis effects. J. Environ. Qual. 1985, 14, 270. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Williams, A.; Davis, A.S.; Jilling, A.; Grandy, A.S.; Koide, R.T.; Mortensen, D.A.; Smith, R.G.; Snapp, S.S.; Spokas, K.A.; Yannarell, A.C.; et al. Reconciling opposing soil processes in row-crop agroecosystems via soil functional zone management. Agric. Ecosyst. Environ. 2017, 236, 99–107. [Google Scholar] [CrossRef]
- Shi, X.H.; Yang, X.M.; Drury, C.F.; Reynolds, W.D.; McLaughlin, N.B.; Zhang, X.P. Impact of ridge tillage on soil organic carbon and selected physical properties of a clay loam in southwestern Ontario. Soil Tillage Res. 2012, 120, 1–7. [Google Scholar] [CrossRef]
- Hansen, N.C.; Moncrief, J.F.; Gupta, S.C.; Capel, P.D.; Olness, A.E. Herbicide banding and tillage system interactions on runoff losses of alachlor and cyanazine. J. Environ. Qual. 2001, 30, 2120. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.D.; MacTavish, D.C.; Findlay, W.I. Surface and subsurface transport of atrazine and alachlor from a Brookston clay loam under continuous corn production. Arch. Environ. Contam. Toxicol. 1992, 23, 240–245. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Lima, I.M.; Klasson, K.T. Sorption of deisopropylatrazine on broiler litter biochars. J. Agric. Food Chem. 2010, 58, 12350–12356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Q.; Sun, K.; Liu, X.; Zheng, W.; Zhao, Y. Sorption of simazine to corn straw biochars prepared at different pyrolytic temperatures. Environ. Pollut. 2011, 159, 2594–2601. [Google Scholar] [CrossRef] [PubMed]
- Giori, F.G.; Tornisielo, V.L.; Pellegrino Cerri, C.E.; Regitano, J.B. Sugarcane straw management and soil attributes on alachlor and diuron sorption in highly weathered tropical soils. J. Environ. Sci. Health Part B 2014, 49, 352–360. [Google Scholar] [CrossRef]
- Levanon, D. Roles of fungi and bacteria in the mineralization of the pesticides atrazine, alachlor, malathion and carbofuran in soil. Soil Biol. Biochem. 1993, 25, 1097–1105. [Google Scholar] [CrossRef]
- Munoz, A.; Koskinen, W.C.; Cox, L.; Sadowsky, M.J. Biodegradation and mineralization of metolachlor and alachlor by Candida xestobii. J. Agric. Food Chem. 2011, 59, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, M.; Dai, J.; Cao, H.; Pan, C.; Qiu, X.; Xu, M. Degradation of acetochlor by four microbial communities. Bioresour. Technol. 2008, 99, 7797–7802. [Google Scholar] [CrossRef] [PubMed]
- Stamper, D.M.; Tuovinen, O.H. Biodegradation of the acetanilide herbicides alachlor, metolachlor, and propachlor. Crit. Rev. Microbiol. 1998, 24, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ferrey, M.L.; Koskinen, W.C.; Blanchette, R.A.; Burnes, T.A. Mineralization of alachlor by lignin-degrading fungi. Can. J. Microbiol. 1994, 40, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Gadagbui, B.; Maier, A.; Dourson, M.; Parker, A.; Willis, A.; Christopher, J.P.; Hicks, L.; Ramasamy, S.; Roberts, S.M. Derived Reference Doses (RfDs) for the environmental degradates of the herbicides alachlor and acetochlor: Results of an independent expert panel deliberation. Regul. Toxicol. Pharmacol. 2010, 57, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cruz, M.S.; Marín-Benito, J.M.; Ordax, J.M.; Azejjel, H.; Sánchez-Martín, M.J. Influence of pine or oak wood on the degradation of alachlor and metalaxyl in soil. J. Environ. Manag. 2012, 95, S228–S232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
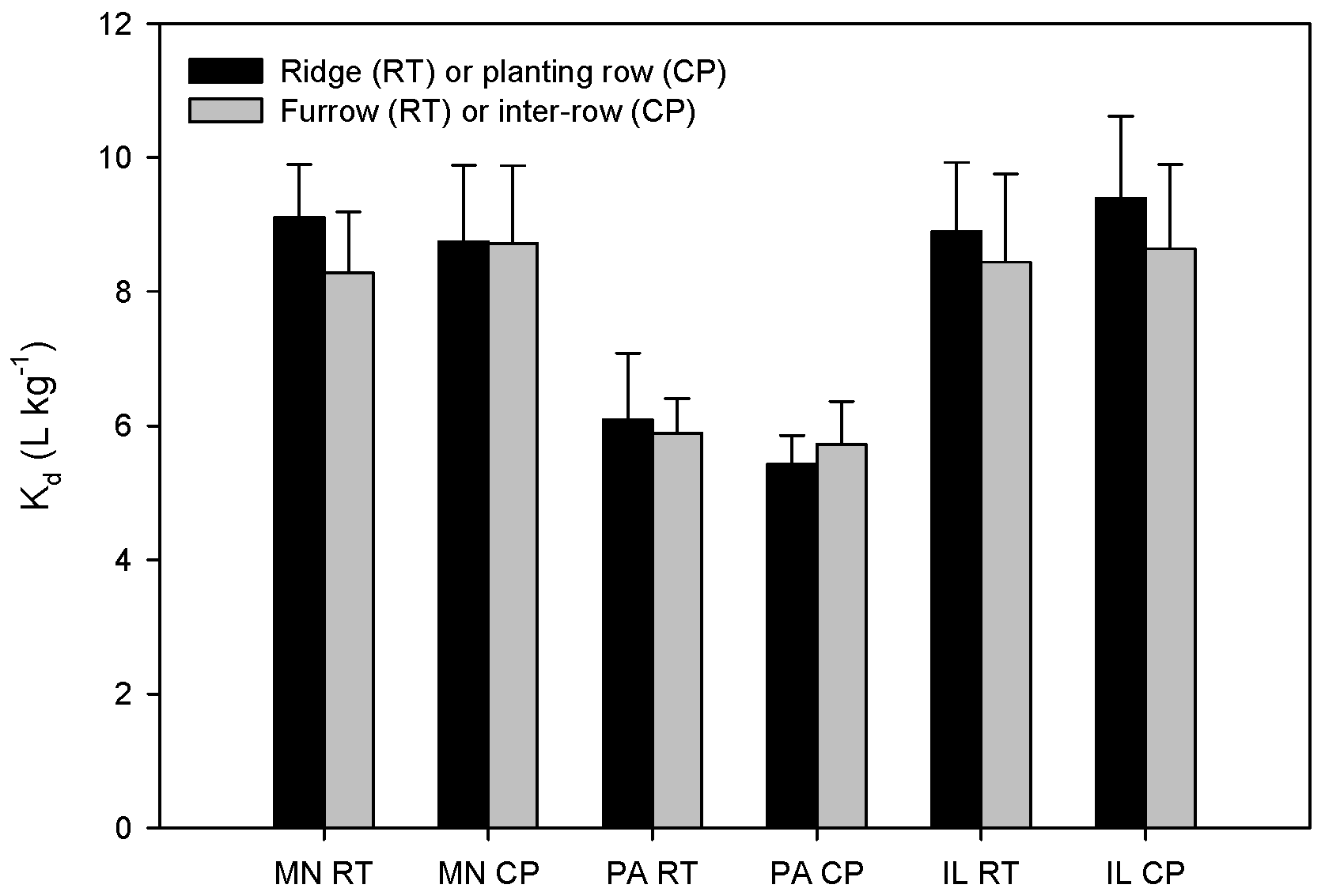
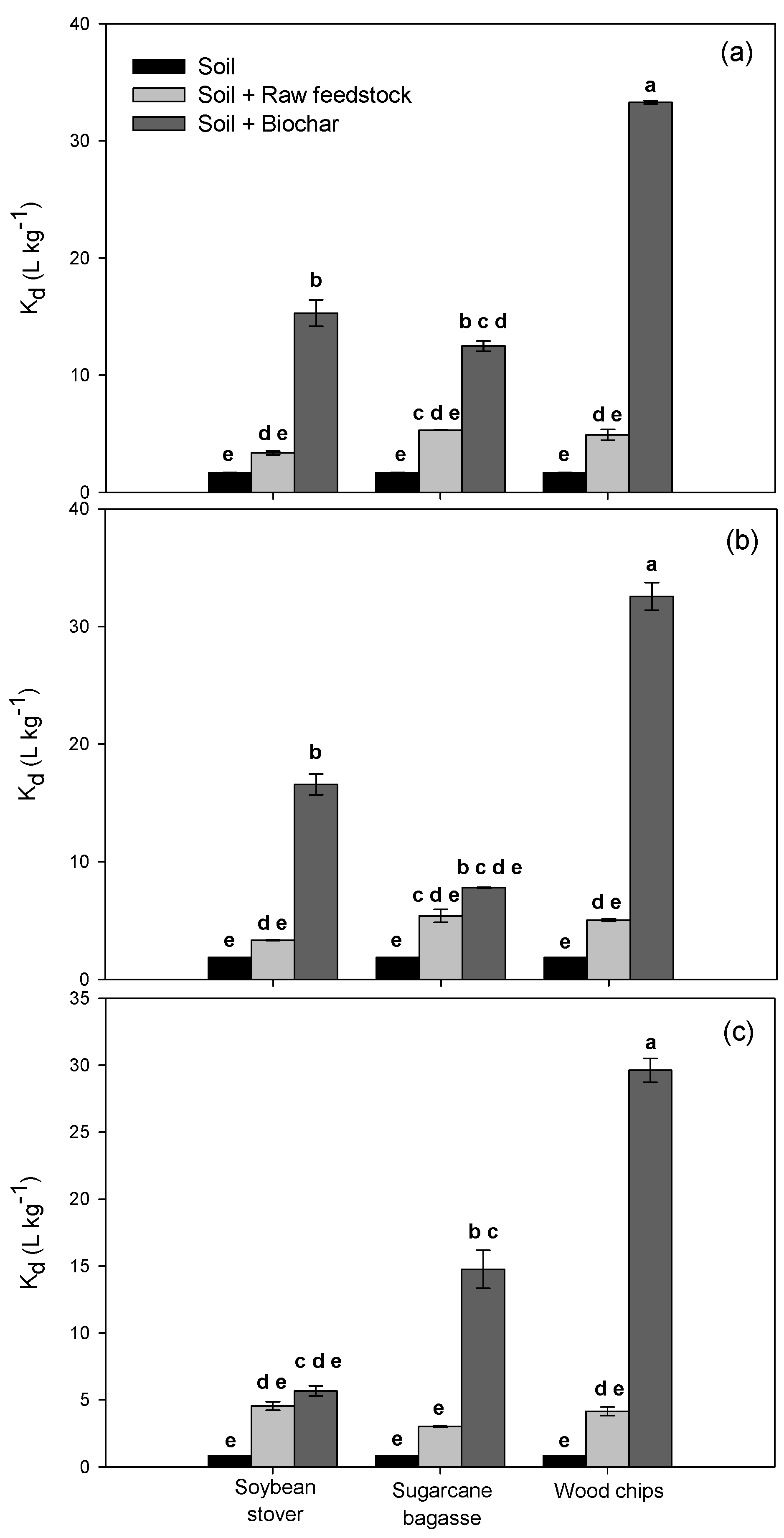
| Location | Tillage | Position | Organic Matter (%) | Cation Exchange Capacity (meq·g−1) | pH | P (mg·kg−1) | K (mg·kg−1) | Ca (mg·kg−1) | Mg (mg·kg−1) | Texture | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | ||||||||||
| MN | RT | Ridge | 4.47 (0.38) | 14.87 (0.51) | 6.27 (0.53) | 26.33 (6.22) | 129.33 (34.2) | 1865.83 (184.51) | 409 (46.52) | 28 | 56 | 16 |
| RT | Furrow | 4.28 (0.35) | 15.1 (0.77) | 6.25 (0.6) | 25.5 (7.37) | 115.67 (42.34) | 1921.00 (253.82) | 400 (59.77) | ||||
| CP | 4.4 (0.68) | 14.65 (0.85) | 6.15 (0.28) | 26.67 (6.74) | 134.00 (26) | 1810.83 (169.67) | 398 (53.24) | |||||
| PA | RT | Ridge | 3.2 (0.17) | 7.73 (1.8) | 6.13 (0.25) | 26.33 (4.16) | 72 (21.7) | 1148.33 (270.3) | 84.33 (11.68) | 10 | 35 | 25 |
| RT | Furrow | 3 (0.2) | 8.27 (1.27) | 6.13 (0.06) | 21.33 (1.53) | 63.33 (19.76) | 1232.33 (207.1) | 92.67 (16.8) | ||||
| CP | 3.2 (0.26) | 7.67 (1.42) | 6.03 (0.15) | 27 (7.81) | 90.33 (57.83) | 1121 (231.14) | 81.33 (9.5) | |||||
| IL | RT | Ridge | 4.9 (0.61) | 16.62 (3.51) | 5.77 (0.24) | 26.5 (12.99) | 201.5 (66.36) | 2389.67 (552.08) | 428.83 (112.23) | 17 | 56 | 27 |
| RT | Furrow | 4.9 (0.61) | 16.62 (3.51) | 5.77 (0.24) | 26.5 (12.99) | 201.5 (66.36) | 2389.67 (552.08) | 428.83 (112.23) | ||||
| CP | 4.77 (0.47) | 15.92 (2.93) | 5.95 (0.4) | 19.83 (6.31) | 176.67 (19.54) | 2412.17 (415.04) | 423.83 (80.26) | |||||
| Amendment | C a (%) | H a (%) | N a (%) | O a (%) | S a (%) | Ash a (%) | VM bc (%) | Fixed C c (%) | Moisture c (%) | pH | Molar Ratios | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H:C | O:C | C:N | |||||||||||
| Soybean | |||||||||||||
| Raw feedstock | 44.7 | 6.4 | 0.8 | 42.0 | 0.1 | 6.0 | 20.0 | 68.0 | 6.0 | 7.3 | 1.72 | 0.70 | 65 |
| Biochar (500 °C) | 48.0 | 2.1 | 1.3 | 11.1 | 0.0 | 37.5 | 17.0 | 41.7 | 3.8 | 8.7 | 0.53 | 0.17 | 43 |
| Sugarcane Bagasse | |||||||||||||
| Raw feedstock | 48.6 | 5.9 | 0.2 | 39.8 | 0.0 | 5.5 | 80.0 | 8.3 | 6.2 | 4.7 | 1.46 | 0.61 | 284 |
| Biochar (350 °C) | 75.2 | 4.6 | 0.6 | 15.8 | 0.1 | 3.8 | 46.5 | 47.0 | 2.7 | 5.0 | 0.73 | 0.16 | 146 |
| Wood chips (grape) | |||||||||||||
| Raw feedstock | 41.6 | 4.9 | 1.2 | 43.5 | 0.2 | 8.7 | 63.1 | 21.2 | 7.0 | 5.8 | 1.42 | 0.78 | 41 |
| Biochar (500 °C) | 70.4 | 2.3 | 0.9 | 9.6 | 0.0 | 16.8 | 19.3 | 64.0 | 4.0 | 7.4 | 0.40 | 0.10 | 88 |
| Feedstock | Amendment | Kd desorption/Kd adsorption | ||
|---|---|---|---|---|
| MN | IL | PA | ||
| Control | 1.7 ± 0.0 | 1.8 ± 0.0 | 2.5 ± 0.0 | |
| Soybean stover | Raw feedstock | 1.7 ± 0.1 | 1.7 ± 0.0 | 1.2 ± 0.1 |
| Biochar | 2.0 ± 0.1 | 2.1 ± 0.1 | 5.8 ± 0.8 | |
| Sugarcane bagasse | Raw feedstock | 1.5 ± 0.0 | 1.5 ± 0.2 | 2.2 ± 0.0 |
| Biochar | 1.3 ± 0.1 | 2.1 ± 0.0 | 1.0 ± 0.0 | |
| Wood chips | Raw feedstock | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 |
| Biochar | 1.9 ± 0.1 | 1.8 ± 0.0 | 1.7 ± 0.0 | |
| Feedstock | Amendment | Total 14CO2 (%) 1 | k (days−1) 2 | R2 |
|---|---|---|---|---|
| Control | 10.4 ± 2.4 a | 0.040 ± 0.017 | 0.981 | |
| Soybean residue | Raw feedstock | 7.0 ± 0.5 ab | 0.042 ± 0.014 | 0.986 |
| Biochar | 4.6 ± 0.4 b | 0.046 ± 0.013 | 0.989 | |
| Sugarcane bagasse | Raw feedstock | 4.0 ± 0.6 b | 0.045 ± 0.008 | 0.995 |
| Biochar | 3.4 ± 0.5 b | 0.042 ± 0.010 | 0.993 | |
| Wood chips | Raw feedstock | 4.2 ± 0.3 b | 0.055 ± 0.007 | 0.997 |
| Biochar | 3.8 ± 1.6 b | 0.017 ± 0.021 | 0.963 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, K.F.; Hall, K.E.; Spokas, K.A.; Koskinen, W.C.; Tornisielo, V.L. Evaluating Agricultural Management Effects on Alachlor Availability: Tillage, Green Manure, and Biochar. Agronomy 2017, 7, 64. https://doi.org/10.3390/agronomy7040064
Mendes KF, Hall KE, Spokas KA, Koskinen WC, Tornisielo VL. Evaluating Agricultural Management Effects on Alachlor Availability: Tillage, Green Manure, and Biochar. Agronomy. 2017; 7(4):64. https://doi.org/10.3390/agronomy7040064
Chicago/Turabian StyleMendes, Kassio F., Kathleen E. Hall, Kurt A. Spokas, William C. Koskinen, and Valdemar L. Tornisielo. 2017. "Evaluating Agricultural Management Effects on Alachlor Availability: Tillage, Green Manure, and Biochar" Agronomy 7, no. 4: 64. https://doi.org/10.3390/agronomy7040064






