Effect of Supplementary Light Source on Quality of Grafted Tomato Seedlings and Expression of Two Photosynthetic Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Grafting Procedures
2.3. Light Treatments
2.4. Data Collection and Analysis
2.5. Quantitative Real-Time PCR Analysis
3. Results
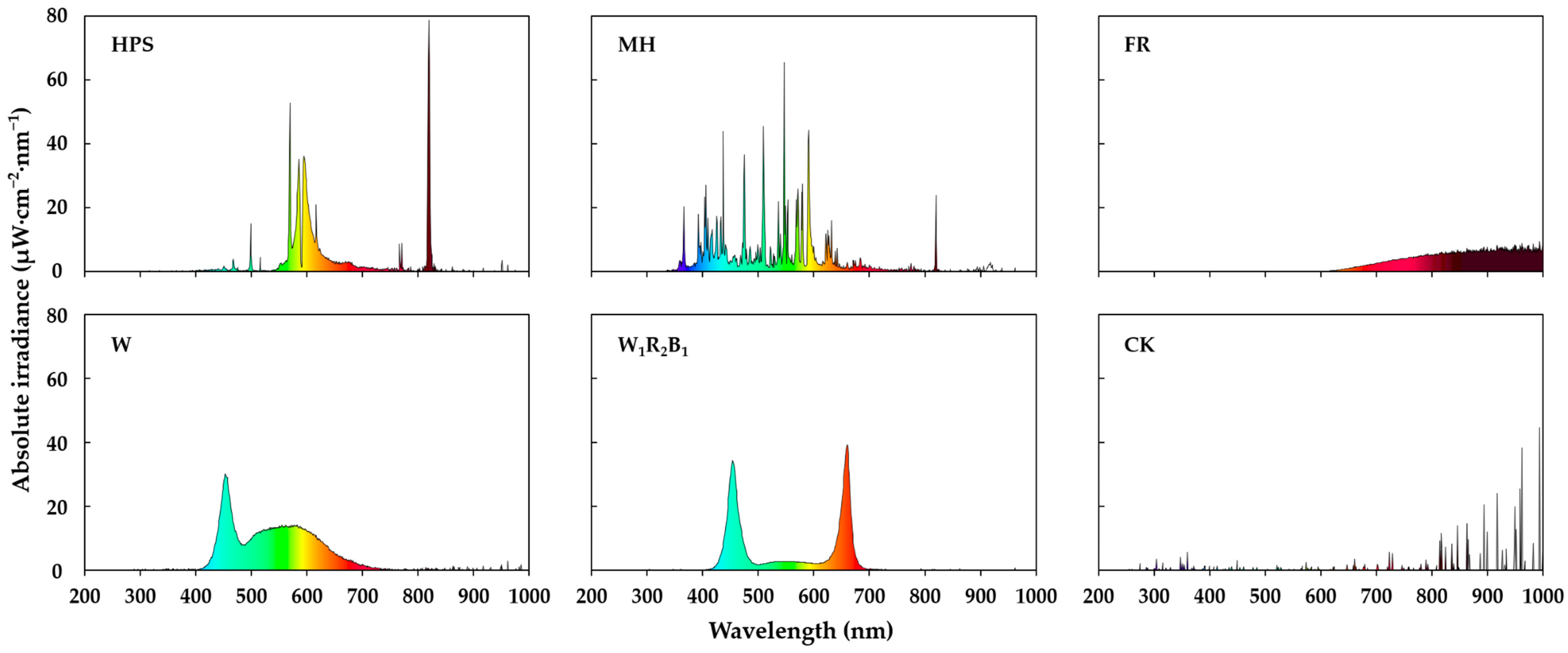
3.1. Spectra of Different Supplementary Light Sources
3.2. Growth and Development of Tomato Seedlings after Supplementary Light Treatments in Glasshouse
3.3. Biomass of Shoots and Roots
3.4. Evaluation of Seedling Quality
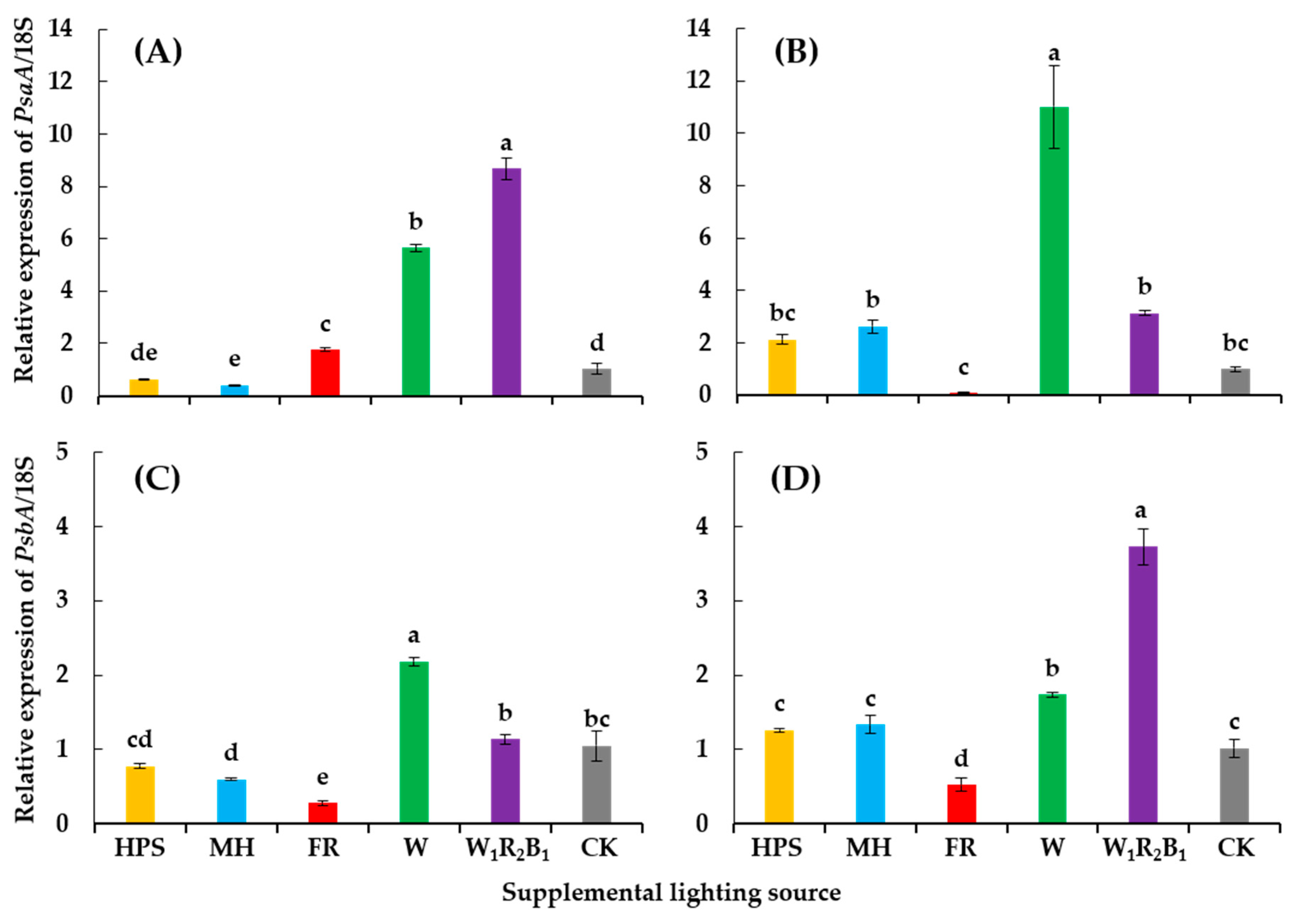
3.5. Expression of Photosysthesis-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, D.; Gao, W.; Ruan, J. Effects of light quality on plant growth and development. Plant Physiol. J. 2015, 51, 1217–1234. [Google Scholar]
- Long, S.P.; Zhu, X.G.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.Q.; Shen, Y.K. Photosynthetic efficiency and crop yield. In Handbook of Plant and Crop Physiology; Marcel Dekker: New York, NY, USA, 2002; pp. 821–834. [Google Scholar]
- Patil, G.G.; Oi, R.; Gissinger, A.; Moe, R. Plant morphology is affected by light quality selective plastic films and alternating day and night temperature. Gartenbauwissenschaft 2001, 66, 53–60. [Google Scholar]
- Murakami, K.; Matsuda, R.; Fujiwara, K. A basis for selecting light spectral distribution for evaluating leaf photosynthetic rates of plants grown under different light spectral distributions. Environ. Control Biol. 2017, 55, 1–6. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Dzakovich, M.P.; Gomez, C.; Lopez, R.; Burr, J.F.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S. Light-emitting diodes in horticulture. Hortic. Rev. 2015, 43, 1–87. [Google Scholar]
- Gómez, C.; Morrow, R.C.; Bourget, C.M.; Massa, G.D.; Mitchell, C.A. Comparison of intracanopy light-emitting diode towers and overhead high-pressure sodium lamps for supplemental lighting of greenhouse-grown tomatoes. Hortic. Technol. 2013, 23, 93–98. [Google Scholar]
- Buchalla, W.; Attin, T. External bleaching therapy with activation by heat, light or laser—A systematic review. Dent. Mater. 2007, 23, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Massa, G.D.; Kim, H.-H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to led lighting. Hortic. Sci. 2008, 43, 1951–1956. [Google Scholar]
- Morrow, R.C. Led lighting in horticulture. Hortic. Sci. 2008, 43, 1947–1950. [Google Scholar]
- Islam, M.A.; Kuwar, G.; Clarke, J.L.; Blystad, D.-R.; Gislerød, H.R.; Olsen, J.E.; Torre, S. Artificial light from light emitting diodes (LEDs) with a high portion of blue light results in shorter poinsettias compared to high pressure sodium (HPS) lamps. Sci. Hortic. 2012, 147, 136–143. [Google Scholar] [CrossRef]
- Radkov, E.; Setlur, A.A. Full Spectrum Phosphor Blends for White Light Generation with Led Chips. U.S. Patent 790,679,0B2, 15 March 2011. [Google Scholar]
- Dhingra, A.; Khurana, J.P.; Tyagi, A.K. Involvement of g-proteins, calmodulin and tagetitoxin-sensitive RNA polymerase in light-regulated expression of plastid genes (psbA, psaA and rbcL) in rice (Oryza sativa L.). Plant Sci. 2004, 166, 163–168. [Google Scholar] [CrossRef]
- Muneer, S.; Park, Y.G.; Jeong, B.R. Red and blue light emitting diodes (LEDs) participate in mitigation of hyperhydricity in in vitro-grown carnation genotypes (Dianthus caryophyllus). J. Plant Growth Regul. 2018, 37, 370–379. [Google Scholar] [CrossRef]
- Wang, Y.; Jensen, L.; Hojrup, P.; Morse, D. Synthesis and degradation of dinoflagellate plastid-encoded psbA proteins are light-regulated, not circadian-regulated. Proc. Natl. Acad. Sci. USA 2005, 102, 2844–2849. [Google Scholar] [CrossRef] [PubMed]
- Trebitsh, T.; Levitan, A.; Sofer, A.; Danon, A. Translation of chloroplast psbA mRNA is modulated in the light by counteracting oxidizing and reducing activities. Mol. Cell. Boil. 2000, 20, 1116–1123. [Google Scholar] [CrossRef]
- Leelavathi, S.; Bhardwaj, A.; Kumar, S.; Dass, A.; Pathak, R.; Pandey, S.S.; Tripathy, B.C.; Padmalatha, K.V.; Dhandapani, G.; Kanakachari, M.; et al. Genome-wide transcriptome and proteome analyses of tobacco psaA and psbA deletion mutants. Plant Mol. Boil. 2011, 76, 407–423. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-M.; Kubota, C.; Tsao, S.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Buschmann, C.; Langsdorf, G.; Lichtenthaler, H. Imaging of the blue, green, and red fluorescence emission of plants: An overview. Photosynthetica 2000, 38, 483–491. [Google Scholar] [CrossRef]
- Nanya, K.; Ishigami, Y.; Hikosaka, S.; Goto, E. In effects of blue and red light on stem elongation and flowering of tomato seedlings. Acta Hortic. 2012, 261–266. [Google Scholar] [CrossRef]
- Lee, J.-G.; Oh, S.-S.; Cha, S.-H.; Jang, Y.-A.; Kim, S.-Y.; Um, Y.-C.; Cheong, S.-R. Effects of red/blue light ratio and short-term light quality conversion on growth and anthocyanin contents of baby leaf lettuce. J. Bio-Environ. Control 2010, 19, 351–359. [Google Scholar]
- Liu, L.; Zabaras, D.; Bennett, L.; Aguas, P.; Woonton, B. Effects of UV-C, red light and sun light on the carotenoid content and physical qualities of tomatoes during post-harvest storage. Food Chem. 2009, 115, 495–500. [Google Scholar] [CrossRef]
- Im, C.S.; Eberhard, S.; Huang, K.; Beck, C.F.; Grossman, A.R. Phototropin involvement in the expression of genes encoding chlorophyll and carotenoid biosynthesis enzymes and LHC apoproteins in chlamydomonas reinhardtii. Plant J. Cell Mol. Boil. 2006, 48, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kreslavski, V.D.; Lyubimov, V.Y.; Shirshikova, G.N.; Shmarev, A.N.; Kosobryukhov, A.A.; Schmitt, F.J.; Friedrich, T.; Allakhverdiev, S.I. Preillumination of lettuce seedlings with red light enhances the resistance of photosynthetic apparatus to UV-A. J. Photochem. Photobiol. B 2013, 122, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.A.; Surrey, K. Red and far-red action on oxidative phosphorylation. Radiat. Res. 1960, 12, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Franklin, K.A.; Whitelam, G.C. Light-quality regulation of freezing tolerance in Arabidopsis thaliana. Nat. Genet. 2007, 39, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Whitelam, G.C.; Halliday, K.J. Annual Plant Reviews, Light and Plant Development; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 30. [Google Scholar]
- Son, K.-H.; Oh, M.-M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. Hortic. Sci. 2013, 48, 988–995. [Google Scholar]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. Hortic. Sci. 2010, 45, 1809–1814. [Google Scholar]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. Var. Capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Batista, D.S.; Felipe, S.H.S.; Silva, T.D.; de Castro, K.M.; Mamedes-Rodrigues, T.C.; Miranda, N.A.; Ríos-Ríos, A.M.; Faria, D.V.; Fortini, E.A.; Chagas, K.; et al. Light quality in plant tissue culture: Does it matter? Cell. Dev. Biol.-Plant 2018, 54, 1–21. [Google Scholar] [CrossRef]
- Macedo, A.F.; Leal-Costa, M.V.; Tavares, E.S.; Lage, C.L.S.; Esquibel, M.A. The effect of light quality on leaf production and development of in vitro-cultured plants of alternanthera brasiliana kuntze. Environ. Exp. Bot. 2011, 70, 43–50. [Google Scholar] [CrossRef]
- Liu, X.; Guo, S.; Xu, Z.; Jiao, X.; Tezuka, T. Regulation of chloroplast ultrastructure, cross-section anatomy of leaves, and morphology of stomata of cherry tomato by different light irradiations of light-emitting diodes. Hortic. Sci. 2011, 46, 217–221. [Google Scholar]
- Jeong, S.W.; Hogewoning, S.W.; van Ieperen, W. Responses of supplemental blue light on flowering and stem extension growth of cut chrysanthemum. Sci. Hortic. 2014, 165, 69–74. [Google Scholar] [CrossRef]
- Jeong, S.W.; Park, S.; Jin, J.S.; Seo, O.N.; Kim, G.-S.; Kim, Y.-H.; Bae, H.; Lee, G.; Kim, S.T.; Lee, W.S.; et al. Influences of four different light-emitting diode lights on flowering and polyphenol variations in the leaves of chrysanthemum (Chrysanthemum morifolium). J. Agric. Food Chem. 2012, 60, 9793–9800. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Mun, B.; Seo, T.; Lee, J.; Oh, S.; Chun, C. Effects of light quality and intensity on the carbon dioxide exchange rate, growth, and morphogenesis of grafted pepper transplants during healing and acclimatization. Korean J. Hortic. Sci. Technol. 2013, 31, 14–23. [Google Scholar] [CrossRef]
- Lanoue, J.; Leonardos, E.D.; Grodzinski, B. Effects of light quality and intensity on diurnal patterns and rates of photo-assimilate translocation and transpiration in tomato leaves. Front. Plant Sci. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lim, C.S.; Muneer, S.; Jeong, B.R. Functional vascular connections and light quality effects on tomato grafted unions. Sci. Hortic. 2016, 201, 306–317. [Google Scholar] [CrossRef]
- Khan, M.S.; Hameed, W.; Nozoe, M.; Shiina, T. Disruption of the psbA gene by the copy correction mechanism reveals that the expression of plastid-encoded genes is regulated by photosynthesis activity. J. Plant Res. 2007, 120, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Lezhneva, L.; Meurer, J. The nuclear factor hcf145 affects chloroplast psaA-psaB-rps14 transcript abundance in Arabidopsis thaliana. Plant J. 2004, 38, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Tozawa, Y.; Teraishi, M.; Sasaki, T.; Sonoike, K.; Nishiyama, Y.; Itaya, M.; Miyao, A.; Hirochika, H. The plastid sigma factor sig1 maintains photosystem I activity via regulated expression of the psaA operon in rice chloroplasts. Plant J. 2007, 52, 124–132. [Google Scholar] [CrossRef] [PubMed]




| Cultivar (C) | Light Source (LS) | Scion | Leaf | Root | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Length (cm) | Stem Diameter (mm) | Number | Length (cm) | Width (cm) | Thickness(mm) | Chlorophyll (SPAD) | Length (cm) | Fresh Weight (g) | ||||
| Super Sunload | HPS | 20.2 ab z | 6.15 abc | 10.3 ab | 12.7 a | 9.0 a | 0.49 b | 51.0 ab | 12.6 a | 0.95 ab | ||
| MH | 18.9 ab | 5.84 abc | 9.3 bc | 13.3 a | 8.6 ab | 0.55 ab | 45.5 bc | 15.3 a | 1.18 ab | |||
| FR | 15.5 b | 5.21 c | 7.7 d | 10.9 bc | 7.5 bc | 0.47 b | 41.0 c | 12.5 a | 0.73 b | |||
| W | 24.6 a | 6.27 ab | 11.0 a | 13.5 a | 8.5 ab | 0.57 ab | 57.0 a | 14.7 a | 1.37 a | |||
| W1R2B1 | 19.0 ab | 6.55 a | 11.0 a | 12.6 ab | 8.3 ab | 0.68 a | 56.8 a | 15.7 a | 1.41 a | |||
| CK | 16.1 b | 5.33 bc | 8.5 cd | 9.7 c | 6.9 c | 0.45 b | 38.9 c | 8.1 b | 0.71 b | |||
| Super Dotaerang | HPS | 17.2 b | 6.01 bc | 9.8 ab | 12.1 a | 7.9 a | 0.46 a | 52.7 ab | 13.4 ab | 0.96 bc | ||
| MH | 15.6 bc | 5.87 bc | 8.8 bc | 11.2 ab | 7.6 a | 0.47 a | 44.9 bc | 11.1 b | 0.80 bc | |||
| FR | 12.3 c | 4.69 c | 7.7 c | 9.7 b | 5.7 b | 0.47 a | 41.0 c | 6.6 c | 0.53 c | |||
| W | 23.3 a | 6.38 b | 9.7 ab | 11.6 ab | 7.3 a | 0.44 a | 48.7 bc | 11.9 ab | 1.18 b | |||
| W1R2B1 | 17.5 b | 7.86 a | 11.2 a | 12.3 a | 7.8 a | 0.58 a | 61.0 a | 14.8 a | 1.79 a | |||
| CK | 12.6 c | 5.60 bc | 7.3 c | 9.8 b | 6.6 ab | 0.51 a | 43.1 c | 7.6 c | 0.57 c | |||
| F-test | ||||||||||||
| LS | *** y | * | *** | *** | *** | * | *** | *** | *** | |||
| C | *** | NS | * | *** | *** | * | NS | * | NS | |||
| LS × C | *** | * | NS | NS | NS | ** | * | *** | * | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Hu, J.; Liu, C.; Wang, M.; Zhao, J.; Kang, D.I.; Jeong, B.R. Effect of Supplementary Light Source on Quality of Grafted Tomato Seedlings and Expression of Two Photosynthetic Genes. Agronomy 2018, 8, 207. https://doi.org/10.3390/agronomy8100207
Wei H, Hu J, Liu C, Wang M, Zhao J, Kang DI, Jeong BR. Effect of Supplementary Light Source on Quality of Grafted Tomato Seedlings and Expression of Two Photosynthetic Genes. Agronomy. 2018; 8(10):207. https://doi.org/10.3390/agronomy8100207
Chicago/Turabian StyleWei, Hao, Jiangtao Hu, Chen Liu, Mengzhao Wang, Jin Zhao, Dong Il Kang, and Byoung Ryong Jeong. 2018. "Effect of Supplementary Light Source on Quality of Grafted Tomato Seedlings and Expression of Two Photosynthetic Genes" Agronomy 8, no. 10: 207. https://doi.org/10.3390/agronomy8100207
APA StyleWei, H., Hu, J., Liu, C., Wang, M., Zhao, J., Kang, D. I., & Jeong, B. R. (2018). Effect of Supplementary Light Source on Quality of Grafted Tomato Seedlings and Expression of Two Photosynthetic Genes. Agronomy, 8(10), 207. https://doi.org/10.3390/agronomy8100207








