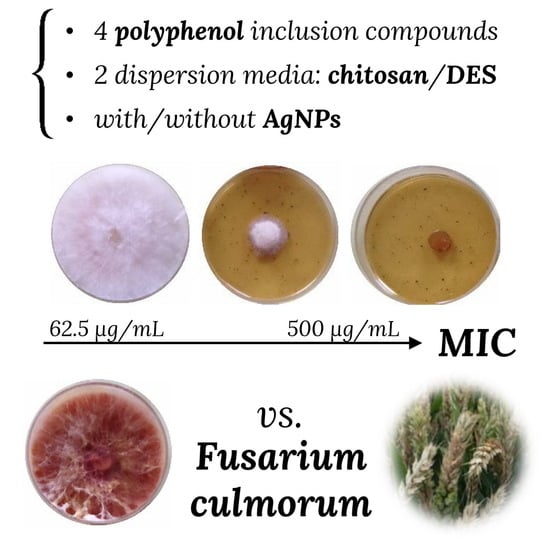
In Vitro Antifungal Activity of Composites of AgNPs and Polyphenol Inclusion Compounds against Fusarium culmorum in Different Dispersion Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Polyphenol Inclusion Compounds
2.3. Preparation of Bioactive Composites with Chitosan Oligomers in Hydroalcoholic Solution
2.4. Preparation of Bioactive Composites in ChCl:urea Deep Eutectic Solvent
2.5. Characterization
2.6. Fungal Isolate and Growth Conditions
2.7. In Vitro Tests of Mycelial Growth Inhibition
2.8. Statistical Analyses
3. Results
4. Discussion
4.1. Efficacy of the Composites
4.2. Mechanism of Action
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.R.; Jefferson, P.G. Fungal populations in roots and crowns of common and durum wheat in Saskatchewan. Can. J. Plant Pathol. 2004, 26, 325–334. [Google Scholar] [CrossRef]
- Pasquali, M.; Beyer, M.; Logrieco, A.; Audenaert, K.; Balmas, V.; Basler, R.; Boutigny, A.-L.; Chrpová, J.; Czembor, E.; Gagkaeva, T.; et al. A European database of Fusarium graminearum and F. culmorum trichothecene genotypes. Front. Microbiol. 2016, 7, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pani, G.; Scherm, B.; Azara, E.; Balmas, V.; Jahanshiri, Z.; Carta, P.; Fabbri, D.; Dettori, M.A.; Fadda, A.; Dessì, A.; et al. Natural and natural-like phenolic inhibitors of type B trichothecene in vitro production by the wheat (Triticum sp.) pathogen Fusarium culmorum. J. Agric. Food Chem. 2014, 62, 4969–4978. [Google Scholar] [CrossRef] [PubMed]
- Chala, A.; Weinert, J.; Wolf, G.A. An integrated approach to the evaluation of the efficacy of fungicides against Fusarium culmorum, the cause of head blight of wheat. J. Phytopathol. 2003, 151, 673–678. [Google Scholar] [CrossRef]
- Picot, A.; Atanasova-Pénichon, V.; Pons, S.; Marchegay, G.; Barreau, C.; Pinson-Gadais, L.; Roucolle, J.; Daveau, F.; Caron, D.; Richard-Forget, F. Maize kernel antioxidants and their potential involvement in Fusarium ear rot resistance. J. Agric. Food. Chem. 2013, 61, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Kadam, A.; Shinde, S.; Saratale, R.G.; Patra, J.; Ghodake, G. Recent developments in nanotechnology transforming the agricultural sector: A transition replete with opportunities. J. Sci. Food Agric. 2018, 98, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Silva-Castro, I.; Martín-García, J.; Diez, J.J.; Flores-Pacheco, J.A.; Martín-Gil, J.; Martín-Ramos, P. Potential control of forest diseases by solutions of chitosan oligomers, propolis and nanosilver. Eur. J. Plant Pathol. 2017, 150, 401–411. [Google Scholar] [CrossRef]
- Bell, A.A.; Hubbard, J.C.; Liu, L.; Davis, R.M.; Subbarao, K.V. Effects of chitin and chitosan on the incidence and severity of Fusarium yellows of celery. Plant Dis. 1998, 82, 322–328. [Google Scholar] [CrossRef]
- Park, R.D.; Jo, K.J.; Jo, Y.; Jin, Y.L.; Kim, K.Y.; Shim, J.H. Variation of antifungal activities of chitosans on plant pathogens. J. Microbiol. Biotechnol. 2002, 12, 84–88. [Google Scholar]
- Al-Hetar, M.Y.; Zainal Abidin, M.A.; Sariah, M.; Wong, M.Y. Antifungal activity of chitosan against Fusarium oxysporum f. sp. cubense. J. Appl. Polym. Sci. 2011, 120, 2434–2439. [Google Scholar] [CrossRef]
- Xing, K.; Shen, X.; Zhu, X.; Ju, X.; Miao, X.; Tian, J.; Feng, Z.; Peng, X.; Jiang, J.; Qin, S. Synthesis and in vitro antifungal efficacy of oleoyl-chitosan nanoparticles against plant pathogenic fungi. Int. J. Biol. Macromol. 2016, 82, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.L.; Atanasova-Pénichon, V.; Benet, M.; Barreau, C.; Richard-Forget, F. Natural phenolic acids from wheat bran inhibit Fusarium culmorum trichothecene biosynthesis in vitro by repressing Tri gene expression. Eur. J. Plant Pathol. 2010, 127, 275–286. [Google Scholar] [CrossRef]
- Nguyen, D.M.C.; Seo, D.J.; Lee, H.B.; Kim, I.S.; Kim, K.Y.; Park, R.D.; Jung, W.J. Antifungal activity of gallic acid purified from Terminalia nigrovenulosa bark against Fusarium solani. Microb. Pathog. 2013, 56, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sarrocco, S.; Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 2018, 13, e0194284. [Google Scholar]
- Gauthier, L.; Bonnin-Verdal, M.N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Richard-Forget, F.; Atanasova-Penichon, V. Fungal biotransformation of chlorogenic and caffeic acids by Fusarium graminearum: New insights in the contribution of phenolic acids to resistance to deoxynivalenol accumulation in cereals. Int. J. Food Microbiol. 2016, 221, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz, M.J.; Kozioł, M.; Gorczyca, A. The effect of silver nanoparticles on phytopathogenic spores of Fusarium culmorum. Can. J. Microbiol. 2010, 56, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Venat, O.; Iacomi, B.; PeticilĂ, A.G. In vitro studies of antifungal activity of colloidal silver against important plants pathogens. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46. [Google Scholar] [CrossRef]
- Gorczyca, A.; Pociecha, E.; Kasprowicz, M.; Niemiec, M. Effect of nanosilver in wheat seedlings and Fusarium culmorum culture systems. Eur. J. Plant Pathol. 2015, 142, 251–261. [Google Scholar] [CrossRef]
- Rashed, A.O.M.; Mohamed, A.E.A.A.R.; Abobakr, M.M. Wheat protection from root rot caused by Fusarium culmorum using silver nanoparticles. J. Chem. Soc. Pak. 2016, 38, 898–903. [Google Scholar]
- Tutaj, K.; Szlazak, R.; Szalapata, K.; Starzyk, J.; Luchowski, R.; Grudzinski, W.; Osinska-Jaroszuk, M.; Jarosz-Wilkolazka, A.; Szuster-Ciesielska, A.; Gruszecki, W.I. Amphotericin B-silver hybrid nanoparticles: Synthesis, properties and antifungal activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Paralikar, P.; Anasane, N.; Gade, R.; Ingle, P. Effective management of soft rot of ginger caused by Pythium spp. and Fusarium spp.: Emerging role of nanotechnology. Appl. Microbiol. Biotechnol. 2018, 102, 6827–6839. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Rascón, R.E.; López-Meneses, A.K.; Plascencia-Jatomea, M.; Cota-Arriola, O.; Moreno-Ibarra, G.M.; Castillón-Campaña, L.G.; Sánchez-Mariñez, R.I.; Cortez-Rocha, M.O. Control of mycotoxigenic fungi with microcapsules of essential oils encapsulated in chitosan. Food Sci. Technol. 2017, 38, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Kalagatur, N.K.; Nirmal Ghosh, O.S.; Sundararaj, N.; Mudili, V. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol. 2018, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Si, J.; Kang, C.; Chung, B.; Chung, D.; Kim, D. Facile preparation of water soluble curcuminoids extracted from turmeric (Curcuma longa L.) powder by using steviol glucosides. Food Chem. 2017, 214, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Okamoto, D.; Sato, H.; Onoue, S.; Otsu, S.; Tozuka, Y. Hybridization of polyvinylpyrrolidone to a binary composite of curcumin/α-glucosyl stevia improves both oral absorption and photochemical stability of curcumin. Food Chem. 2016, 213, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhou, D.; Xie, J.; Mao, F. Preparation of chitosan oligomers and their antioxidant activity. Eur. Food Res. Technol. 2007, 225, 451–456. [Google Scholar] [CrossRef]
- Biswas, A.; Shogren, R.L.; Stevenson, D.G.; Willett, J.L.; Bhowmik, P.K. Ionic liquids as solvents for biopolymers: Acylation of starch and zein protein. Carbohydr. Polym. 2006, 66, 546–550. [Google Scholar] [CrossRef]
- Matei, P.; Martín-Gil, J.; Michaela Iacomi, B.; Pérez-Lebeña, E.; Barrio-Arredondo, M.; Martín-Ramos, P. Silver nanoparticles and polyphenol inclusion compounds composites for Phytophthora cinnamomi mycelial growth inhibition. Antibiotics 2018, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gil, J.; Matei Petruta, M.; Pérez Lebeña, E. Complejo de Inclusión Para Mejorar la Biodisponibilidad de Compuestos Biológicamente Activos No Hidrosolubles. Patent P201731489, 28 December 2017. [Google Scholar]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, I.; Edel-Hermann, V.; Gautheron, N.; Durling, M.B.; Kolseth, A.-K.; Steinberg, C.; Persson, P.; Friberg, H.; Cullen, D. Genus-specific primers for study of Fusarium communities in field samples. Appl. Environ. Microbiol. 2016, 82, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.; Curfs-Breuker, I.; de Hoog, G.; Meis, J.; Verweij, P. Antifungal susceptibility testing of Fusarium: A practical approach. J. Fungi 2017, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, H.; Banerjee, T.; Walia, S. In vitro screening of Curcuma longa L and its derivatives as antifungal agents against Helminthosporrum oryzae and Fusarium solani. Pestic. Res. J. 2008, 20, 6–9. [Google Scholar]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food. Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Georgantzi, C.; Lioliou, A.E.; Paterakis, N.; Makris, D. Combination of lactic acid-based deep eutectic solvents (DES) with β-cyclodextrin: Performance screening using ultrasound-assisted extraction of polyphenols from selected native Greek medicinal plants. Agronomy 2017, 7, 54. [Google Scholar] [CrossRef]
- Ziedan, E.H.E.; Saad, M.M. Efficacy of nanoparticles on seed borne fungi and their pathological potential of cucumber. Int. J. PharmTech Res. 2016, 9, 16–24. [Google Scholar]
- Aleksandrowicz-Trzcińska, M.; Szaniawski, A.; Olchowik, J.; Drozdowski, S. Effects of copper and silver nanoparticles on growth of selected species of pathogenic and wood-decay fungi in vitro. For. Chron. 2018, 94, 109–116. [Google Scholar] [CrossRef]
- Soltanloo, H.; Alimohammadi, M.; Ramezanpour, S.; Bagher, M.; Najar, B. Nanosilver colloid: A novel antimicrobial candidate applicable in plant tissue culture. Aust. J. Basic Appl. Sci. 2010, 4, 5338–5345. [Google Scholar]
- Villamizar-Gallardo, R.; Cruz, J.F.O.; Ortíz-Rodriguez, O.O. Fungicidal effect of silver nanoparticles on toxigenic fungi in cocoa. Pesqui. Agropecu. Bras. 2016, 51, 1929–1936. [Google Scholar] [CrossRef]
- Kasprowicz, M.J.; Gorczyca, A.; Frandsen, R.J. The effect of nanosilver on pigments production by Fusarium culmorum (WG Sm.) Sacc. Pol. J. Microbiol. 2013, 62, 365–372. [Google Scholar] [PubMed]
- Dananjaya, S.H.S.; Erandani, W.K.C.U.; Kim, C.H.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Comparative study on antifungal activities of chitosan nanoparticles and chitosan silver nano composites against Fusarium oxysporum species complex. Int. J. Biol. Macromol. 2017, 105, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, X.; Sun, Y.; Yang, M.; Song, K.; Zheng, Z.; Chen, Y.; Liu, X.; Jia, Z.; Dong, R.; et al. Antimicrobial activity of ferulic acid against Cronobacter sakazakii and possible mechanism of action. Foodborne Pathog. Dis. 2016, 13, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Yun, D.G.; Lee, D.G. Silymarin exerts antifungal effects via membrane-targeted mode of action by increasing permeability and inducing oxidative stress. Biochim. Biophys. Acta 2017, 1859, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.G.; Lee, D.G. Assessment of silibinin as a potential antifungal agent and investigation of its mechanism of action. IUBMB Life 2017, 69, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.; Sánchez, N.S.; Calahorra, M. Effects of chitosan on Candida albicans: Conditions for its antifungal activity. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar]
- Ivask, A.; ElBadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano 2013, 8, 374–386. [Google Scholar] [CrossRef] [PubMed]


| Medium | Polyphenol | Difference | Standardized Difference | Critical Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| COS | Curcumin | −1.042 | −1.650 | 2.093 | 0.115 | No |
| Ferulic acid | −3.459 | −2.182 | 2.093 | 0.042 | Yes | |
| Gallic acid | −5.375 | −6.521 | 2.093 | <0.0001 | Yes | |
| Silymarin | −3.333 | −2.157 | 2.093 | 0.044 | Yes | |
| DES | Curcumin | −9.825 | −3.697 | 2.093 | 0.002 | Yes |
| Ferulic acid | −6.979 | −5.126 | 2.093 | <0.0001 | Yes | |
| Gallic acid | −4.771 | −4.494 | 2.093 | 0.000 | Yes | |
| Silymarin | −6.508 | −4.603 | 2.093 | 0.000 | Yes |
| Contrast | Difference | Standardized Difference | Critical Value | p-Value | Significant | |||
| DES vs. COS | −12.599 | −13.888 | 1.973 | <0.0001 | Yes | |||
| Category | LS Means | Standard Error | Lower Bound (95%) | Upper Bound (95%) | Groups | |||
| DES | 20.667 | 0.641 | 19.401 | 21.932 | A | |||
| COS | 33.266 | 0.641 | 32.000 | 34.531 | B | |||
| Contrast | Difference | Standardized Difference | Critical Value | p-Value | Significant | |||
| EC50 | DES vs. COS | −164.188 | −4.642 | 2.228 | 0.001 | Yes | ||
| EC90 | DES vs. COS | −617.013 | −2.709 | 2.228 | 0.022 | Yes | ||
| Category | LS Means | Standard Error | Lower Bound (95%) | Upper Bound (95%) | Groups | |||
| EC50 | DES | 159.450 | 25.012 | 103.721 | 215.179 | A | ||
| COS | 323.638 | 25.012 | 267.908 | 379.367 | B | |||
| EC90 | DES | 376.550 | 161.058 | 17.690 | 735.410 | A | ||
| COS | 993.563 | 161.058 | 634.702 | 1352.423 | B | |||
| Treatments with Chitosan Oligomers | ||||||||
| Curcumin | Ferulic Acid | Gallic Acid | Silymarin | |||||
| Without AgNPs | With AgNPs | Without AgNPs | With AgNPs | Without AgNPs | With AgNPs | Without AgNPs | With AgNPs | |
| EC50 (µg·mL−1) | 239.4 | 227.3 | 291.7 | 240.4 | 579.1 | 312.9 | 396.2 | 302.1 |
| EC90 (µg·mL−1) | 540.5 | 502.6 | 712.9 | 543.7 | 2604.5 | 973.2 | 1240.5 | 830.6 |
| Treatments with DES | ||||||||
| Curcumin | Ferulic Acid | Gallic Acid | Silymarin | |||||
| Without AgNPs | With AgNPs | Without AgNPs | With AgNPs | Without AgNPs | With AgNPs | Without AgNPs | With AgNPs | |
| EC50 (µg·mL−1) | 208.8 | 129.0 | 174.4 | 117.9 | 167.5 | 160.8 | 182.4 | 134.8 |
| EC90 (µg·mL−1) | 501.6 | 332.8 | 376.5 | 333.8 | 365.3 | 381.7 | 393.6 | 327.1 |
| Dispersion Medium | Composite | Chitosan Oligomers | DES | Polyphenol * | Stevioside | AgNPs * |
|---|---|---|---|---|---|---|
| COS | 62.5 | 7.75 | - | 7.75 | 46.75 | 0.25 |
| 125 | 15.5 | - | 15.5 | 93.5 | 0.5 | |
| 250 | 31 | - | 31 | 187 | 1 | |
| 500 | 62 | - | 62 | 374 | 2 | |
| DES | 62.5 | - | 30.75 | 7.9 | 23.62 | 0.25 |
| 125 | - | 61.5 | 15.8 | 47.25 | 0.5 | |
| 250 | - | 123 | 31.5 | 94.5 | 1 | |
| 500 | - | 246 | 63 | 189 | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, P.M.; Iacomi, B.M.; Martín-Gil, J.; Pérez-Lebeña, E.; Ramos-Sánchez, M.C.; Barrio-Arredondo, M.T.; Martín-Ramos, P. In Vitro Antifungal Activity of Composites of AgNPs and Polyphenol Inclusion Compounds against Fusarium culmorum in Different Dispersion Media. Agronomy 2018, 8, 239. https://doi.org/10.3390/agronomy8110239
Matei PM, Iacomi BM, Martín-Gil J, Pérez-Lebeña E, Ramos-Sánchez MC, Barrio-Arredondo MT, Martín-Ramos P. In Vitro Antifungal Activity of Composites of AgNPs and Polyphenol Inclusion Compounds against Fusarium culmorum in Different Dispersion Media. Agronomy. 2018; 8(11):239. https://doi.org/10.3390/agronomy8110239
Chicago/Turabian StyleMatei, Petruta Mihaela, Beatrice Michaela Iacomi, Jesús Martín-Gil, Eduardo Pérez-Lebeña, M. Carmen Ramos-Sánchez, M. Teresa Barrio-Arredondo, and Pablo Martín-Ramos. 2018. "In Vitro Antifungal Activity of Composites of AgNPs and Polyphenol Inclusion Compounds against Fusarium culmorum in Different Dispersion Media" Agronomy 8, no. 11: 239. https://doi.org/10.3390/agronomy8110239