Corn Tassel: A New Source of Phytochemicals and Antioxidant Potential for Value-Added Product Development in the Agro-Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. Chemicals and Reagents
2.3. Sample Extraction
2.4. Determination of Total Phenolic Content (TPC)
2.5. Determination of Total Anthocyanin Content (TAC)
2.6. Determination of Total Carotenoid Content (TCC)
2.7. Determination of Antioxidant Capacity
2.8. Statistical Analysis
3. Results and Discussion
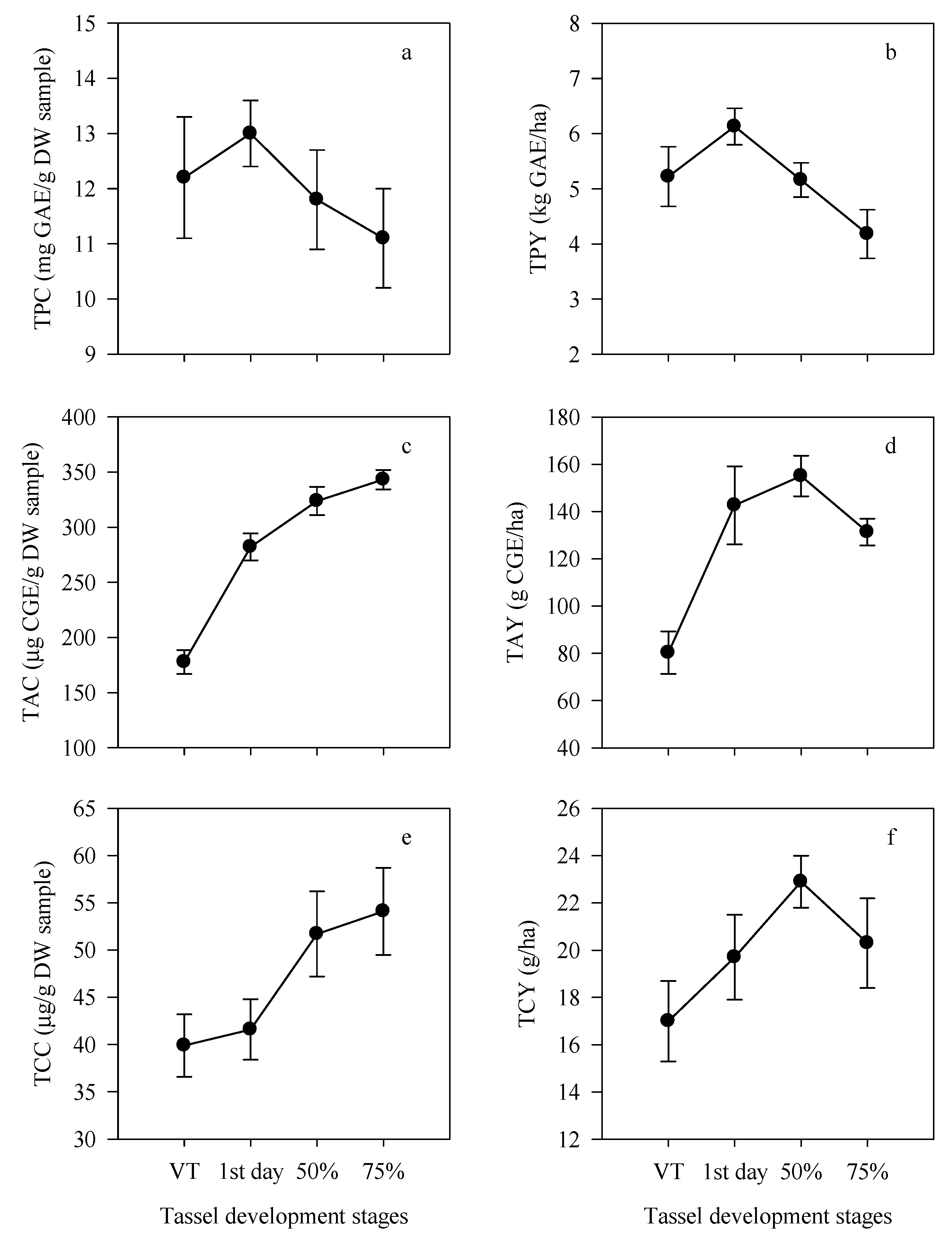
3.1. Variation in Tassel Weight, Phytochemicals and Antioxidant Activity
3.2. Effect of Development Stages on Tassel Weight and Phenolic Compounds
3.3. Anthocyanins in Corn Tassels
3.4. Carotenoids in Corn Tassels
3.5. Antioxidant Capacity in Corn Tassels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarepoua, E.; Tangwongchai, R.; Suriharn, B.; Lertrat, K. Influence of variety and harvest maturity on phytochemical content in corn silk. Food Chem. 2015, 169, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Thapphasaraphong, S.; Rimdusit, T.; Priprem, A.; Puthongking, P. Crops of waxy purple corn: A valuable source of antioxidative phytochemicals. Int. J. Adv. Agric. Environ. Eng. 2016, 3, 73–77. [Google Scholar]
- Dong, J.; Cai, L.; Zhu, X.; Huang, X.; Yin, T.; Fang, H.; Ding, Z. Antioxidant activities and phenolic compounds of cornhusk, corncob and stigma maydis. J. Braz. Chem. Soc. 2014, 25, 1956–1964. [Google Scholar] [CrossRef]
- Li, C.Y.; Kim, H.W.; Won, S.R.; Min, H.K.; Park, K.J.; Park, J.Y.; Ahn, M.S.; Rhee, H.I. Corn husk as a potential source of anthocyanins. J. Agric. Food Chem. 2008, 56, 11413–11416. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Kiesselbach, T.A. The Structure and Reproduction of Corn; Niversity of Nebraska Press: Lincoln, NE, USA, 1949; p. 101. [Google Scholar]
- Aylor, D.E. Survival of maize (Zea mays) pollen exposed in the atmosphere. Agric. Forest Meteorol. 2004, 123, 125–133. [Google Scholar] [CrossRef]
- MacRobert, J.F.; Setimela, P.S.; Gethi, J.; Worku, M. Maize Hybrid Seed Production Manual; CIMMYT: Mexico, Mexico, 2014; p. 26. [Google Scholar]
- Žilic´, S.; Vancˇetovic´, J.; Jankovic´, M.; Maksimovic´, V. Chemical composition, bioactive compounds, antioxidant capacity and stability of floral maize (Zea mays L.) pollen. J. Funct. Foods 2014, 10, 65–74. [Google Scholar] [CrossRef]
- Khider, M.; Elbanna, K.; Mahmoud, A.; Owayss, A.A. Egyptian honeybee pollen as antimicrobial, antioxidant agents, and dietary food supplements. Food Sci. Biotechnol. 2013, 22, 1461–1469. [Google Scholar] [CrossRef]
- Mohsen, S.M.; Ammar, A.S.M. Total phenolic contents and antioxidant activity of corn tassel extracts. Food Chem. 2009, 112, 595–598. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; Fang, M.; Zhan, C.; Pan, H.; Wu, Y.; Gong, Z. Antioxidant and antigenotoxic activity of bioactive extracts from corn tassel. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Wille, J.J.; Berhow, M.A. Bioactives derived from ripe corn tassels: A possible new natural skin whitener, 4-hydroxy-1-oxindole-3-acetic acid. Curr. Bioact. Compd. 2011, 7, 126–134. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Osterc, G.; Stampar, F. Changes in the phenolic concentration during flower development of rose ‘KORcrisett’. J. Am. Soc. Hortic. Sci. 2009, 134, 491–496. [Google Scholar]
- Joshi, R.; Poonam; Gulati, A. Biochemical attributes of tea flowers (Camellia sinensis) at different developmental stages in the Kangra region of India. Sci. Hortic. 2011, 130, 266–274. [Google Scholar] [CrossRef]
- Salem, N.; Msaada, K.; Hamdaoui, G.; Limam, F.; Marzouk, B. Variation in phenolic composition and antioxidant activity during flower development of safflower (Carthamus tinctorius L.). J. Agric. Food Chem. 2011, 59, 4455–4463. [Google Scholar] [CrossRef] [PubMed]
- Ammar, I.; Ennouri, M.; Khemakhem, B.; Yangui, T.; Attia, H. Variation in chemical composition and biological activities of two species of Opuntia flowers at four stages of flowering. Ind. Crops Prod. 2012, 37, 34–40. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, G.; Gu, Z.; Han, Y.; Chen, Z. Optimization extraction of anthocyanins from purple corn (Zea mays L.) cob using tristimulus colorimetry. Eur. Food Res. Technol. 2008, 227, 409–415. [Google Scholar] [CrossRef]
- Alfieri, M.; Hidalgo, A.; Berardo, N.; Redaelli, R. Carotenoid composition and heterotic effect in selected Italian maize germplasm. J. Cereal Sci. 2014, 59, 181–188. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungistic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Hu, Q.-P.; Xu, J.-G. Profiles of carotenoids, anthocyanins, phenolics, and antioxidant activity of selected color waxy corn grains during maturation. J. Agric. Food Chem. 2011, 59, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.A., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar]
- Rivera, S.; Canera, R. Influence of sample processing on the analysis of carotenoids in maize. Molecules 2012, 17, 11255–11268. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gomaz, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley and Sons: Singapore, 1984; p. 680. [Google Scholar]
- Harakotr, B.; Suriharn, B.; Scott, M.P.; Lertrat, K. Genotypic variability in anthocyanins, total phenolics, and antioxidant activity among diverse waxy corngermplasm. Euphytica 2014, 203, 237–248. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasak, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; Westgate, M.; Grass, L.; Dornbos, D. Tassel morphology as an indicator of potential pollen production in maize. Crop Manage. 2003. [Google Scholar] [CrossRef]
- Ricci, B.; Monod, H.; Guérin, D.; Messéan, A.; Maton, C.; Balique, B.; Angevin, F. Predicting maize pollen production using tassel morphological characteristics. Field Crops Res. 2012, 136, 107–115. [Google Scholar] [CrossRef]
- Xu, G.; Wang, X.; Huang, C.; Xu, D.; Li, D.; Tian, J.; Chen, Q.; Wang, C.; Liang, Y.; Wu, Y.; et al. Complex genetic architecture underlies maize tassel domestication. New Phytol. 2017, 214, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V. Phenolic compounds: Introduction, In Natural Products; Ramawat, K.G., Mérillon, J.M., Eds.; Springer-Verlag: Heidelber, Germany, 2013; pp. 1543–1580. [Google Scholar]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Shetty, K. Role of proline-linked pentose phosphate pathway in biosynthesis of plant phenolics for functional food and environmental applications: A review. Process Biochem. 2004, 39, 789–804. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanins and antioxidant activity in coloured waxy corn at different maturation stages. J. Func.Foods. 2014, 9, 109–118. [Google Scholar] [CrossRef]
- Bakó, E.; Deli, J.; Tóth, G. HPLC study on the carotenoid composition of Calendula products. J. Biochem. Biophys. Methods 2002, 53, 241–250. [Google Scholar] [CrossRef]
- Bogdanov, S. Pollen: Nutrition, Functional Properties, Health: A review. Available online: http://www.bee-hexagon.net/files/file/fileE/Health/PollenBook2Review.pdf (accessed on 19 May 2017).
- Menkir, A.; Liu, W.; White, W.S.; Maziya-Dixon, B.; Rocheford, T. Carotenoid diversity in tropical-adapted yellow maize inbred lines. Food Chem. 2008, 109, 521–529. [Google Scholar] [CrossRef]
- Hwang, T.; Ndolo, V.U.; Katundu, M.; Nyirenda, B.; Bezner-Kerr, R.; Arntfield, S.; Beta, T. Provitamin A potential of landrace orange maize variety (Zea mays L.) grown in different geographical locations of central Malawi. Food Chem. 2016, 196, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Aličić, D.; Šubarić, D.; Jašić, M.; Pašalić, H.; Ačkar, Đ. Antioxidant Properties of Pollen. Available online: http://www.hrcak.srce.hr/file/186516 (accessed on 16 February 2018).





| Entry No. | Varieties | Type | Glume Color | Anther Color | Origin |
|---|---|---|---|---|---|
| 1 | PAC339 | Field corn | Red-green | Yellow-pink | Pacific Seeds (Thai) Co., Ltd. |
| 2 | P4546 | Field corn | Green | Pink | Pioneer Hi-Bred (Thailand) Co., Ltd. |
| 3 | S6248 | Field corn | Red-green | Pink | Syngenta Seed Co., Ltd. |
| 4 | Hibrix3 | Sweet corn | Green | Yellow | Pacific Seeds (Thai) Co., Ltd. |
| 5 | Sugar75 | Sweet corn | Green | Yellow | Syngenta Seed Co., Ltd. |
| 6 | Sweet violet | Waxy corn | Green | Light green | East West Seed Co., Ltd. |
| 7 | Muang tam | Waxy corn | Red-green | Green-pink | Syngenta Seed Co., Ltd. |
| 8 | KGW1 | Waxy corn | Purple-green | Purple | Khon Kaen University |
| Source of Variation | df | Tassel Weight | Total Phytochemical Content | Total Phytochemical Yield | Antioxidant Capacity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | Dry | Phenolics | Anthocyanins | Carotenoids | Phenolics | Anthocyanins | Carotenoids | TEAC | DPPH | ||
| Block | 2 | 5.56 | 0.24 | 20.15 | 3971 | 477 | 5.04 | 1844 | 80.1 | 728 | 359 |
| (0.6) a | (0.3) | (5.6) | (0.0) | (3.9) | (4.3) | (0.1) | (2.5) | (6.6) | (7.1) | ||
| Development stages (S) | 3 | 253.64 ** | 15.03 ** | 15.75 ** | 130,783 ** | 1204 ** | 15.15 ** | 25,833 ** | 144.3 ** | 246 ** | 212 ** |
| (41.3) | (23.9) | (6.6) | (1.6) | (14.6) | (19.2) | (1.3) | (7.0) | (3.4) | (6.3) | ||
| Varieties(V) | 7 | 96.73 ** | 13.97 ** | 64.09 ** | 3,385,556 ** | 2459 ** | 17.68 ** | 776,941 ** | 635.1 ** | 2184 ** | 827 ** |
| (36.7) | (51.9) | (62.2) | (95.0) | (69.5) | (52.2) | (93.7) | (71.2) | (69.3) | (57.1) | ||
| S*V | 21 | 3.58 ns | 0.62 ns | 2.96 ns | 39,989 ** | 68 ** | 1.01 ns | 11,539 ** | 26.9 ** | 114 ** | 36 ns |
| (4.1) | (6.9) | (8.6) | (3.4) | (5.8) | (9.0) | (4.2) | (9.1) | (10.8) | (7.4) | ||
| Error | 62 | 5.16 | 0.52 | 1.98 | 364 | 25 | 0.59 | 735 | 10.5 | 35 | 36 |
| (17.3) | (17.0) | (17.0) | (0.1) | 6.25 | (15.4) | (0.8) | (10.2) | (9.9) | (22.1) | ||
| C.V. (%) | 8.54 | 8.43 | 11.70 | 6.78 | 10.67 | 14.81 | 21.30 | 16.24 | 10.15 | 7.56 | |
| Varieties | Tassel Weight | Phenolic Compounds | ||
|---|---|---|---|---|
| Fresh (g) | Dry (g) | Total Phenolic Content | Total Phenolic Yield | |
| (mg GAE/g DW Sample) | (kg GAE/ha) | |||
| Field corn | ||||
| PAC339 | 26.5 ± 1.9 c | 8.7 ± 0.6 b | 12.8 ± 0.7 bc | 5.59 ± 0.7 b |
| P4546 | 23.4 ± 0.7 d | 7.3 ± 0.2 d | 15.8 ± 1.1 a | 5.80 ± 0.5 b |
| S6248 | 24.2 ± 0.9 d | 8.2 ± 0.3 bc | 12.9 ± 1.4 bc | 5.34 ± 0.4 b |
| Mean | 24.7 | 8.1 | 13.8 | 5.58 |
| Sweet corn | ||||
| Hibrix3 | 31.2 ± 2.4 a | 10.1 ± 0.6 a | 13.4 ± 1.2 bc | 6.78 ± 1.0 a |
| Sugar75 | 29.2 ± 1.7 b | 9.5 ± 0.4 a | 11.0 ± 0.6 d | 5.25 ± 0.1 b |
| Mean | 30.2 | 9.8 | 12.2 | 6.01 |
| Waxy corn | ||||
| Sweet violet | 24.4 ± 0.2 d | 7.2 ± 0.1 d | 8.4 ± 0.6 f | 3.02 ± 0.2 d |
| Muang tam | 24.9 ± 0.9 cd | 7.7 ± 0.2 cd | 9.7 ± 0.9 e | 3.75 ± 0.4 c |
| KGW1 | 28.8 ± 0.3 b | 9.5 ± 0.5 a | 12.2 ± 1.8 c | 5.86 ± 0.9 b |
| Mean | 26.0 | 8.2 | 10.1 | 4.21 |
| Varieties | Tassel Development Stages | ||||
|---|---|---|---|---|---|
| VT Tassel | 1st day of Pollen Shed | 50% of Pollen Shed | 75% of Pollen Shed | Mean | |
| Total Anthocyanin Content (μg CGE/g DW Sample) | |||||
| Field corn | |||||
| PAC339 | 76.0 ± 4.2 kl | 84.2 ± 5.5 j–l | 219.3 ± 17.2 fg | 208.0 ± 2.5 g | 146.9 ± 4.6 C |
| P4546 | 59.8 ± 6.6 lm | 244.6 ± 43.6 f | 338.9 ± 46.5 e | 325.0 ± 22.9 e | 242.1 ± 26.7 B |
| S6248 | 93.9 ± 28.3 jk | 132.5 ± 49.5 i | 128.1 ± 8.8 i | 176.5 ± 4.8 h | 132.7 ± 20.4 C |
| Mean | 76.6 | 153.8 | 228.8 | 236.5 | 173.9 |
| Sweet corn | |||||
| Hibrix3 | 26.4 ± 2.5 no | 18.1 ± 6.4 no | 17.4 ± 8.1 no | 16.7 ± 9.8 no | 19.6 ± 6.7 E |
| Sugar75 | 12.7 ± 6.4 no | 39.1 ± 2.9 m–o | 23.1 ± 1.4 no | 27.4 ± 6.5 no | 25.6 ± 2.1 E |
| Mean | 19.6 | 28.6 | 20.3 | 22.1 | 22.6 |
| Waxy corn | |||||
| Sweet violet | 11.5 ± 2.5 no | 11.1 ± 0.8 o | 18.7 ± 2.7 no | 17.9 ± 3.6 no | 14.8 ± 2.0 E |
| Muang tam | 42.3 ± 15.3 mn | 80.3 ± 6.7 j–k | 127.9 ± 6.5 i | 108.9 ± 7.5 ij | 89.9 ± 5.7 D |
| KGW1 | 1099.3 ± 44.0 d | 1647.2 ± 25.1 c | 1716.6 ± 35.5 b | 1864.6 ± 49.3 a | 1582.0 ± 38.5 A |
| Mean | 384.4 | 579.5 | 621.1 | 663.8 | 562.2 |
| Total Anthocyanin Yield (g CGE/ha) | |||||
| Field corn | |||||
| PAC339 | 33.6 ± 1.5 h–k | 39.5 ± 1.7 h–k | 88.6 ± 11.5 d–g | 86.6 ± 10.5 d–g | 62.1 ± 4.2 C |
| P4546 | 22.5 ± 1.9 h–k | 99.9 ± 21.3 d–f | 121.2 ± 8.8 d | 105.4 ± 17.2 de | 87.2 ± 11.0 B |
| S6248 | 40.4 ± 10.9 h–k | 58.1 ± 23.4 f–j | 52.6 ± 6.8 g–j | 64.8 ± 5.6 e–h | 54.0 ± 9.0 CD |
| Mean | 32.2 | 65.8 | 87.5 | 85.6 | 67.8 |
| Sweet corn | |||||
| Hibrix3 | 13.3 ± 2.1 jk | 10.1 ± 4.3 jk | 9.3 ± 4.8 jk | 7.2 ± 4.4 k | 10.0 ± 3.9 E |
| Sugar75 | 6.0 ± 2.8 k | 20.7 ± 1.8 h–k | 11.6 ± 1.8 jk | 10.7 ± 2.4 jk | 12.2 ± 0.5 E |
| Mean | 9.6 | 15.4 | 10.4 | 9.0 | 11.1 |
| Waxy corn | |||||
| Sweet violet | 3.9 ± 0.7 k | 4.5 ± 0.3 k | 6.7 ± 1.2 k | 6.0 ± 0.7 k | 5.3 ± 0.6 E |
| Muang tam | 16.6 ± 7.5 i–k | 34.5 ± 3.4 h–k | 51.6 ± 6.4 g–j | 35.9 ± 3.5 h–k | 34.7 ± 2.5 D |
| KGW1 | 506.1 ± 61.1 c | 873.4 ± 114.5 a | 898.7 ± 50.4 a | 733.7 ± 56.8 b | 753.0 ± 48.1 A |
| Mean | 175.5 | 304.1 | 319.0 | 258.6 | 264.3 |
| Varieties | Tassel Development Stages | ||||
|---|---|---|---|---|---|
| VT Tassel | 1st Day of Pollen Shed | 50% of Pollen Shed | 75% of Pollen Shed | Mean | |
| Total Carotenoid Content (μg/g DW Sample) | |||||
| Field corn | |||||
| PAC339 | 32.0 ± 5.8 o–r | 31.9 ± 4.2 o–r | 49.3 ± 10.6 e–j | 50.6 ± 5.7 d–g | 41.0 ± 4.5 D |
| P4546 | 23.7 ± 5.2 s | 31.3 ± 8.4 o–s | 34.6 ± 8.2 m–q | 38.5 ± 5.8 k–m | 32.0 ± 6.6 E |
| S6248 | 33.8 ± 7.4 n–r | 29.8 ± 5.5 p–s | 36.4 ± 4.6 l–p | 41.6 ± 5.9 j–n | 35.4 ± 5.4 E |
| Mean | 29.8 | 31.0 | 40.1 | 43.6 | 36.1 |
| Sweet corn | |||||
| Hibrix3 | 26.8 ± 5.9 q–s | 26.1 ± 5.4 rs | 43.9 ± 10.0 g–l | 41.7 ± 3.4 i–n | 34.6 ± 6.1 E |
| Sugar75 | 47.8 ± 1.8 f–j | 46.9 ± 9.9 f–j | 46.5 ± 5.5 f–k | 56.4 ± 2.7 c–e | 49.4 ± 3.6 C |
| Mean | 37.3 | 36.5 | 45.2 | 49.1 | 42.0 |
| Waxy corn | |||||
| Sweet violet | 54.3 ± 3.4 c–f | 50.3 ± 3.4 e–h | 59.5 ± 3.7 bc | 61.4 ± 7.8 bc | 56.4 ± 3.3 B |
| Muang tam | 42.4 ± 6.3 h–m | 49.8 ± 6.8 e–i | 54.5 ± 2.7 c–f | 56.5 ± 5.3 c–e | 50.8 ± 4.6 C |
| KGW1 | 58.5 ± 7.6 cd | 66.9 ± 6.3 b | 88.4 ± 4.3 a | 85.7 ± 8.0 a | 74.9 ± 6.2 A |
| mean | 51.7 | 55.7 | 67.5 | 67.9 | 60.7 |
| Total Carotenoid Yield (g/ha) | |||||
| Field corn | |||||
| PAC339 | 14.0 ± 1.4 j–m | 15.1 ± 2.6 h–l | 19.8 ± 4.4 d–i | 21.2 ± 4.8 d–g | 17.5 ± 2.3 D |
| P4546 | 8.9 ± 2.0 m | 12.7 ± 3.6 k–m | 12.5 ± 3.2 lm | 12.6 ± 3.1 l–m | 11.7 ± 2.8 E |
| S6248 | 14.6 ± 2.8 i–l | 13.0 ± 3.1 k–m | 15.0 ± 2.4 h–l | 15.2 ± 1.2 h–l | 14.4 ± 2.1 E |
| Mean | 12.5 | 13.6 | 15.8 | 16.3 | 14.6 |
| Sweet corn | |||||
| Hibrix3 | 13.5 ± 3.9 j–m | 14.4 ± 3.5 j–l | 23.6 ± 7.4 c–e | 18.0 ± 2.4 f–k | 17.4 ± 4.3 D |
| Sugar75 | 22.7 ± 2.0 c–f | 24.8 ± 4.8 cd | 23.2 ± 3.2 c–f | 22.0 ± 0.6 c–f | 23.2 ± 2.2 B |
| Mean | 18.1 | 23.4 | 23.4 | 20.0 | 20.3 |
| Waxy corn | |||||
| Sweet violet | 18.5 ± 2.1 e–j | 21.2 ± 1.5 d–h | 21.2 ± 1.2 d–g | 20.9 ± 3.9 d–g | 20.2 ± 1.1 C |
| Muang tam | 16.2 ± 2.7 g–l | 21.9 ± 3.4 d–g | 21.9 ± 1.6 d–f | 18.7 ± 2.7 e–j | 19.6 ± 1.8 CD |
| KGW1 | 27.2 ± 6.1 c | 35.8 ± 7.7 b | 46.3 ± 3.7 a | 33.6 ± 2.5 b | 35.7 ± 4.3 A |
| Mean | 20.6 | 29.8 | 29.8 | 24.4 | 25.2 |
| Varieties | TEAC (μmol TE/g DW Sample) | ||||
|---|---|---|---|---|---|
| VT Tassel | 1st Day of Pollen Shed | 50% of Pollen Shed | 75% of Pollen Shed | Mean | |
| Field corn | |||||
| PAC339 | 61.2 ± 9.9 d–g | 62.3 ± 10.5 d–f | 77.2 ± 3.9 a–c | 59.0 ± 7.3 e–h | 64.9 ± 6.3 C |
| P4546 | 69.8 ± 11.9 b–d | 79.1 ± 10.3 ab | 79.3 ± 1.8 ab | 76.2 ± 4.7 a–c | 76.1 ± 6.0 A |
| S6248 | 64.9 ± 8.3 d–f | 68.6 ± 3.9 c–e | 57.4 ± 4.7 f–i | 58.7 ± 3.9 f–h | 62.4 ± 3.8 C |
| Mean | 65.3 | 70.0 | 71.3 | 64.6 | 67.8 |
| Sweet corn | |||||
| Hibrix3 | 70.3 ± 0.3 b–d | 68.8 ± 1.2 cd | 55.5 ± 9.3 f–i | 59.1 ± 10.8 e–g | 63.4 ± 5.2 C |
| Sugar75 | 52.3 ± 3.6 g–j | 44.2 ± 9.4 jk | 57.2 ± 7.7 f–i | 49.3 ± 0.3 h–j | 50.8 ± 1.8D |
| Mean | 61.3 | 56.5 | 56.3 | 54.2 | 57.1 |
| Waxy corn | |||||
| Sweet violet | 47.9 ± 10.0 ij | 36.8 ± 10.6 k–m | 44.3 ± 0.1 jk | 34.3 ± 5.4 lm | 40.8 ± 6.4 E |
| Muang tam | 37.4 ± 10.6 k–m | 42.8 ± 9.3 j–l | 43.6 ± 2.2 j–l | 33.4 ± 4.4 m | 39.3 ± 6.4 E |
| KGW1 | 63.0 ± 7.2 d–f | 81.3 ± 7.6 a | 74.9 ± 9.1 a–c | 62.5 ± 11.1 d–f | 70.4 ± 5.8 B |
| Mean | 49.4 | 53.6 | 54.3 | 43.4 | 50.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duangpapeng, P.; Ketthaisong, D.; Lomthaisong, K.; Lertrat, K.; Scott, M.P.; Suriharn, B. Corn Tassel: A New Source of Phytochemicals and Antioxidant Potential for Value-Added Product Development in the Agro-Industry. Agronomy 2018, 8, 242. https://doi.org/10.3390/agronomy8110242
Duangpapeng P, Ketthaisong D, Lomthaisong K, Lertrat K, Scott MP, Suriharn B. Corn Tassel: A New Source of Phytochemicals and Antioxidant Potential for Value-Added Product Development in the Agro-Industry. Agronomy. 2018; 8(11):242. https://doi.org/10.3390/agronomy8110242
Chicago/Turabian StyleDuangpapeng, Prakasit, Danupol Ketthaisong, Khomsorn Lomthaisong, Kamol Lertrat, Marvin Paul Scott, and Bhalang Suriharn. 2018. "Corn Tassel: A New Source of Phytochemicals and Antioxidant Potential for Value-Added Product Development in the Agro-Industry" Agronomy 8, no. 11: 242. https://doi.org/10.3390/agronomy8110242
APA StyleDuangpapeng, P., Ketthaisong, D., Lomthaisong, K., Lertrat, K., Scott, M. P., & Suriharn, B. (2018). Corn Tassel: A New Source of Phytochemicals and Antioxidant Potential for Value-Added Product Development in the Agro-Industry. Agronomy, 8(11), 242. https://doi.org/10.3390/agronomy8110242






