Exogenously Applied Nitric Oxide Enhances Salt Tolerance in Rice (Oryza sativa L.) at Seedling Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growth Conditions
2.2.1. Gene Expression Experiment
2.2.2. Phenotypic Experiment
2.3. Quantitative Real Time PCR (qPCR) Analysis
2.4. Total Protein and Antioxidant Enzyme Assay
2.5. Statistical Analysis
3. Results
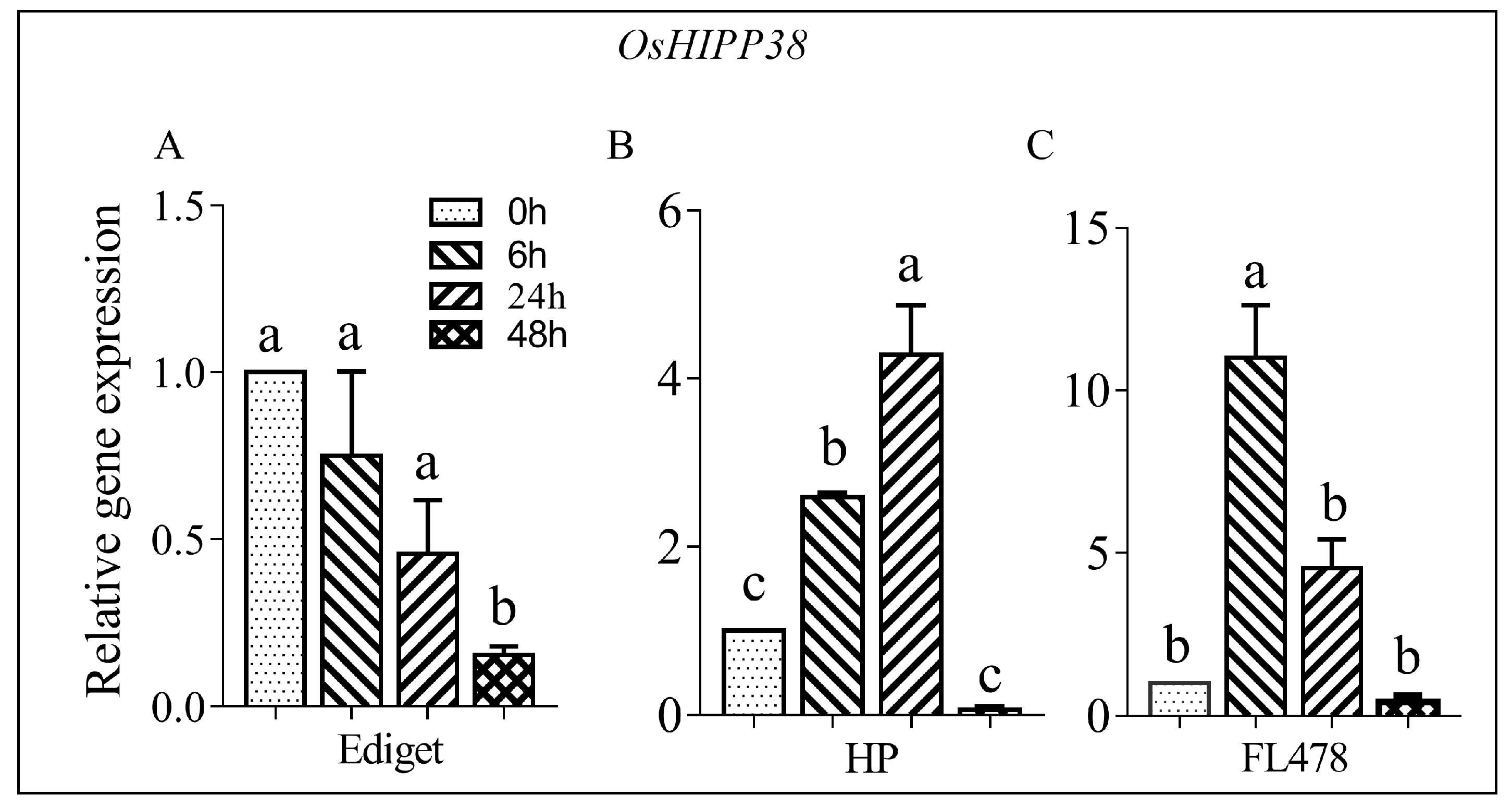
3.1. Salt Induced Change in the Expression of OsHIPP38 in Contrasting Genotypes
3.2. NO Induced Response of OsHIPP38, OsGR1, and OsP5CS2 to Salinity in Rice
3.3. Effects of NO on Protein and Enzyme Activity in Rice Genotypes Exposed to Salinity
3.4. Effect of NO on Phenotypic Traits of Rice Genotypes under Salinity
4. Discussion
4.1. OsHIPP38 Shows Differential Response to Salinity in Contrasting Genotypes
4.2. NO Enhances the Expression level of OsHIPP38, OsGR1 and OsP5CS2 in Susceptible Genotype under Salinity
4.3. NO Counteracts Salt-Induced Gene Expression in the Tolerant Genotype in Salt Stress Conditions
4.4. NO Enhances Protein Content and Enzymatic Activities in the Contrasting Genotypes
4.5. NO Maintains the Production of Biomass in Salt-Stressed Seedlings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gross, B.L.; Zhao, Z.J. Archaeological and genetic insights into the origins of domesticated rice. Proc. Natl. Acad. Sci. USA 2014, 111, 6190–6197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idso Craig, D. Estimates of Global Food Production in the Year 2050: Will We Produce Enough to Adequately Feed the World? Center for the Study of Carbon Dioxide and Global Change 2011. Available online: www.Co2science.Org (accessed on 10 June 2018).
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Ramankutty, N.; Mueller, N.D.; West, P.C.; Foley, J.A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 2012, 3, 1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korres, N.E.; Norsworthy, J.K.; Burgos, N.R.; Oosterhuis, D.M. Temperature and drought impacts on rice production: An agronomic perspective regarding short- and long-term adaptation measures. Water Resour. Rural Dev. 2017, 9, 12–27. [Google Scholar] [CrossRef]
- Chakrabortya, S.; Newtonb, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Masutomi, Y.; Takahashi, K.; Harasawa, H.; Matsuoka, Y. Impact assessment of climate change on rice production in asia in comprehensive consideration of process/parameter uncertainty in general circulation models. Agric. Ecosyst. Environ. 2009, 131, 281–291. [Google Scholar] [CrossRef]
- Ashikari, M.; Ma, J.F. Exploring the power of plants to overcome environmental stresses. Rice 2015, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugh, T.A.M.; Muller, C.; Elliott, J.; Deryng, D.; Folberth, C.; Olin, S.; Schmid, E.; Arneth, A. Climate analogues suggest limited potential for intensification of production on current croplands under climate change. Nat. Commun. 2016, 7, 12608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devkota, M.; Martius, C.; Gupta, R.K.; Devkota, K.P.; McDonald, A.J.; Lamers, J.P.A. Managing soil salinity with permanent bed planting in irrigated production systems in central asia. Agric. Ecosyst. Environ. 2015, 202, 90–97. [Google Scholar] [CrossRef]
- Lafitte, H.R.; Ismail, A.; Bennett, J. Abiotic stress tolerance in rice for asia-progress and the future. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Qadir, M.; Noble, A.D.; Qureshi, A.S.; Gupta, R.K.; Yuldashev, T.; Karimov, A. Salt-induced land and water degradation in the aral sea basin:A challenge to sustainable agriculture in central asia. Nat. Resour. Forum 2009, 33, 134–149. [Google Scholar] [CrossRef]
- Castillo, E.G.; Tuong, T.P.; Ismail, A.; Inubushi, K. Response to salinity in rice: Comparative effects of osmotic and ionic stresses. Plant Prod. Sci. 2015, 10, 159–170. [Google Scholar] [CrossRef]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ros-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Das, P.; Nutan, K.K.; Singla-Pareek, S.-U.L; Pareek, A. Understanding salinity responses and adopting ‘omics-based’ approaches to generate salinity tolerant cultivars of rice. Front. Plant Sci. 2015, 6, 712. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.H.; Shannon, M.C. Salinity effects on seedling growth and yield components of rice. Crop Sci. 2000, 40, 996–1003. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrao, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Gurmani, A.R.; Bano, A.; Khan, S.U.; Din, J.; Zhang, J.L. Alleviation of salt stress by seed treatment with abscisic acid (aba), 6-benzylaminopurine (ba) and chlormequat chloride (ccc) optimizes ion and organic matter accumulation and increases yield of rice (Oryza sativa L.). Aust. J. Crop. Sci. 2011, 5, 1278–1285. [Google Scholar]
- Roy, D.; Bhunia, A.; Basu, N.; Banerjee, S.K. Effect of nacl-salinity on metabolism of proline in salt-sensitive and salt-resistant cultivars of rice. Biol. Plantarum 1992, 34, 159–162. [Google Scholar] [CrossRef]
- Verslues, P.E.; Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book 2010, 8, e0140. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Su, J.; Chang, M.; Verma, D.P.S.; Fan, Y.-L.; Wu, R. Overexpression of a δ1-pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water-and salt-stress in transgenic rice. Plant Sci. 1998, 139, 41–48. [Google Scholar] [CrossRef]
- Hasan, A.; Hafiz, H.R.; Siddiqui, N.; Khatun, M.; Islam, R.; Mamun, A.A. Evaluation of wheat genotypes for salt tolerance based on some physiological traits. J. Crop Sci. Biotechnol. 2016, 18, 333–340. [Google Scholar] [CrossRef]
- Hur, J.; Jung, K.H.; Lee, C.H.; An, G.H. Stress-inducible osp5cs2 gene is essential for salt and cold tolerance in rice. Plant Sci. 2004, 167, 417–426. [Google Scholar] [CrossRef]
- Guo, Z.; Ou, W.; Lu, S.; Zhong, Q. Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol. Biochem. PPB 2006, 44, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Jain, S.; Jain, V. Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J. Plant Biochem. Biot. 2013, 22, 27–34. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Ismail, M.R.; Uddin, M.K.; Islam, M.Z.; Ashrafuzzaman, M. Efficacy of ascorbate-glutathione cycle for scavenging h2o2 in two contrasting rice genotypes during salinity stress. Aust. J. Crop Sci. 2013, 7, 1801–1808. [Google Scholar]
- Huang, M.; Guo, Z. Responses of antioxidative system to chilling stress in two rice cultivars differing in sensitivity. Biol. Plantarum 2005, 49, 81–84. [Google Scholar] [CrossRef]
- De Abreu-Neto, J.B.; Turchetto-Zolet, A.C.; de Oliveira, L.F.V.; Zanettini, M.H.B.; Margis-Pinheiro, M. Heavy metal-associated isoprenylated plant protein (hipp): Characterization of a family of proteins exclusive to plants. FEBS J. 2013, 280, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Barth, O.; Vogt, S.; Uhlemann, R.; Zschiesche, W.; Humbeck, K. Stress induced and nuclear localized hipp26 from Arabidopsis thaliana interacts via its heavy metal associated domain with the drought stress related zinc finger transcription factor athb29. Plant Mol. Biol. 2009, 69, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Suh, J.P.; Lee, C.K.; Lee, J.H.; Kim, Y.G.; Jena, K.K. Qtl mapping and development of candidate gene-derived DNA markers associated with seedling cold tolerance in rice (Oryza sativa L.). Mol. Genet. Genom. MGG 2014, 289, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Fancy, N.N.; Bahlmann, A.K.; Loake, G.J. Nitric oxide function in plant abiotic stress. Plant Cell Environ. 2017, 40, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Miranda, K.M.; Colton, C.A.; Citrin, D.; Espey, M.G.; Wink, D.A. Heme proteins and nitric oxide (no): The neglected, eloquent chemistry in no redox signaling and regulation. Antioxid. Redox Signal. 2003, 5, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.N.; Misra, M.; Singh, R. Nitric oxide ameliorates stress responses in plants. Plant Soil Environ. 2011, 57, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Beligni, M.V.; Lamattina, L. Nitric oxide interferes with plant photo-oxidative stress by detoxifying reactive oxygen species. Plant Cell Environ. 2002, 25, 737–748. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Kao, C.H. Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul. 2004, 42, 227–238. [Google Scholar] [CrossRef]
- Kausar, F.; Shahbaz, M.; Ashraf, M. Protective role of foliar-applied nitric oxide in triticum aestivum under saline stress. Turk. J. Bot 2013, 37, 1155–1165. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Rehman, H. Exogenously applied nitric oxide enhances the drought tolerance in fine grain aromatic rice (Oryza sativa L.). J. Agron. Crop. Sci. 2009, 195, 254–261. [Google Scholar] [CrossRef]
- Ahmad, P.; Latef, A.A.A.; Hashem, A.; Abd Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci 2016, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Egbichi, I.; Keyster, M.; Ludidi, N. Effect of exogenous application of nitric oxide on salt stress responses of soybean. S. Afr. J. Bot. 2014, 90, 131–136. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, G.; Arora, K.; Kohli, R.K. Nitric oxide (as sodium nitroprusside) supplementation ameliorates cd toxicity in hydroponically grown wheat roots. Environ. Exp. Bot. 2008, 63, 158–167. [Google Scholar] [CrossRef]
- Esim, N.; Atici, O.; Mutlu, S. Effects of exogenous nitric oxide in wheat seedlings under chilling stress. Toxicol. Ind. Health 2014, 30, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Wang, L.; Liu, L.Y.; Guo, Y.D.; Ren, H.Z. Alleviating effect of exogenous nitric oxide in cucumber seedling against chilling stress. Afr. J. Biotechnol. 2011, 10, 4380–4386. [Google Scholar]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carrion, A.I.; Castellano, R.; Rosales, M.A.; Ruiz, J.M.; Romero, L. Role of nitric oxide under saline stress: Implications on proline metabolism. Biol. Plantarum 2008, 52, 587–591. [Google Scholar] [CrossRef]
- Ruan, H.H.; Shen, W.B.; Xu, L.L. Nitric oxide involved in the abscisic acid induced proline accumulation in wheat seedling leaves under salt stress. Acta Bot. Sin. 2004, 46, 1307–1315. [Google Scholar]
- Fan, H.F.; Du, C.X.; Guo, S.R. Effect of nitric oxide on proline metabolism in cucumber seedlings under salinity stress. J. Am. Soc. Hortic. Sci. 2012, 137, 127–133. [Google Scholar]
- Glenn, B.G.; Senadhira, D. Genetic-analysis of salinity tolerance in rice (Oryza-sativa L.). Theor. Appl. Gen. 1993, 86, 333–338. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Packer, L., Ed.; Academic Press: San Diego, CA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Imran, Q.M.; Falak, N.; Hussain, A.; Mun, B.G.; Sharma, A.; Lee, S.U.; Kim, K.M.; Yun, B.W. Nitric oxide responsive heavy metal-associated gene athmad1 contributes to development and disease resistance in arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1712. [Google Scholar] [CrossRef] [PubMed]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. Myb transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.K.; Thapar, U.; Kundnani, P.; Panwar, P.; Grover, A. Functional relevance of j-protein family of rice (Oryza sativa). Cell. Stress Chaperon 2013, 18, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Mun, B.-G.; Lee, S.-U.; Hussain, A.; Kim, H.-H.; Rolly, N.K.; Jung, K.-H.; Yun, B.-W. S-nitrosocysteine-responsive genes modulate diverse regulatory pathways in Oryza sativa: A transcriptome profiling study. Funct. Plant Biol. 2018, 45, 630–644. [Google Scholar] [CrossRef]
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Sanchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Perez, C.; Padilla, M.N.; Corpas, F.J.; Barroso, J.B. Antioxidant systems are regulated by nitric oxide-mediated post-translational modifications (no-ptms). Front. Plant Sci. 2016, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Yoshiba, Y.; Kiyosue, T.; Nakashima, K.; YamaguchiShinozaki, K.; Shinozaki, K. Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol. 1997, 38, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Seraj, Z.I.; Fujita, M. Interactive effects of nitric oxide and glutathione in mitigating copper toxicity of rice (Oryza sativa L.) seedlings. Plant Signal. Behav. 2015, 10, e991570. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.; Nath, S.; Chanu, T.T.; Sharma, G.D.; Panda, S.K. Cadmium stress-induced oxidative stress and role of nitric oxide in rice (Oryza sativa L.). Acta Physiol. Plant 2011, 33, 1737–1747. [Google Scholar] [CrossRef]
- Baena-Gonzalez, E. Energy signaling in the regulation of gene expression during stress. Mol. Plant 2010, 3, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Chutipaijit, S.; Cha-um, S.; Sompornpailin, K. High contents of proline and anthocyanin increase protective response to salinity in Oryza sativa L. Spp. Indica. Aust. J. Crop. Sci. 2011, 5, 1191–1198. [Google Scholar]
- Kibria, M.G.; Hossain, M.; Murata, Y.; Hoque, M.A. Antioxidant defense mechanisms of salinity tolerance in rice genotypes. Rice Sci. 2017, 24, 155–162. [Google Scholar] [CrossRef]
- Pal, M.; Singh, D.K.; Rao, L.S.; Singh, K.P. Photosynthetic characteristics and activity of antioxidant enzymes in salinity tolerant and sensitive rice cultivars. Indian J. Plant Physiol. 2004, 9, 407–412. [Google Scholar]
- Garg, N.; Kaur, H. Response of antioxidant enzymes, phytochelatins and glutathione production towards cd and zn stresses in Cajanus cajan (L.) millsp genotypes colonized by arbuscular mycorrhizal fungi. J. Agron. Crop. Sci. 2013, 199, 118–133. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Ros, L.V.G.; Belchi-Navarro, S.; Bru, R.; Barcelo, A.R.; Pedreno, M.A. Class iii peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Pattanagul, W.; Thitisaksakul, M. Effect of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. Indian J. Exp. Biol. 2008, 46, 736–742. [Google Scholar] [PubMed]
- Sudhir, P.; Murthy, S.D.S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Tiwari, B.S.; Bose, A.; Ghosh, B. Photosynthesis in rice under a salt stress. Photosynthetica 1997, 34, 303–306. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between reactive oxygen species production and photochemistry of photosystems i and ii in Lemna gibba L. Plants under salt stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, S.; Kawasaki, M.; Taniguchi, M.; Miyake, H. Light dependency of salinity-induced chloroplast degradation. Plant Prod. Sci. 2003, 6, 219–223. [Google Scholar] [CrossRef]
- Wei, L.L.; Derrien, B.; Gautier, A.; Houille-Vernes, L.; Boulouis, A.; St-Marcoux, D.; Malnoe, A.; Rappaport, F.; de Vitry, C.; Vallon, O.; et al. Nitric oxide-triggered remodeling of chloroplast bioenergetics and thylakoid proteins upon nitrogen starvation in Chlamydomonas reinhardtii. Plant Cell 2014, 26, 353–372. [Google Scholar] [CrossRef] [PubMed]
- Balotf, S.; Islam, S.; Kavoosi, G.; Kholdebarin, B.; Juhasz, A.; Ma, W.J. How exogenous nitric oxide regulates nitrogen assimilation in wheat seedlings under different nitrogen sources and levels. PLoS ONE 2018, 13, e0190269. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.D.; Lamattina, L.; Spoel, S.H.; Loake, G.J. Nitric oxide function in plant biology: A redox cue in deconvolution. New Phytol. 2014, 202, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.L.; Li, M.M.; Yao, T.; Wang, H.; Zhang, Y.C.; Xiao, L.H.; Wang, J.Z.; Zhang, Z.; Hu, Y.; Liu, W.Z.; et al. Nitric oxide restrains root growth by DNA damage induced cell cycle arrest in Arabidopsis thaliana. Nitric Oxide 2012, 26, 54–60. [Google Scholar] [CrossRef] [PubMed]



| No | Gene | F-Primer | R-Primer | Size |
|---|---|---|---|---|
| 1 | OsHIPP38 | TCTCGGAGTACGGCTACGTC | GGTGCATGCATTAGGGTTCT | 158 |
| 2 | OsGR1 | GGCAGGCAGTTTGGTTGATG | GTTGAGCTCGGCTACCAGTT | 110 |
| 3 | OsP5CS2 | TAGCAGGACTGTTGGCACTG | CCGCTATTTGAAGCCAAGAC | 223 |
| 4 | OsUBQ1 | GACGGACGCACCCTGGCTGA | TGCTGCCAATTACCATATACC | 396 |
| Genotype | Trait | Dry Biomass (mg) | Reduction (%) † | |||
|---|---|---|---|---|---|---|
| Control | NaCl | SNP+NaCl | NaCl | SNP+NaCl | ||
| HP | Root | 22.50 | 15.25 | 23.25 | 32.22 | −3.33 |
| Ediget | 25.00 | 18.50 | 15.50 | 26.00 | 38.00 | |
| HP | Shoot | 82.75 | 60.75 | 90.00 | 26.59 | −8.76 |
| Ediget | 102.75 | 58.25 | 73.00 | 43.31 | 28.95 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamu, T.A.; Mun, B.-G.; Lee, S.-U.; Hussain, A.; Yun, B.-W. Exogenously Applied Nitric Oxide Enhances Salt Tolerance in Rice (Oryza sativa L.) at Seedling Stage. Agronomy 2018, 8, 276. https://doi.org/10.3390/agronomy8120276
Adamu TA, Mun B-G, Lee S-U, Hussain A, Yun B-W. Exogenously Applied Nitric Oxide Enhances Salt Tolerance in Rice (Oryza sativa L.) at Seedling Stage. Agronomy. 2018; 8(12):276. https://doi.org/10.3390/agronomy8120276
Chicago/Turabian StyleAdamu, Teferi Alem, Bong-Gyu Mun, Sang-Uk Lee, Adil Hussain, and Byung-Wook Yun. 2018. "Exogenously Applied Nitric Oxide Enhances Salt Tolerance in Rice (Oryza sativa L.) at Seedling Stage" Agronomy 8, no. 12: 276. https://doi.org/10.3390/agronomy8120276





