Towards Remote Estimation of Radiation Use Efficiency in Maize Using UAV-Based Low-Cost Camera Imagery
Abstract
:1. Introduction
- incoming radiation (RUEinc) calculated aswith PARinc = incident PAR and DM = dry matter produced,
- total absorbed light (RUEtotal), calculated aswith fAPAR as the fraction of daily PAR absorbed, and
- radiation absorbed by photosynthetically active vegetation (RUEgreen), calculated aswith fAPARgreen calculated as
- Does UAV-based commercial off-the-shelf (COTS) digital camera imagery reflectance data allow for fAPAR estimation support, for ultimately determining RUEs of maize in small-scale experimental plots of variable LAI and biomass development (measured destructively)?
- Do RUE values of maize derived from this technique differ substantially from field-collected ones reported in the literature?
- Is there a difference between RUEtotal and RUEgreen in the treatments?
2. Material and Methods
2.1. Study Site and Field Experiment
2.2. Leaf Area Index and Dry Biomass Measurements
2.3. Collection of Spectral Data and Preprocessing
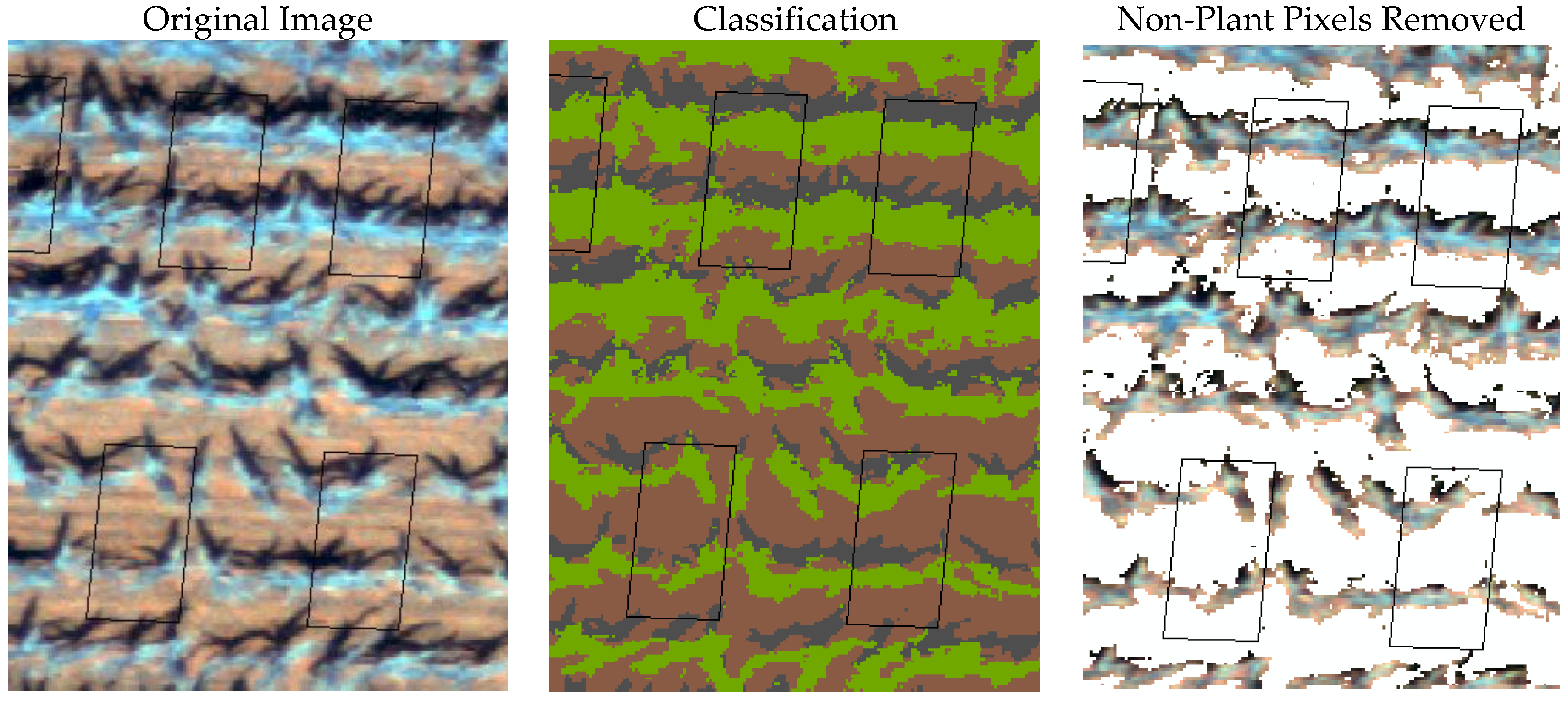
2.4. Image Classification and Estimation of Fractional Cover
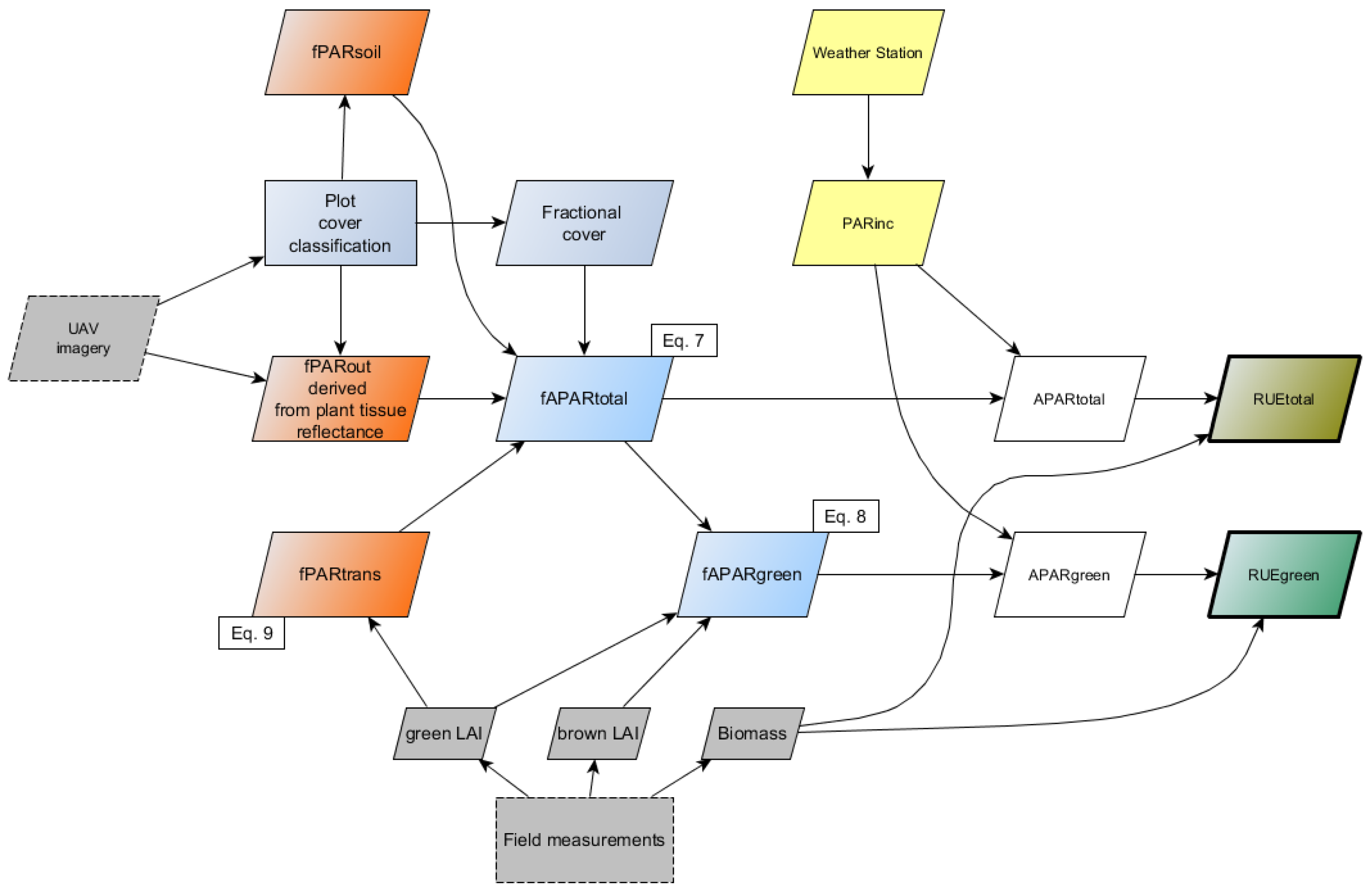
2.5. Derivation of fAPAR and APAR
- The spectrum captured by the blue, red and green bands of the cameras corresponded roughly to the spectrum of photosynthetically active radiation (PAR, 400–700 nm).
- PARinc (derived as 0.5× total solar radiation) measured by the weather station corresponded to PARinc above the canopy in the field.
- fPARout corresponded to the average reflectance value (%) of plant tissue that was derived from the red, green and blue bands of the converted camera imagery.
- fPARtrans was not measured directly. We estimated the fraction of PAR transmitted through the canopy by applying the Lambert–Beer law Equation (9), where k is the extinction coefficient and gLAI the green leaf area index:A number of k values have been reported for maize, with recent publications suggesting a range between 0.49 [7], 0.63 [36] and 0.65 [37] for maize hybrid plants. We selected a k value of 0.55 for our analysis.
- fPARsoil reflects a fixed amount of fPARtrans back into the direction of the canopy, where it is absorbed by the plants. We averaged all pixel values in the class ‘illuminated soil’, thereby neglecting those influences that vary soil moisture levels and so affect soil reflectance. An average reflectance of 10% was assumed, based on the average of soil reflectance within the class ‘illuminated soil’.
- Temporal increase in fAPAR between sampling dates follows a linear relationship.
- The plants grew free from environmental stresses.
2.6. Calculation of RUE
3. Results
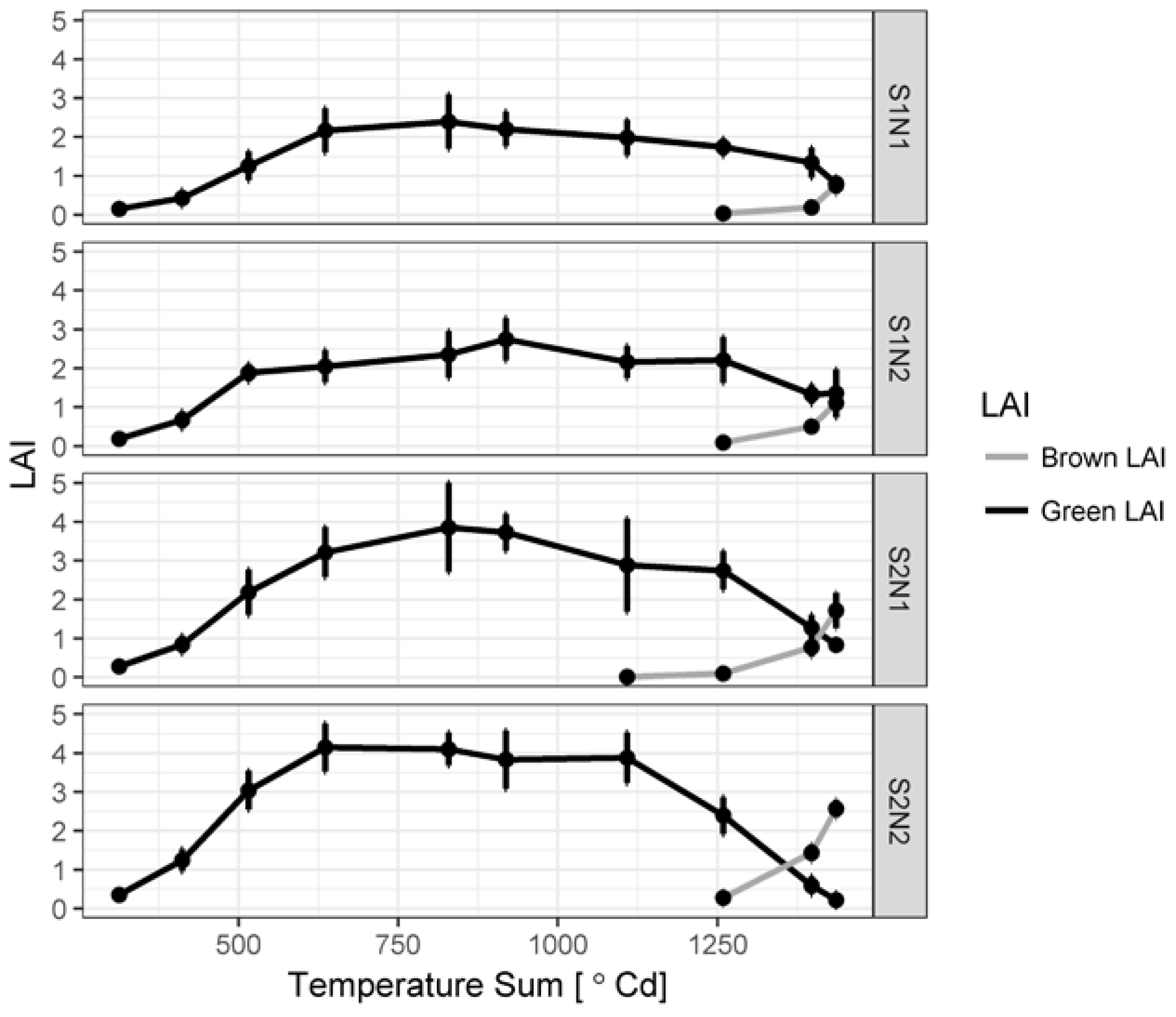
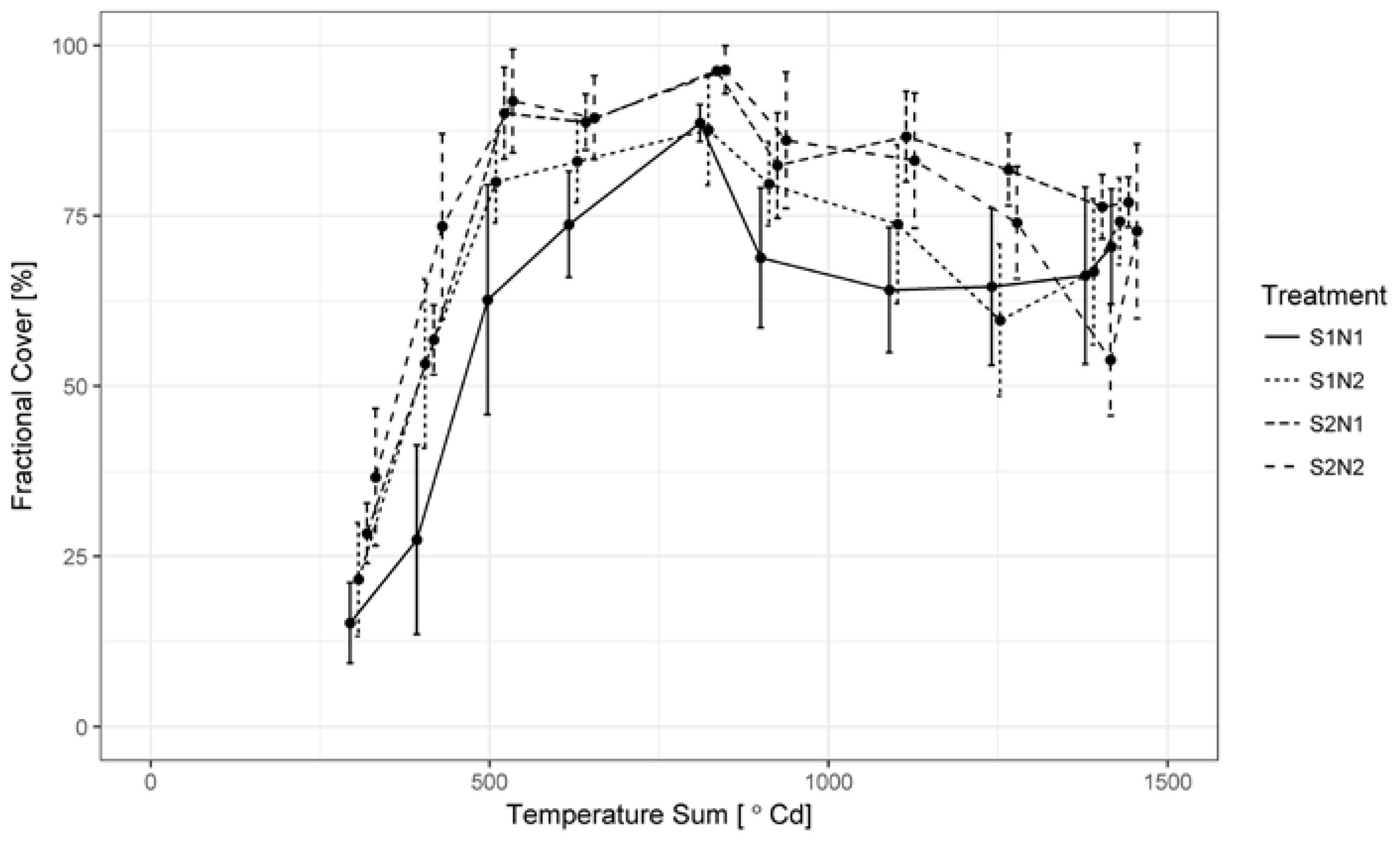
3.1. Green LAI, Brown LAI and Fractional Cover Development
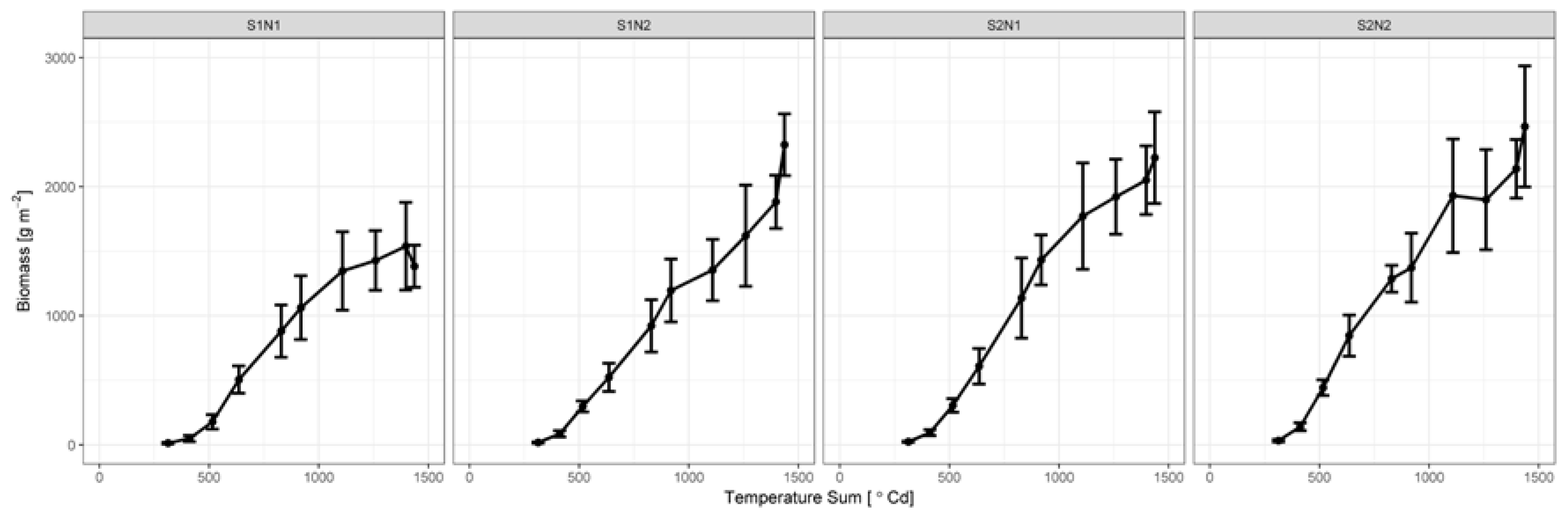
3.2. Biomass Development
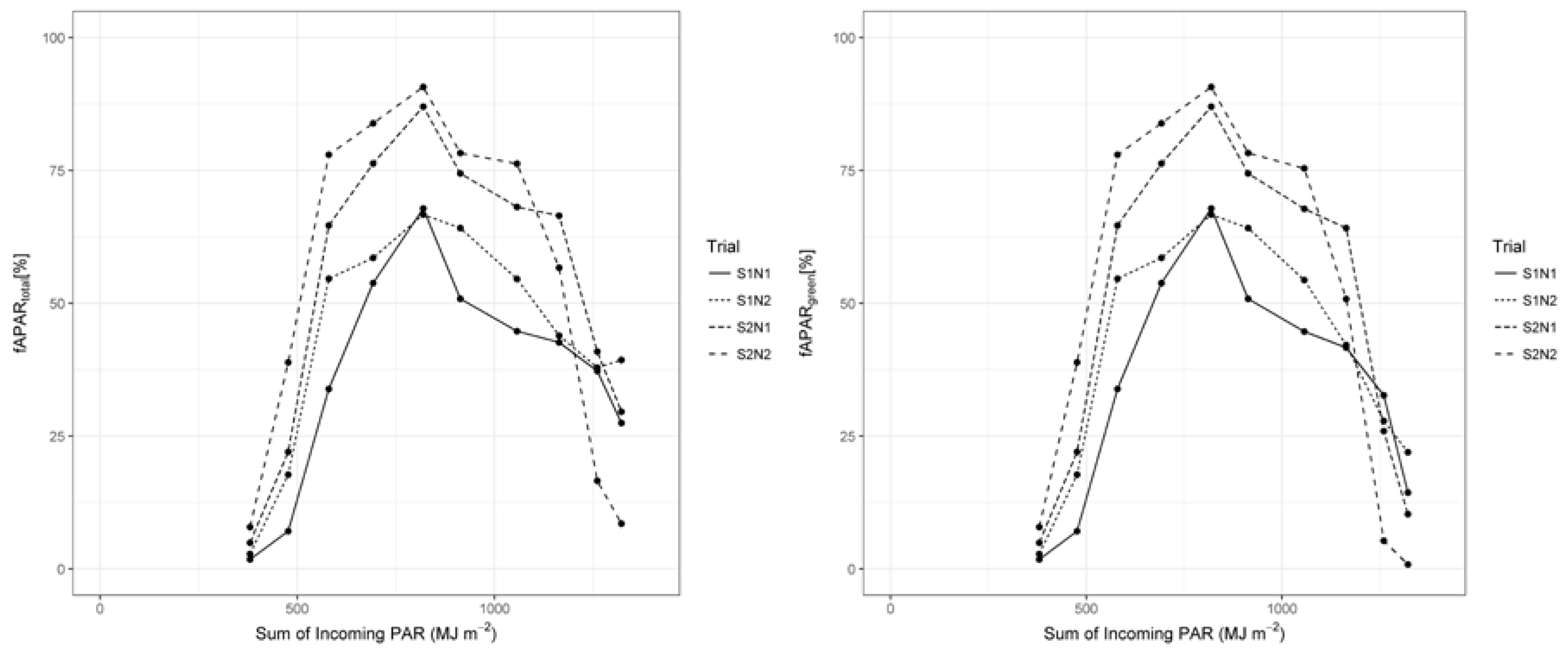
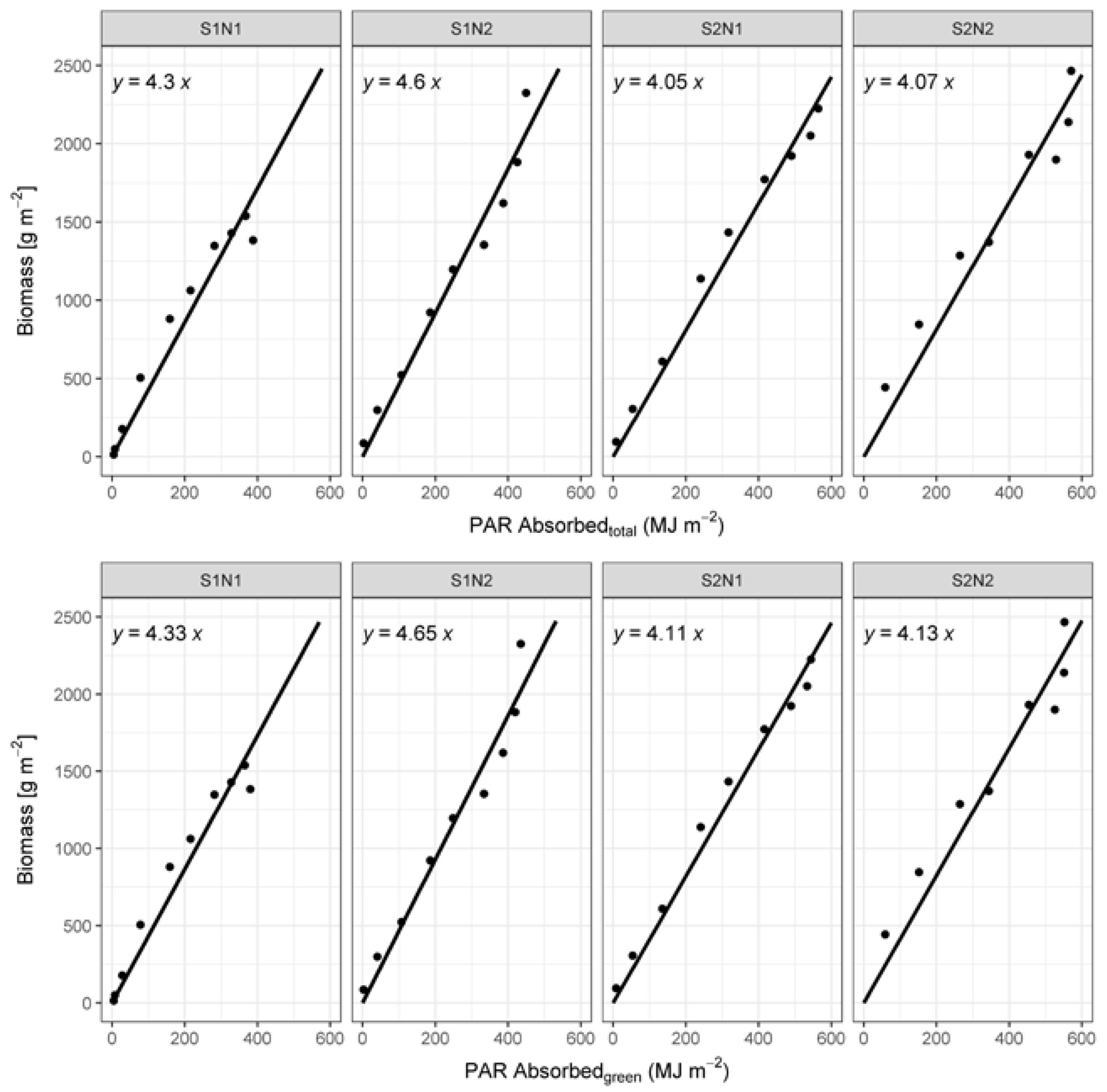
3.3. Radiation Use Efficiency Development
4. Discussion
4.1. Camera Sensitivity
4.2. Green and Brown LAI Development and Measurement Techniques
4.3. fAPAR Calculation Assumptions
4.4. RUE Values
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stöckle, C.O.; Kemanian, A.R. Crop Radiation Capture and Use Efficiency: A Framework for Crop Growth Analysis. In Crop Physiology; Academic Press: San Diego, CA, USA, 2009; Chapter 7; pp. 145–170. ISBN 978-0-12-374431-9. [Google Scholar]
- Monteith, J.L. The management of inputs for yet greater agricultural yield and efficiency-Climate and the efficiency of crop production in Britain. Philos. Trans. R. Soc. Lond. B 1977, 281, 277–294. [Google Scholar] [CrossRef]
- Morel, J.; Bégué, A.; Todoroff, P.; Martiné, J.-F.; Lebourgeois, V.; Petit, M. Coupling a sugarcane crop model with the remotely sensed time series of fIPAR to optimise the yield estimation. Eur. J. Agron. 2014, 61, 60–68. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Muchow, R.C. Radiation Use Efficiency. Adv. Agron. 1999, 65, 215–265. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gamon, J.A. The need for a common basis for defining light-use efficiency: Implications for productivity estimation. Remote Sens. Environ. 2015, 156, 196–201. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Peng, Y.; Arkebauer, T.J.; Suyker, A.E. Productivity, absorbed photosynthetically active radiation, and light use efficiency in crops: Implications for remote sensing of crop primary production. J. Plant Physiol. 2015, 177, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, J.L.; Arkebauer, T.J.; Walters, D.T.; Cassman, K.G.; Dobermann, A. Maize radiation use efficiency under optimal growth conditions. Agron. J. 2005, 97, 72–78. [Google Scholar] [CrossRef]
- Viña, A.; Gitelson, A.A. New developments in the remote estimation of the fraction of absorbed photosynthetically active radiation in crops. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Luche, H.D.S.; Silva, J.A.G.D.; Maia, L.C.D.; Oliveira, A.C.D. Stay-green: A potentiality in plant breeding. Ciênc. Rural 2015, 45, 1755–1760. [Google Scholar] [CrossRef]
- Antonietta, M.; Acciaresi, H.A.; Guiamet, J.J. Responses to N Deficiency in Stay Green and Non-Stay Green Argentinean Hybrids of Maize. J. Agron. Crop Sci. 2016, 202, 231–242. [Google Scholar] [CrossRef]
- Swanckaert, J.; Pannecoucque, J.; Waes, J.V.; Steppe, K.; Labeke, M.-C.V.; Reheul, D. Stay-green characterization in Belgian forage maize. J. Agric. Sci. 2017, 155, 766–776. [Google Scholar] [CrossRef]
- Singer, J.W.; Meek, D.W.; Sauer, T.J.; Prueger, J.H.; Hatfield, J.L. Variability of light interception and radiation use efficiency in maize and soybean. Field Crops Res. 2011, 121, 147–152. [Google Scholar] [CrossRef]
- Claverie, M.; Demarez, V.; Duchemin, B.; Hagolle, O.; Ducrot, D.; Marais-Sicre, C.; Dejoux, J.-F.; Huc, M.; Keravec, P.; Béziat, P.; et al. Maize and sunflower biomass estimation in southwest France using high spatial and temporal resolution remote sensing data. Remote Sens. Environ. 2012, 124, 844–857. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.; Liu, J.; Qian, B.; Jing, Q.; Croft, H.; Chen, J.; Wang, J.; Huffman, T.; Shang, J.; Chen, P. Deriving Maximum Light Use Efficiency From Crop Growth Model and Satellite Data to Improve Crop Biomass Estimation. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2017, 10, 104–117. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Peng, Y.; Arkebauer, T.J.; Schepers, J. Relationships between gross primary production, green LAI, and canopy chlorophyll content in maize: Implications for remote sensing of primary production. Remote Sens. Environ. 2014, 144, 65–72. [Google Scholar] [CrossRef]
- Jones, H.G.; Vaughan, R.A. Remote Sensing of Vegetation: Principles, Techniques, and Applications; Oxford University Press: Oxford, UK, 2010; ISBN 978-0-19-920779-4. [Google Scholar]
- Bendig, J.; Bolten, A.; Bennertz, S.; Broscheit, J.; Eichfuss, S.; Bareth, G. Estimating Biomass of Barley Using Crop Surface Models (CSMs) Derived from UAV-Based RGB Imaging. Remote Sens. 2014, 6, 10395–10412. [Google Scholar] [CrossRef]
- Yue, J.; Yang, G.; Li, C.; Li, Z.; Wang, Y.; Feng, H.; Xu, B. Estimation of Winter Wheat Above-Ground Biomass Using Unmanned Aerial Vehicle-Based Snapshot Hyperspectral Sensor and Crop Height Improved Models. Remote Sens. 2017, 9, 708. [Google Scholar] [CrossRef]
- Córcoles, J.I.; Ortega, J.F.; Hernández, D.; Moreno, M.A. Estimation of leaf area index in onion (Allium cepa L.) using an unmanned aerial vehicle. Biosyst. Eng. 2013, 115, 31–42. [Google Scholar] [CrossRef]
- Hunt, E.R.; Hively, W.D.; Fujikawa, S.J.; Linden, D.S.; Daughtry, C.S.T.; McCarty, G.W. Acquisition of NIR-Green-Blue Digital Photographs from Unmanned Aircraft for Crop Monitoring. Remote Sens. 2010, 2, 290–305. [Google Scholar] [CrossRef]
- Burkart, A.; Hecht, V.L.; Kraska, T.; Rascher, U. Phenological analysis of unmanned aerial vehicle based time series of barley imagery with high temporal resolution. Precis. Agric. 2017, 1–13. [Google Scholar] [CrossRef]
- Haghighattalab, A.; Crain, J.; Mondal, S.; Rutkoski, J.; Singh, R.P.; Poland, J. Application of Geographically Weighted Regression to Improve Grain Yield Prediction from Unmanned Aerial System Imagery. Crop Sci. 2017, 57, 2478–2489. [Google Scholar] [CrossRef]
- Maresma, Á.; Ariza, M.; Martínez, E.; Lloveras, J.; Martínez-Casasnovas, J.A. Analysis of Vegetation Indices to Determine Nitrogen Application and Yield Prediction in Maize (Zea mays L.) from a Standard UAV Service. Remote Sens. 2016, 8, 973. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, H.B.; Xu, X.Q.; He, J.Y.; Ge, X.K.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.X.; Tian, Y.C. Predicting grain yield in rice using multi-temporal vegetation indices from UAV-based multispectral and digital imagery. ISPRS J. Photogramm. Remote Sens. 2017, 130, 246–255. [Google Scholar] [CrossRef]
- Gaiser, T.; Perkons, U.; Küpper, P.M.; Puschmann, D.U.; Peth, S.; Kautz, T.; Pfeifer, J.; Ewert, F.; Horn, R.; Köpke, U. Evidence of improved water uptake from subsoil by spring wheat following lucerne in a temperate humid climate. Field Crops Res. 2012, 126, 56–62. [Google Scholar] [CrossRef]
- Kautz, T.; Stumm, C.; Kösters, R.; Köpke, U. Effects of perennial fodder crops on soil structure in agricultural headlands. J. Plant Nutr. Soil Sci. 2010, 173, 490–501. [Google Scholar] [CrossRef]
- Birch, C.J.; Vos, J.; van der Putten, P.E.L. Plant development and leaf area production in contrasting cultivars of maize grown in a cool temperate environment in the field. Eur. J. Agron. 2003, 19, 173–188. [Google Scholar] [CrossRef] [Green Version]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of methods for in situ leaf area index determination: Part I. Theories, sensors and hemispherical photography. Agric. For. Meteorol. 2004, 121, 19–35. [Google Scholar] [CrossRef]
- Kelcey, J.; Lucieer, A. Sensor Correction of a 6-Band Multispectral Imaging Sensor for UAV Remote Sensing. Remote Sens. 2012, 4, 1462–1493. [Google Scholar] [CrossRef]
- Smith, G.M.; Milton, E.J. The use of the empirical line method to calibrate remotely sensed data to reflectance. Int. J. Remote Sens. 1999, 20, 2653–2662. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Leutner, B.; Horning, N. RStoolbox: Tools for Remote Sensing Data Analysis. 2017. CRAN–Package RStoolbox. Available online: https://cran.r-project.org/web/packages/RStoolbox/index.html (accessed on 5 February 2018).
- Ghamisi, P.; Plaza, J.; Chen, Y.; Li, J.; Plaza, A.J. Advanced Spectral Classifiers for Hyperspectral Images: A review. IEEE Geosci. Remote Sens. Mag. 2017, 5, 8–32. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.F.; Ghimire, B.; Rogan, J.; Chica-Olmo, M.; Rigol-Sanchez, J.P. An assessment of the effectiveness of a random forest classifier for land-cover classification. ISPRS J. Photogramm. Remote Sens. 2012, 67, 93–104. [Google Scholar] [CrossRef]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. 2016. CRAN–Package Raster. Available online: https://cran.r-project.org/web/packages/raster/index.html (accessed on 5 February 2018).
- Liu, G.; Hou, P.; Xie, R.; Ming, B.; Wang, K.; Xu, W.; Liu, W.; Yang, Y.; Li, S. Canopy characteristics of high-yield maize with yield potential of 22.5 Mgha−1. Field Crops Res. 2017, 213, 221–230. [Google Scholar] [CrossRef]
- Maddonni, G.A.; Otegui, M.E.; Cirilo, A.G. Plant population density, row spacing and hybrid effects on maize canopy architecture and light attenuation. Field Crops Res. 2001, 71, 183–193. [Google Scholar] [CrossRef]
- Timlin, D.J.; Fleisher, D.H.; Kemanian, A.R.; Reddy, V.R. Plant Density and Leaf Area Index Effects on the Distribution of Light Transmittance to the Soil Surface in Maize. Agron. J. 2014, 106, 1828. [Google Scholar] [CrossRef]
- Berra, E.; Gibson-Poole, S.; MacArthur, A.; Gaulton, R.; Hamilton, A. Estimation of the spectral sensitivity functions of un-modified and modified commercial off-the-shelf digital cameras to enable their use as a multispectral imaging system for UAVs. ISPRS-Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, XL-1/W4, 207–214. [Google Scholar] [CrossRef]
- Ross, J.; Sulev, M. Sources of errors in measurements of PAR. Agric. For. Meteorol. 2000, 100, 103–125. [Google Scholar] [CrossRef]
- Bréda, N.J.J. Ground-based measurements of leaf area index: A review of methods, instruments and current controversies. J. Exp. Bot. 2003, 54, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, W.W.; Ruwe, K.; Schlemmer, M.R. Comparison of three leaf area index meters in a corn canopy. Crop Sci. 2000, 40, 1179–1183. [Google Scholar] [CrossRef]
- Baret, F.; de Solan, B.; Lopez-Lozano, R.; Ma, K.; Weiss, M. GAI estimates of row crops from downward looking digital photos taken perpendicular to rows at 57.5° zenith angle: Theoretical considerations based on 3D architecture models and application to wheat crops. Agric. For. Meteorol. 2010, 150, 1393–1401. [Google Scholar] [CrossRef]
- Verger, A.; Vigneau, N.; Chéron, C.; Gilliot, J.-M.; Comar, A.; Baret, F. Green area index from an unmanned aerial system over wheat and rapeseed crops. Remote Sens. Environ. 2014, 152, 654–664. [Google Scholar] [CrossRef]
- Andrade, F.H.; Calviño, P.; Cirilo, A.; Barbieri, P. Yield Responses to Narrow Rows Depend on Increased Radiation Interception. Agron. J. 2002, 94, 975. [Google Scholar] [CrossRef]
- Huang, S.; Gao, Y.; Li, Y.; Xu, L.; Tao, H.; Wang, P. Influence of plant architecture on maize physiology and yield in the Heilonggang River valley. Crop J. 2017, 5, 52–62. [Google Scholar] [CrossRef]
- Ma, D.L.; Xie, R.Z.; Niu, X.K.; Li, S.K.; Long, H.L.; Liu, Y.E. Changes in the morphological traits of maize genotypes in China between the 1950s and 2000s. Eur. J. Agron. 2014, 58, 1–10. [Google Scholar] [CrossRef]
- Gabaldón-Leal, C.; Webber, H.; Otegui, M.E.; Slafer, G.A.; Ordóñez, R.A.; Gaiser, T.; Lorite, I.J.; Ruiz-Ramos, M.; Ewert, F. Modelling the impact of heat stress on maize yield formation. Field Crops Res. 2016, 198, 226–237. [Google Scholar] [CrossRef]
- Lobell, D.B.; Asner, G.P. Moisture effects on soil reflectance. Soil Sci. Soc. Am. J. 2002, 66, 722–727. [Google Scholar] [CrossRef]
- Tollenaar, M.; Aguilera, A. Radiation use efficiency of an old and a new maize hybrid. Agron. J. 1992, 84, 536–541. [Google Scholar] [CrossRef]
- Gallo, K.P.; Daughtry, C.S.T. Techniques for Measuring Intercepted and Absorbed Photosynthetically Active Radiation in Corn Canopies. Agron. J. 1986, 78, 752–756. [Google Scholar] [CrossRef]
- Antonietta, M.; Fanello, D.D.; Acciaresi, H.A.; Guiamet, J.J. Senescence and yield responses to plant density in stay green and earlier-senescing maize hybrids from Argentina. Field Crops Res. 2014, 155, 111–119. [Google Scholar] [CrossRef]
- Gou, L.; Xue, J.; Qi, B.; Ma, B.; Zhang, W. Morphological Variation of Maize Cultivars in Response to Elevated Plant Densities. Agron. J. 2017, 109, 1443. [Google Scholar] [CrossRef]
- Anderson, E.L. Tillage and N fertilization effects on maize root growth and root:shoot ratio. Plant Soil 1988, 108, 245–251. [Google Scholar] [CrossRef]
- Mitchell, P.L.; Sheehy, J.E.; Woodward, F.I. Potential Yields and the Efficiency of Radiation Use in Rice; IRRI Discussion Paper Series; International Rice Research Institute: Manila, Philippines, 1998; Volume 32. [Google Scholar]
- Li, W.; Niu, Z.; Chen, H.; Li, D.; Wu, M.; Zhao, W. Remote estimation of canopy height and aboveground biomass of maize using high-resolution stereo images from a low-cost unmanned aerial vehicle system. Ecol. Indic. 2016, 67, 637–648. [Google Scholar] [CrossRef]
| Source | Average RUE | Based on | Location | Comments |
|---|---|---|---|---|
| [7] | 3.8 g MJ−1 | APAR | Sterling, NE, and Lincoln NE, USA | Near-optimal growth conditions, five growing seasons, irrigated |
| [6] | 2.24 gC MJ−1 | APARgreen | Mead, NE, USA | Multiyear observations, irrigated and rainfed, high variability within maize cultivars |
| [12] | 3.35 g MJ−1 | IPAR | Ames, IA, USA | One growing season, rainfed |
| [4] | 1.6 g MJ−1 during vegetative growth, 1.7 g MJ−1 during reproductive growth | Solar radiation | Various | Review of publications from different locations with different measurement techniques |
| [13] | 3.3 g MJ−1 | APAR | Toulouse, France | Three growing seasons, irrigated |
| [14] | 3.41 g MJ−1 | APAR | Southern Ontario, Canada | One growing season, rainfed, nitrogen/no nitrogen treatment |
| BBCH Stage | Date | Temperature Sum [°Cd] |
|---|---|---|
| Planting Date | 4 May 2016 | - |
| Emergence | 10 May 2016 | 44.45 |
| Begin Flowering | 15 July 2016 | 585.72 |
| Fruit Development: Milk-ripe stage | 15 August 2016 | 918.41 |
| Full ripening | 23 September 2016 | 1346.67 |
| Harvest | 29 October 2016 | 1454.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tewes, A.; Schellberg, J. Towards Remote Estimation of Radiation Use Efficiency in Maize Using UAV-Based Low-Cost Camera Imagery. Agronomy 2018, 8, 16. https://doi.org/10.3390/agronomy8020016
Tewes A, Schellberg J. Towards Remote Estimation of Radiation Use Efficiency in Maize Using UAV-Based Low-Cost Camera Imagery. Agronomy. 2018; 8(2):16. https://doi.org/10.3390/agronomy8020016
Chicago/Turabian StyleTewes, Andreas, and Jürgen Schellberg. 2018. "Towards Remote Estimation of Radiation Use Efficiency in Maize Using UAV-Based Low-Cost Camera Imagery" Agronomy 8, no. 2: 16. https://doi.org/10.3390/agronomy8020016
APA StyleTewes, A., & Schellberg, J. (2018). Towards Remote Estimation of Radiation Use Efficiency in Maize Using UAV-Based Low-Cost Camera Imagery. Agronomy, 8(2), 16. https://doi.org/10.3390/agronomy8020016




