The Effect of Community-Based Soil and Water Conservation Practices on Abundance and Diversity of Soil Macroinvertebrates in the Northern Highlands of Ethiopia
Abstract
1. Introduction
2. Materials and Methods
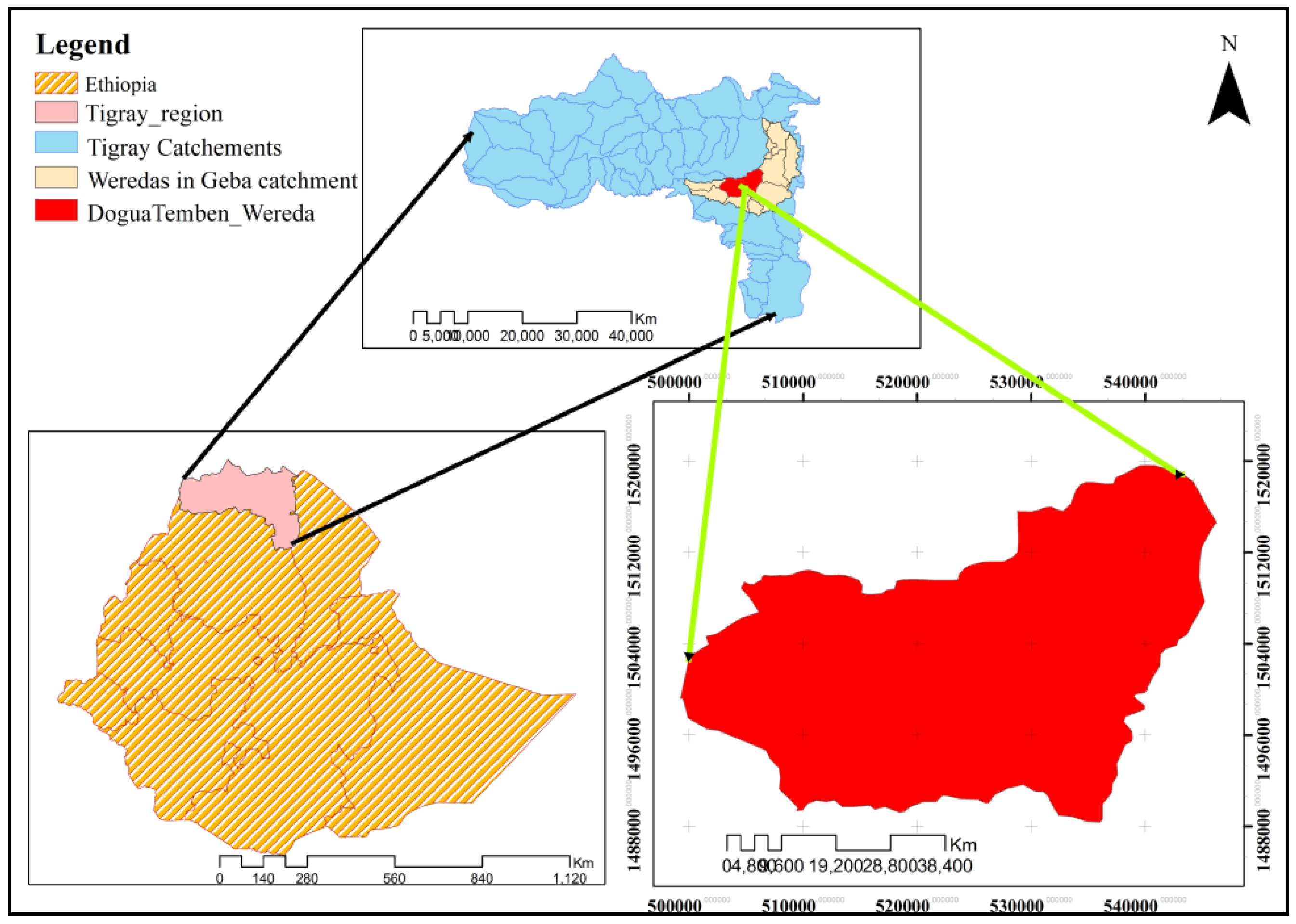
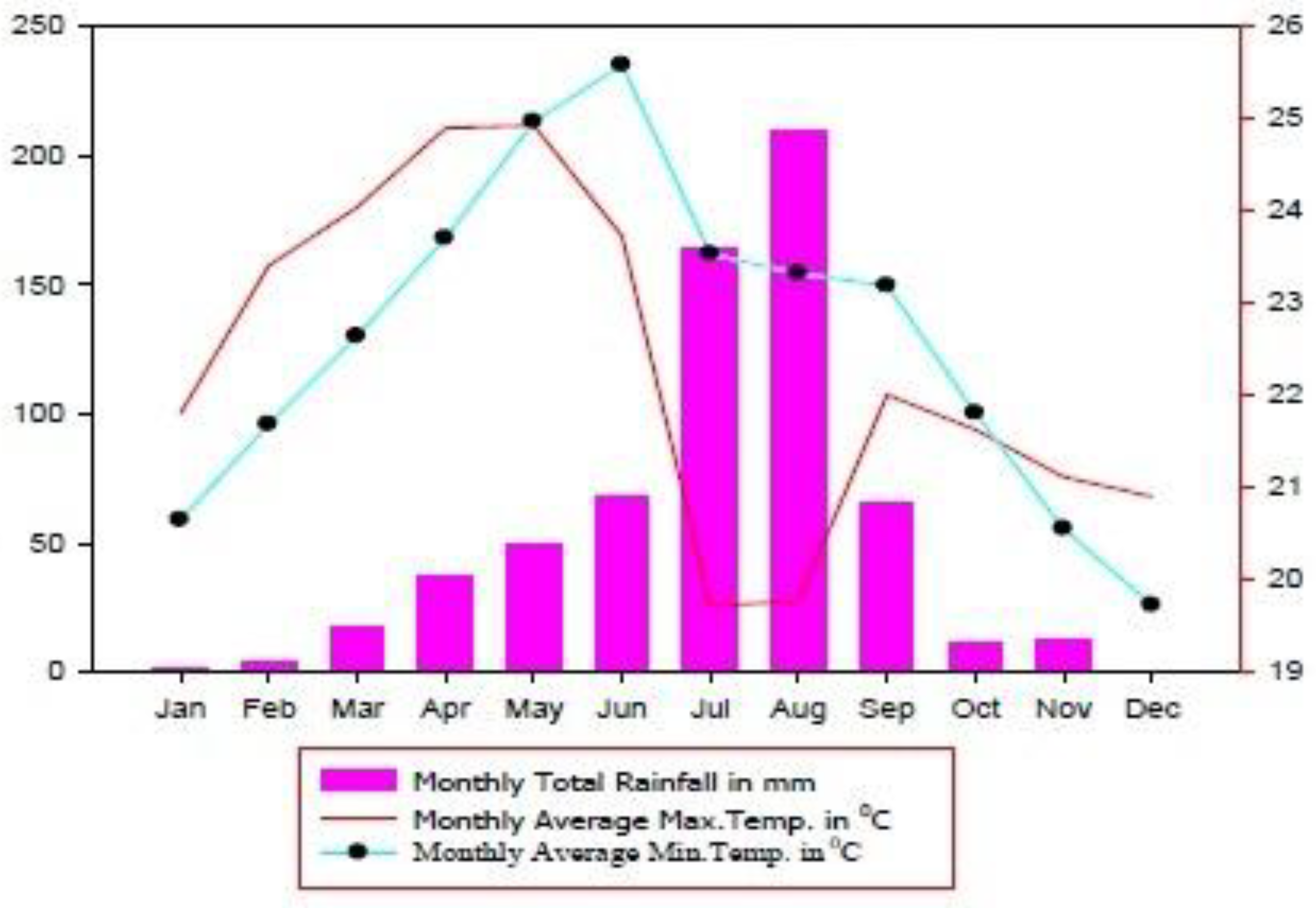
2.1. Description of the Study Area
2.2. Experimental Design
2.3. Soil Macro-Invertebrate Sampling and Identification
2.4. Determination of Diversity Indices of Soil Macroinvertebrates
2.5. Soil Sampling and Analysis
3. Statistical Analysis
4. Results and Discussion
4.1. Effect of Soil and Water Conservation Measures on Abundance and Diversity of Soil Macroinvertebrates
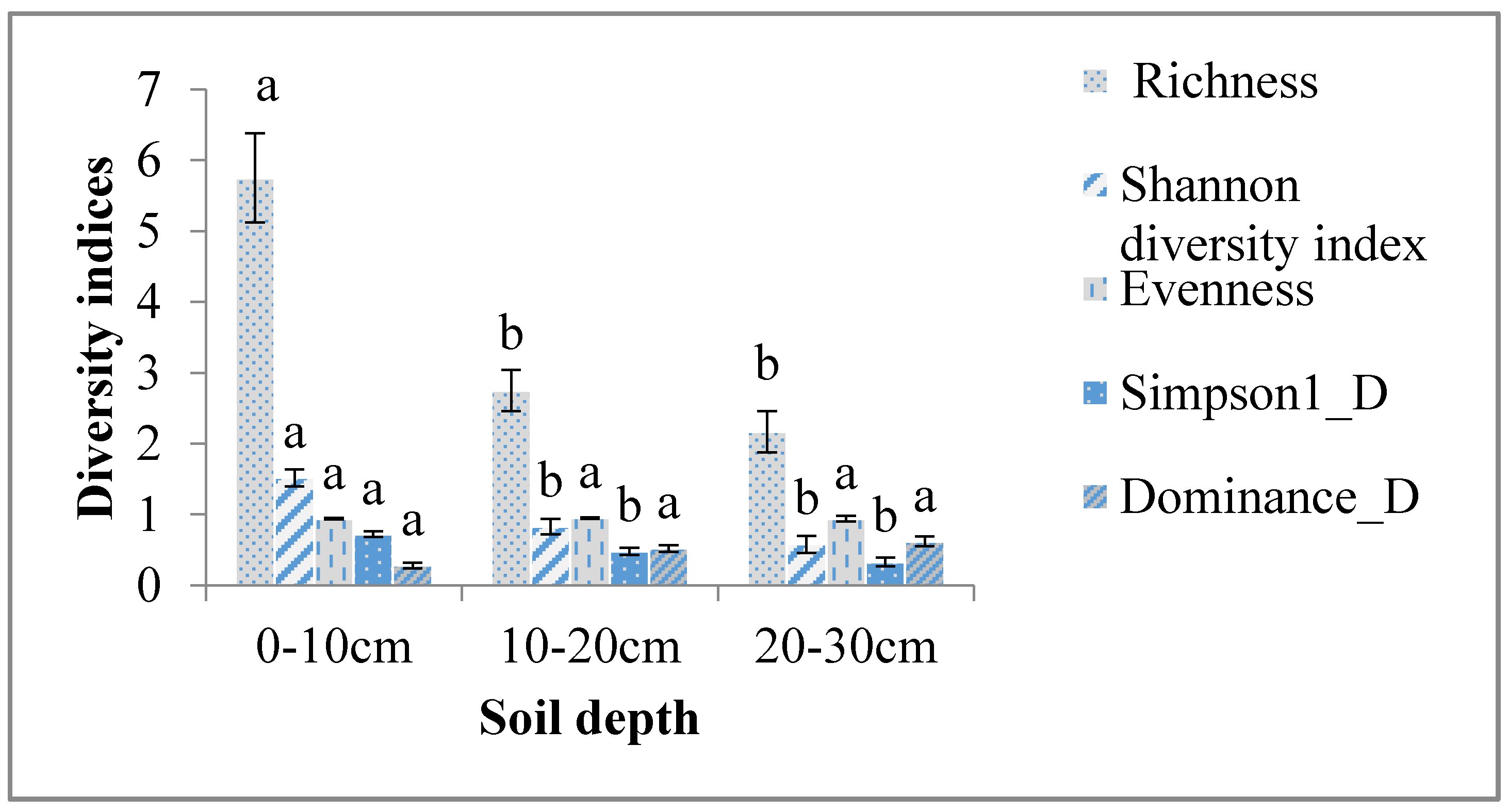
4.2. Effect of Soil Depth on Abundance and Diversity of Soil Macroinvertebrates
4.3. Effect of Seasonal Changes on Abundance and Diversity of Soil Macroinvertebrates
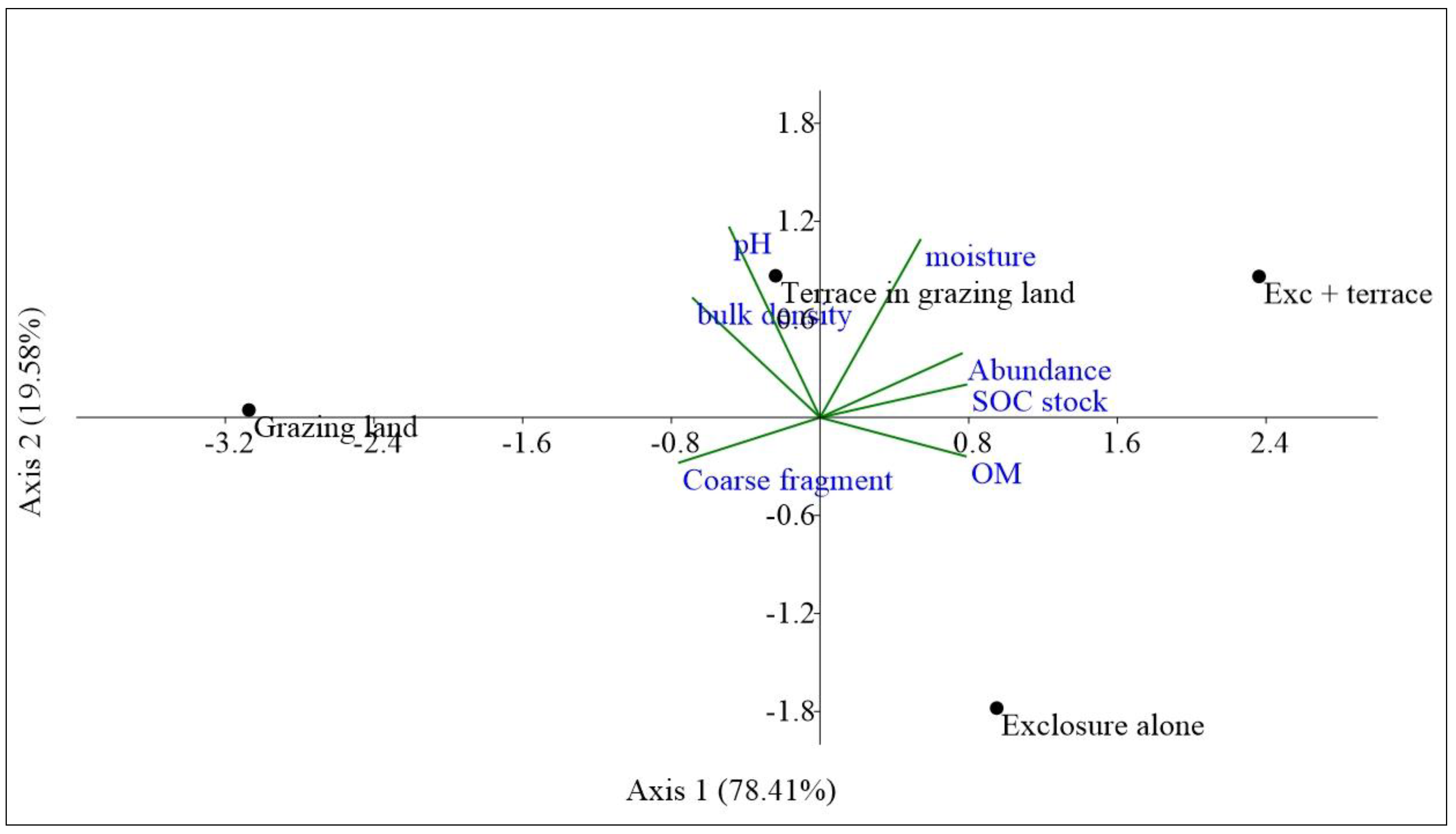
4.4. Relative Contribution of Soil Properties to Soil Macroinvertebrates
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nyssen, J.; Munro, N.; Haile, M.; Poesen, J.; Descheemaeker, K.; Haregeweyn, N.; Decker, J. Understanding the Environmental Changes in Tigray: A Photographic Record Over 30 Years. Tigray Livelihood Papers No. 3, VLIR – Mekelle University IUC Programme and Zala-Daget Project. 2007; 82 p. [Google Scholar]
- Carolyn, T.; Kwadwo, A. Responding to Land Degradation in the Highlands of Tigray Northern Ethiopia; Discussion Paper; International Food Policy Research Institute (IFPRI) Eastern and Southern Africa Regional Office: Washington, DC, USA, 2011. [Google Scholar]
- Gebreegziabher, T.; Nyssen, J.; Govaerts, B.; Getnet, F.; Behailu, M.; Haile, M.; Deckers, J. Contour furrows for in situ soil and water conservation, Tigray, northern Ethiopia. Soil Tillage Res. 2009, 103, 257–264. [Google Scholar] [CrossRef]
- Mekuria, W. Effectiveness of exclosures to restore ecosystem carbon stock and vegetation in the highlands of Tigray, northern Ethiopia. J. Arid Environ. 2007, 69, 270–284. [Google Scholar] [CrossRef]
- Gebrehiwot, T.; Veen, A.V.D. The effect of enclosures in rehabilitating degraded vegetation: A case of Enderta District, northern Ethiopia. For. Res. 2014, 3, 128. [Google Scholar]
- Amdemariam, T.; Selassie, YG.; Haile, M.; Yamoh, C. Effect of soil and water conservation measures on selected soil physical and chemical properties and barley (Hordeum spp.) yield. J. Environ. Sci. 2011, 5, 1483–1495. [Google Scholar]
- Mastewal, Y.; Mekuria, W.; Hauser, M. The effectiveness of village bylaws in sustainable management of community-managed exclosures in northern Ethiopia. Sustain. Sci. 2013, 8, 73–86. [Google Scholar]
- Descheemaeker, K.; Muys, B.; Nyssen, J.; Poesen, J.; Raes, D.; Haile, M.; Deckers, J. Litter production and organic matter accumulation in exclosures of the Tigray highlands, Ethiopia. For. Ecol. Manag. 2006, 233, 21–35. [Google Scholar] [CrossRef]
- Begum, F.; Bajracharya, R.M.; Sitaula, B.K.; Sharma, S.; Ali, S.; Ali, H. Seasonal dynamics and land use effect on soil microarthropod communities in the mid-hills of Nepal. Int. J. Sustain. Dev. World Ecol. 2014, 5, 114–123. [Google Scholar]
- Menta, C. Soil fauna diversity function, soil degradation, biological indices, soil restoration. Environ. Sci. 2012. [Google Scholar] [CrossRef]
- Paolo, A.G.; Raffaella, B.; Danio, A.M.D.; Ettore, C. Assessment of soil-quality index based on microarthropods in corn cultivation in northern Italy. Ecol. Indic. 2010, 10, 129–135. [Google Scholar]
- Ali, A.; Bhat, GA.; Ali, M. Epigeal and hypogeal macroinvertebrate diversity in different microhabitats of the Yusmarg Hill Resort (Kashmir, India). Ecol. Balk. 2012, 4, 21–30. [Google Scholar]
- Ayuke, F.O.; Karanja, N.K.; Muya, E.M.; Musombi, B.K.; Mungatu, J.; Nyamasyo, G.H.N. Macrofauna diversity and abundance across land use systems in Embu, Kenya. Trop. Subtrop. Agroecosyst. 2009, 11, 371–384. [Google Scholar]
- Jouquet, P.; Dauber, J.; Lagerlof, J.; Lavelle, P.; Lepage, M. Soil invertebrates as ecosystem engineers: Intended and accidental effects on soil and feedback loops. Appl. Soil Ecol. 2006, 32, 153–164. [Google Scholar] [CrossRef]
- Lal, R. Effect of macrofauna on soil properties in tropical ecosystems. Agric. Ecosyst. Environ. 1988, 24, 101–116. [Google Scholar] [CrossRef]
- Ruiz, N.; Lavelle, P. Soil Macro-Fauna Field Manual; FAO: Rome, Italy, 2008; pp. 1–100. [Google Scholar]
- Maharning, A.R.; Mills, A.A.; Adl, S.M. Soil community changes during secondary succession to naturalized grasslands. Appl. Soil Ecol. 2008, 41, 137–147. [Google Scholar] [CrossRef]
- Nyssen, J.; Poesen, J.; Moeyersons, J.; Luyten, E.; Veyret-Picot, M.; Deckers, J.; Mitiku, H.; Govers, G. Impact of road building on gully erosion risk: A case study from the northern Ethiopian highlands. Earth Surf. Process. Landf. 2002, 27. [Google Scholar] [CrossRef]
- Beyth, M. Paleozoic–mesozoic sedimentary basin of Makalle outlier. Am. Assoc. Pet. Geol. Bull. 1972, 56, 2426–2439. [Google Scholar]
- World Reference Base (WRB). A Framework for International Classification, Correlation and Communication; FAO: Rome, Italy, 2006. [Google Scholar]
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods, 2nd ed.; CABI: Wallingford, UK, 1993. [Google Scholar]
- Magurran, A.E. Measuring of Biological Diversity; Blackwell Publishing: London, UK, 2004. [Google Scholar]
- Sorensen, T.A. A method of establishing groups of equal amplitude in plant sociology based on similarity of species contents, and its application to analyses of vegetation of Danish commons. Biol. Skr. Kongl. Danske Vidensk. Selsk. 1948, 5, 1–34. [Google Scholar]
- Chopra, S.H.; Kanwar, J.S. Analytical Agricultural Chemistry; Kalyani Publisher Ludhiana: New Delhi, India, 1976. [Google Scholar]
- Walkeley, A.; Black, I. An examination of Degtjareff methods for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Lal, R.; Follett, R.F.; Stewart, B.A.; KimbIe, J.M. Soil carbon sequestration to mitigate climate change and advance food security. Soil Sci. 2007, 172, 943–956. [Google Scholar] [CrossRef]
- Wiwatwitaya, D.; Takeda, H. Seasonal changes in soil arthropod abundance in the dry evergreen forest of north-east Thailand, with special reference to collembolan communities. Ecol. Soc. Japan 2005, 20, 59–70. [Google Scholar] [CrossRef]
- Mehraj, S.S.; Bhat, G.A. Epigeal macroinvertebrate diversity in different microhabitats of the Sonamarg Hill Resort (Kashmir, India). Ecol. Balk. 2013, 4, 392–401. [Google Scholar]
- Adeniyi, S. Diversity and abundance of soil mesofauna and microbial population in southwestern Nigeria. Afr. J. Plant Sci. 2009, 3, 210–216. [Google Scholar]
- Liu, R.-T.; ZHAO, H-L.; Zhao, X.Y. Influence of grazing exclusion on soil macroinvertebrate diversity in degraded sandy grassland (Inner Mongolia, China). J. Ecol. 2012, 60, 375–385. [Google Scholar]
- Mekuria, W.; Aynekulu, E. Exclosure land management for restoration of the soils in degraded communal grazing lands in northern Ethiopia. Land Degrad. Dev. 2010. [Google Scholar] [CrossRef]
- Anagement, M.; State, I. Evaluation of arthropod diversity and abundance in contrasting habitat Uyo, Akwa. J. Appl. Sci. Environ. 2014, 18, 403–408. [Google Scholar]
- Mills, A.A.; Adl, M.S. Changes in nematode abundances and body length in response to management intensive grazing in a low-input temperate pasture. Soil Biol. Biochem. 2011, 43, 150–158. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, H.; Zhao, X. Desertification impact on macroinvertebrate diversity in grassland soil in Horqin, northern China. Proced. Environ. Sci. 2011, 10, 1401–1409. [Google Scholar] [CrossRef][Green Version]
- Bugalho, M.N.; Lecomte, X.; Goncalves, M.; Branco, M.C.; Caldeira, M. Establishing grazing and grazing excluded patches increases plant and invertebrate diversity in a Mediterranean oak woodland. For. Ecol. Manag. 2011, 261, 2133–2139. [Google Scholar] [CrossRef]
- Parfitta, R.L.; Yeates, G.W.; Rossa, D.J.; Schon, N.L.; Mackay, A.D.; Wardle, D.A. Effect of fertilizer, herbicide and grazing management of pastures on plant and soil communities. App. Soil. Ecol. 2010, 45, 175–186. [Google Scholar] [CrossRef]
- Lehman, R.M.; Cambardella, C.A.; Stott, D.E.; Acosta-Martinez, V.; Halvorson, J.J.; Kremer, R.J.; Ducey, T.F. Understanding and enhancing soil biological health: The solution for reversing soil degradation. Sustainability 2015, 7, 988–1027. [Google Scholar] [CrossRef]
- Balan, A.; Senicovscaia, I.; Daniliuc, R. Contribution of soil invertebrates to the stability of eroded soils in the south of the Republic of Moldova. Ser. A Agron 2012, LV-2012, 19–24. [Google Scholar]
- Barrios, E. Soil biota, ecosystem services and land productivity. Ecol. Econ. 2007, 64, 269–285. [Google Scholar] [CrossRef]
- Kooch, Y.; Haghverdi, K. Earthworms—good indicators for forest disturbance. J. Bibliogr. Sci. Biotech. 2014, 3, 155–162. [Google Scholar]
- Barros, E.; Grimaldi, M.; Sarrazin, M.; Chauvel, A.; Mitja, D.; Desjardins, T.; Lavelle, P. Soil physical degradation and changes in macrofaunal communities in central Amazon. Appl. Soil Ecol. 2004, 26, 157–168. [Google Scholar] [CrossRef]
- Decaëns, T. Macro-ecological patterns in soil communities. Glob. Ecol. Biogeogr. 2010, 19, 287–302. [Google Scholar] [CrossRef]
- Folgarait, P.J. Ant biodiversity and its relationship to ecosystem functioning: A review biodiversity and conservation. Biodivers. Conserv. 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Santorufo, L.; Cornelis, A.M.; Gestel, V.; Rocco, A.; Maisto, G. Soil invertebrates as bio indicators of urban soil quality. Environ. Pollut. 2012, 16, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, H.; Mahdieh, E. Effects of rangeland exclusion on plant cover and soil properties in a steppe rangeland of southeastern Iran. Arid Land Res. Manag. 2017. [Google Scholar] [CrossRef]


| SWC Measures | Characteristics |
|---|---|
| Non-conserved communal grazing lands | Low vegetation cover (Figure 3) High proportion of bare soil (personal observation) High stone cover/coarse fragment (Table 2) Sheet, rill, and gully erosion very common(personal observation) Low organic carbon (Table 2) Low moisture content (Table 2) Approximately not more than 10% vegetation cover (from plot wise estimation) |
| Stone terraces in grazing lands | Relatively more stable and durable than other physical SWC measures Higher organic matter than non-terraced fields (Table 2) Significantly higher moisture content than non-conserved grazing lands (Table 2) High sediment deposit (personal observation) Approximately not more than 10%vegetation cover (from plot wise estimation) |
| Exclosures + terraces | Closed from the interference of humans and livestock Enrichment planting common Supported with terraces (Figure 3) High organic carbon (Table 2) Sheet, rill, and gully erosion are less common (personal observation) Approximately greater than 50% vegetation cover (from plot wise estimation) Significantly higher moisture content than non-conserved grazing lands (Table 2) |
| Exclosures alone | Closed from humans and livestock interference No enrichment planting No physical SWC measures (Figure 3) High organic carbon (Table 2) Sheet, rill, and gully erosion are less common than non-conserved grazing lands (personal observation) Approximately greater than 50% vegetation cover (from plot wise estimation) |
| SWC | Soil Moisture (%) | pH | Bulk Density (kg m−3) | SOC (%) | Coarse Fragment (%) | SOC Stock (kg m−2) |
|---|---|---|---|---|---|---|
| Non-conserved grazing lands | 8.59 ± 0.02 b | 8.22 ± 0.04 b | 1290 ± 31.9 a | 1.9 ± 0.1 c | 57.03 ± 1.6 b | 160.7 ± 11.2 c |
| Terraces in grazing lands | 9.61 ± 0.01 a | 8.21 ± 0.04 b | 1260 ± 27.8 a | 2.5 ± 0.1 b | 53.2 ± 2.3 b | 210 ± 12.5 b |
| Exclosures + terraces | 10.06 ± 0.01 a | 8.08 ± 0.03 a | 1240 ± 20.7 a | 2.9 ± 0.1 a | 45.5 ± 2.0 a | 288.7 ± 14.0 a |
| Exclosures alone | 8.70 ± 0.03 b | 7.9 ± 0.04 a | 1220 ± 47.6 a | 2.8 ± 0.1 a | 54.4 ± 2.1b | 236 ± 12.6 b |
| SMI | Non Conserved Communal Grazing Lands | % | Terraces in Grazed Lands | % | Exclosures with Terraces | % | Exclosure alone | % |
|---|---|---|---|---|---|---|---|---|
| Formicidae | 745.8 | 23.8 | 755.6 | 24.1 | 858.7 | 27.4 | 780.0 | 24.8 |
| Termitidae | 0.9 | 0.1 | 250.7 | 37.5 | 241.3 | 36.1 | 175.1 | 26.2 |
| Carabidae | 10.4 | 16.3 | 3.6 | 5.6 | 22.2 | 34.9 | 27.6 | 43.3 |
| Rhinotermitidae | 0.0 | 0.0 | 8.9 | 20.8 | 29.3 | 68.8 | 4.4 | 10.4 |
| Spirostreptidae | 9.8 | 16.7 | 11.5 | 19.7 | 12.4 | 21.2 | 24.9 | 42.4 |
| Gryllidae | 12.4 | 17.9 | 18.7 | 26.9 | 24.0 | 34.6 | 14.2 | 20.5 |
| Lycosidae | 6.2 | 17.5 | 7.1 | 20.0 | 15.1 | 42.5 | 7.1 | 20.0 |
| Scarabaeidae | 10.7 | 26.7 | 13.3 | 33.3 | 11.6 | 28.9 | 4.4 | 11.1 |
| Scorpionidae | 2.7 | 9.1 | 16.0 | 54.5 | 3.6 | 12.1 | 7.1 | 24.2 |
| Cryptopidae | 9.8 | 28.2 | 2.7 | 7.7 | 13.3 | 38.5 | 8.9 | 25.6 |
| Blattidae | 2.7 | 15.0 | 6.2 | 34.9 | 7.1 | 39.9 | 1.8 | 10.0 |
| Lumbricidae | 0.0 | 0.0 | 4.4 | 31.3 | 5.3 | 37.5 | 4.4 | 31.3 |
| Acrididae | 0.0 | 0.0 | 0.9 | 9.1 | 6.2 | 63.6 | 2.7 | 27.3 |
| Dytiscidae | 0.0 | 0.0 | 0.0 | 0.0 | 2.7 | 100.0 | 0.0 | 0.0 |
| Staphylinidae | 0.0 | 0.0 | 1.8 | 25.0 | 2.7 | 37.5 | 2.7 | 37.5 |
| Scutigeridae | 0.0 | 0.0 | 2.6 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ligiidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 100.0 |
| Bostrichidae | 0.9 | 33.3 | 0.9 | 33.3 | 0.0 | 0.0 | 0.9 | 33.3 |
| Argasidae | 0.0 | 0.0 | 0.9 | 50.0 | 0.9 | 50.0 | 0.0 | 0.0 |
| Japygidae | 0.0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | 0.9 | 100 |
| Total | 811.6 | 19.1 | 1105.7 | 26.1 | 1256.4 | 29.6 | 1068.0 | 25.2 |
| Indices | SWC Measures | |||
|---|---|---|---|---|
| Non Conserved Communal Grazing Land | Terraces in Grazed Land | Exclosures with Terraces | Exclosures alone | |
| Abundance | 811.6 ± 318.9 a | 1105.7 ± 318.9 bc | 1256.4 ± 318.9 a | 1067.1 ± 318.9 ab |
| Family richness | 2.8 ± 0.32 b | 3.3 ± 0.76 b | 4.4 ± 0.68 a | 3.7 ± 0.61 ab |
| Shannon diversity index | 0.9 ± 0.12 b | 0.9 ± 0.19 b | 1.21 ± 0.16 a | 1.0 ± 0.17 ab |
| Evenness | 0.9 ± 0.01 a | 0.9 ± 0.05 a | 0.9 ± 0.01 a | 0.9 ± 0.01 a |
| Simpson index (1_D) | 0.5 ± 0.06 a | 0.4 ± 0.08 a | 0.6 ± 0.07 a | 0.5 ± 0.07 a |
| Dominance_D | 0.5 ± 0.06 a | 0.5 ± 0.08 a | 0.4 ± 0.07 a | 0.5 ± 0.07 a |
| SMI | Soil Depth | ||
|---|---|---|---|
| 0–10 cm | 10–20 cm | 20–30 cm | |
| Formicidae | 1424.4 ± 300.04 a | 664.4 ± 117.33 b | 266.2 ± 31.19 b |
| Termitidae | 207.3 ± 156.97 a | 177.0 ± 130.51 a | 116.7 ± 107.09 a |
| Carabidae | 32.0 ± 15.51 a | 15.3 ± 8.14 ab | 1.3 ± 0.92 b |
| Rhinotermitidae | 8.7 ± 6.16 a | 0.7 ± 0.66 a | 22.7 ± 19.99 a |
| Spirostreptidae | 35.3 ± 13.44 a | 8.7 ± 3.72 b | 0.0 ± 0 b |
| Gryllidae | 36.7 ± 11.00 a | 10.7 ± 6.22 b | 4.7 ± 2.80 b |
| Lycosidae | 23.3 ± 8.28 a | 2.0 ± 1.46 b | 1.3 ± 0.92 b |
| Scarabaeidae | 25.3 ± 7.69 a | 1.3 ± 1.33 b | 3.3 ± 2.15 b |
| Scorpionidae | 18.7 ± 6.78 a | 1.3 ± 0.92 b | 2.0 ± 1.46 b |
| Cryptopidae | 14.0 ± 4.00 a | 6.0 ± 2.69 a | 6.0 ± 3.02 a |
| Blattoidae | 6.0 ± 2.11 a | 6.0 ± 3.01 a | 1.3 ± 0.92 a |
| Lumbricidae | 6.0 ± 2.69 a | 4.7 ± 3.53 a | 0.0 ± 0 a |
| Acrididae | 6.0 ± 2.85 a | 1.3 ± 1.33 b | 0.0 ± 0.0 b |
| Dytiscidae | 2.0 ± 2.00 a | 0.0 ± 0.0 a | 0.0 ± 0 a |
| Staphylinidae | 2.7 ± 1.57 a | 1.3 ± 1.33 a | 1.3 ± 0.92 a |
| Scutigeridae | 2.0 ± 1.08 a | 0.0 ± 0.0 b | 0.0 ± 0 b |
| Ligiidae | 0.7 ± 0.66 a | 0.0 ± 0.0 a | 0.0 ± 0 a |
| Bostrichidae | 1.3 ± 0.92 a | 0.7 ± 0.66 a | 0.0 ± 0 a |
| Argasidae | 1.3 ± 0.92 a | 0.0 ± 0.0 a | 0.0 ± 0 a |
| Japygidae | 0.0 ± 0 a | 0.7 ± 0.66 a | 0.0 ± 0 a |
| Mean total | 1852.4 ± 350.59 a | 902.0 ± 218.58 b | 326.9 ± 112.66 c |
| Soil Macroinvertebrates | Wet Season | Dry Season |
|---|---|---|
| Formicidae | 1289.3 ± 10.00 a | 280.7 ± 21.22 b |
| Termitidae | 326.4 ± 147.93 a | 7.6 ± 5.11 b |
| Carabidae | 28.0 ± 11.13 a | 3.7 ± 1.61 b |
| Rhinotermitidae | 21.3 ± 13.79 a | 0.0 ± 0.00 a |
| Spirostreptidae | 24.0 ± 9.29 a | 5.3 ± 2.62 a |
| Gryllidae | 16.4 ± 5.96 a | 18.2 ± 6.98 a |
| Lycosidae | 16.9 ± 5.77 a | 0.9 ± 0.61 b |
| Scarabaeidae | 15.6 ± 5.31 a | 4.4 ± 2.35 b |
| Scorpionidae | 12.4 ± 4.73 a | 2.2 ± 1.13 b |
| Cryptopidae | 8.9 ± 2.95 a | 8.4 ± 2.50 a |
| Blattoidae | 6.7 ± 2.24 a | 2.2 ± 1.13 a |
| Lumbricidae | 7.1 ± 2.88 a | 0.0 ± 0.00 b |
| Acrididae | 3.6 ± 1.92 a | 1.3 ± 0.98 a |
| Dytiscidae | 1.3 ± 1.33 a | 0.0 ± 0.00 a |
| Staphylinidae | 3.6 ± 1.44 a | 0.0 ± 0.00 b |
| Scutigeridae | 1.3 ± 0.73 a | 0.0 ± 0.00 b |
| Ligiidae | 0.4 ± 0.44 a | 0.0 ± 0.00 a |
| Bostrichidae | 0.4 ± 0.44 a | 0.9 ± 0.61 a |
| Argasidae | 0.4 ± 0.44 a | 0.4 ± 0.44 a |
| Japygidae | 0.4 ± 0.44 a | 0.0 ± 0.00 a |
| Mean total | 1784.6 ± 263.87 a | 336.2 ± 26.06 b |
| SWC Measures | Wet Season | |||
|---|---|---|---|---|
| Non-Conserved Grazing Lands | Terraces in Grazed Lands | Exclosures with Terraces | Exclosures alone | |
| Non-conserved grazing land | ||||
| Terraces in grazed lands | 0.37 ± 0.04 ab | |||
| Exclosures with terraces | 0.38 ± 0.02 ab | 0.39 ± 0.0 a | ||
| Exclosures without terraces | 0.33 ± 0.05 ab | 0.38 ± 0.02 ab | 0.41 ± 0.01 a | |
| Dry Season | ||||
| Non conserved grazing lands | ||||
| Terraces | 0.32 ± 0.03 ab | |||
| Exclosures with terraces | 0.36 ± 0.04 ab | 0.20 ± 0.0 c | ||
| Exclosures without terraces | 0.39 ± 0.03 ab | 0.26 ± 0.07 bc | 0.35 ± 0.05 ab | |
| Parameters | Abundance | Soil Moisture | pH | Bulk Density | Coarse Fragment | Soc | Soc Stock |
|---|---|---|---|---|---|---|---|
| Abundance | 1 | ||||||
| Soil moisture | 0.85 ns | 1 | |||||
| pH | −0.46 ns | 0.85 ns | 1 | ||||
| Bulk density | −0.71 ns | −0.22 ns | 0.30 ns | 1 | |||
| Coarse fragment | −0.89 ns | −0.89 ns | 0.58 ns | 0.45 ns | 1 | ||
| SOC | 0.90 ns | 0.54 ns | 0.03 ns | −0.93 ns | −0.72 ns | 1 | |
| SOC stock | 0.97 * | 0.76 ns | 0.31 ns | −0.77 ns | −0.92 ns | 0.94 ns | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welemariam, M.; Kebede, F.; Bedadi, B.; Birhane, E. The Effect of Community-Based Soil and Water Conservation Practices on Abundance and Diversity of Soil Macroinvertebrates in the Northern Highlands of Ethiopia. Agronomy 2018, 8, 56. https://doi.org/10.3390/agronomy8040056
Welemariam M, Kebede F, Bedadi B, Birhane E. The Effect of Community-Based Soil and Water Conservation Practices on Abundance and Diversity of Soil Macroinvertebrates in the Northern Highlands of Ethiopia. Agronomy. 2018; 8(4):56. https://doi.org/10.3390/agronomy8040056
Chicago/Turabian StyleWelemariam, Mengistu, Fassil Kebede, Bobe Bedadi, and Emiru Birhane. 2018. "The Effect of Community-Based Soil and Water Conservation Practices on Abundance and Diversity of Soil Macroinvertebrates in the Northern Highlands of Ethiopia" Agronomy 8, no. 4: 56. https://doi.org/10.3390/agronomy8040056
APA StyleWelemariam, M., Kebede, F., Bedadi, B., & Birhane, E. (2018). The Effect of Community-Based Soil and Water Conservation Practices on Abundance and Diversity of Soil Macroinvertebrates in the Northern Highlands of Ethiopia. Agronomy, 8(4), 56. https://doi.org/10.3390/agronomy8040056





