Variation of Agronomic Traits of Ravenna Grass and Its Potential as a Biomass Crop
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sucrose Concentration of Leaf and Culm Sap
2.2. Percentage Seed Set and Number of Caryopses Per Panicle
2.3. Biomass Harvest
2.4. Data Analysis
3. Results
3.1. Sucrose Concentration of Leaf and Culm Sap
3.2. Percentage Seed Set and Number of Caryopses Per Panicle
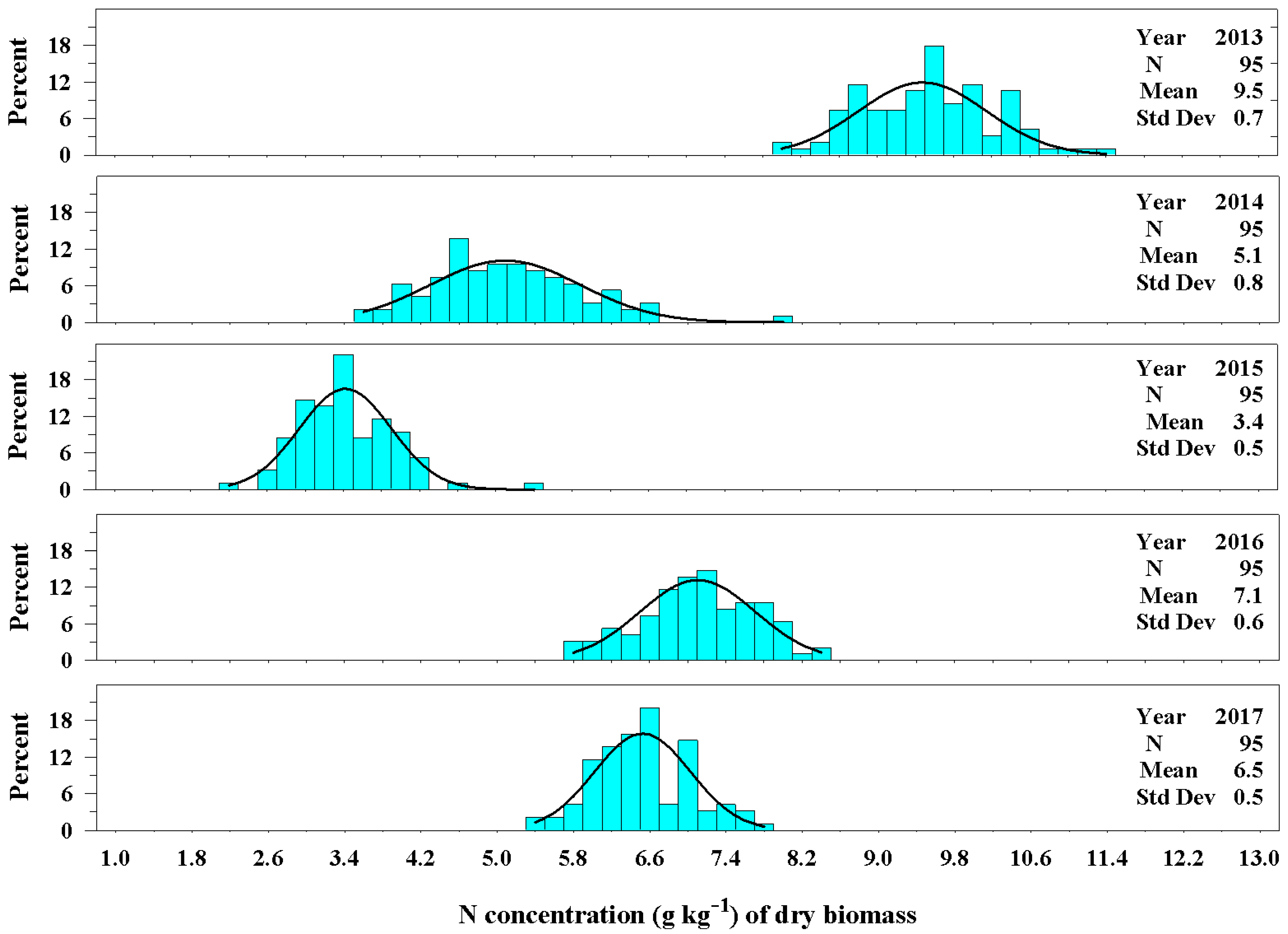
3.3. Biomass Harvested Per Plant; and C, N, and Ash Concentration of Biomass
4. Discussion
4.1. Sucrose Concentration of Leaf and Culm Sap
4.2. Percentage Seed Set and Number of Caryopses Per Panicle
4.3. Biomass Harvested Per Plant; and C, N, and Ash Concentration of Biomass
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Phillips, S.M. Saccharum ravennae (L.) L. Flora of China. 1994. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=242345942 (accessed on 10 January 2018).
- USDA National Plant Germplasm System. Taxon: Saccharum ravennae (L.) L. USDA Germplasm Resource Information Network. Available online: http://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?103325 (accessed on 8 January 2018).
- Valdés, B.; Scholz, H. The Euro+Med treatment of Gramineae—A generic synopsis and some new names. Willdenowia 2006, 36, 657–669. [Google Scholar] [CrossRef]
- CABI Invasive Species Compendium. Saccharum ravennae (Ravenna grass). Available online: http//www.cabi.org/isc/datasheet/109359 (accessed on 10 January 2018).
- Meyer, M.H. Ornamental Grasses in the United States. Hort. Rev. 2011, 39, 121–152. [Google Scholar] [CrossRef]
- Besse, P.; McIntyre, C.L.; Berding, N. Characterization of Erianthus sect. Ripidium and Saccharum germplasm (Andropogoneae-Saccharinae) using RFLP markers. Euphytica 1997, 93, 283–292. [Google Scholar] [CrossRef]
- Janaki-Ammal, E.K. Intergeneric hybrids of Saccharum. J. Genet. 1941, 41, 217–253. [Google Scholar] [CrossRef]
- Hattori, T.; Shiotsu, F.; Doi, T.; Morita, S. Suppression of tillering in Erianthus ravennae (L.) Beauv. due to drought stress at establishment. Plant Prod. Sci. 2010, 13, 252–255. [Google Scholar] [CrossRef]
- Palmer, I.E.; Gehl, R.J.; Ranney, T.G.; Touchel, D.; George, N. Biomass yield, nitrogen response, and nutrient uptake of perennial bioenergy grasses in North Carolina. Biomass Bioenergy 2014, 63, 218–228. [Google Scholar] [CrossRef]
- Springer, T.L.; Goldman, J.J. Germination of Saccharum ravennae (L.) L. (Poaceae) Caryopses and Intact Spikelets. Crop Sci. 2016, 56, 682–688. [Google Scholar] [CrossRef]
- Shimura, K.; Kawatake, M.; Nishimura, G.; Okamoto, K. Studies on utilization of Ravenna grass (Erianthus ravennae) as a forage crop. Bull. Tokai-Kinki Natl. Agric. Exp. Stat. 1973, 26, 71–78. [Google Scholar]
- Shedayi, A.A.; Begum, S.; Sadia, S.; Xu, M.; Ilihi, I. Plant species consumed by Ibex and chemical analysis of Saccharum ravennae L. from three different locations of Gilgit, Pakistan. J. Environ. Agric. Sci. 2016, 9, 21–27. [Google Scholar]
- Nozoye, T.; Aung, S.A.; Masuda, H.; Nakanishi, H.; Nishizawa, N.K. Bioenergy grass [Erianthus ravennae (L.) Beauv.] secretes two members of mugineic acid family phytosidrophores which involved in their tolerance to Fe deficiency. Soil Sci. Plant Nutr. 2017, 63, 543–552. [Google Scholar] [CrossRef]
- SAS Institute, Inc. SAS/STAT® User’s Guide, version 9.4; SAS Institute, Inc.: Cary, NC, USA, 2010. [Google Scholar]
- Sacheva, M.; Mann, A.P.S.; Batta, S.K. Sucrose metabolism and expression of key enzyme activities in low and high sucrose storing sugarcane genotypes. Sugar Tech. 2003, 5, 265–271. [Google Scholar] [CrossRef]
- Glasziou, K.T.; Gayler, K.R. Storage of sugars in stalks of sugar cane. Bot. Rev. 1972, 38, 471–488. [Google Scholar] [CrossRef]
- Janaki-Ammal, E.K. Cytogentics analysis of Saccharum spontaneum chromosome studies in some Indian forms. Indian J. Agric. Sci. 1936, 6, 1–8. [Google Scholar]
- Breaux, R.D. Breeding to enhance sucrose content of sugarcane varieties in Louisiana. Field Crops Res. 1984, 9, 59–67. [Google Scholar] [CrossRef]
- Springer, T.L.; Dewald, C.L.; Sims, P.L.; Gillen, R.L. How does plant population density affect the forage yield of eastern gamagrass? Crop Sci. 2003, 43, 2206–2211. [Google Scholar] [CrossRef]
- Springer, T.L. Effect of nitrogen fertilization and residual nitrogen on biomass yield of switchgrass. Bioenergy Res. 2017, 10, 648–656. [Google Scholar] [CrossRef]
- Heaton, E.; Voight, T.; Long, S.P. A quantitative review comparing the yields of two candidate C4 perennial biomass crops in relation to nitrogen, temperature, and water. Biomass Bioenergy 2004, 27, 21–30. [Google Scholar] [CrossRef]
- Monti, A.; Virgilio, N.D.; Venturi, G. Mineral composition and ash content of six major energy crops. Biomass Bioenergy 2008, 32, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, B.M. Physical properties of biomass. In Biomass Handbook; Kitani, O., Hall, C.W., Eds.; Gordon & Breach: New York, NY, USA, 1989; pp. 860–891. [Google Scholar]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Urquiaga, S.; Cruz, K.H.S.; Boddy, R.M. Contribution of nitrogen fixation to sugar cane: Nitrogen-15 and nitrogen-balance estimates. Soil Sci. Soc. Am. J. 1992, 56, 105–114. [Google Scholar] [CrossRef]
- Cherney, J.H.; Baker, E.V. Ash Content of Grasses for Biofuel; Bioenergy Information Sheet #5; Cornell University Cooperative Extension: Ithaca, NY, USA, 2006. [Google Scholar]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]








© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Springer, T.L. Variation of Agronomic Traits of Ravenna Grass and Its Potential as a Biomass Crop. Agronomy 2018, 8, 70. https://doi.org/10.3390/agronomy8050070
Springer TL. Variation of Agronomic Traits of Ravenna Grass and Its Potential as a Biomass Crop. Agronomy. 2018; 8(5):70. https://doi.org/10.3390/agronomy8050070
Chicago/Turabian StyleSpringer, Tim L. 2018. "Variation of Agronomic Traits of Ravenna Grass and Its Potential as a Biomass Crop" Agronomy 8, no. 5: 70. https://doi.org/10.3390/agronomy8050070



