Extension of Aquaponic Water Use for NFT Baby-Leaf Production: Mizuna and Rocket Salad
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Set-Up
2.2. Plant Material and Samplings
2.3. Water Quality Monitoring
2.4. Extraction of Phenols for Analysis
2.5. Determining Total Phenols Using the Folin–Ciocalteu Assay
2.6. Determining Total Antioxidant Activity Using Ferric Reducing Antioxidant Power
2.7. Quantitative Determination of Anions and Cations Using Ion Chromatography (IC)
2.8. Statistical Analysis
3. Results
3.1. Nutrient Solutions
3.2. Vegetables Species
4. Discussion
4.1. Water Quality
4.2. Vegetable Species
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Rakocy, J.; Shultz, R.C.; Bailey, D.S.; Thoman, E.S. Aquaponic production of tilapia and basil: Comparing a batch and staggered cropping system. In Proceedings of the South Pacific Soilless Culture Conference (SPSCC), Palmerston North, New Zealand, 10–13 February 2003; Volume 648, pp. 63–69. [Google Scholar]
- Junge, R.; König, B.; Villarroel, M.; Komives, T.; Jijakli, M.H. Strategic Points in Aquaponics. Water 2017, 9, 182. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Junge, R.; Schmautz, Z.; Sambo, P.; Borin, M. Hydroponic system sand water management in aquaponics: A review. Ital. J. Agron. 2018, 13, 1–11. [Google Scholar]
- Graber, A.; Junge, R. Aquaponic Systems: Nutrient recycling from fish wastewater by vegetable production. Desalination 2009, 246, 147–156. [Google Scholar] [CrossRef]
- Shete, A.P.; Verma, A.K.; Chadha, N.K.; Prakash, C.; Peter, R.M.; Ahmad, I.; Nuwansi, K.K.T. Optimization of hydraulic loading rate in aquaponic system with Common carp (Cyprinus carpio) and Mint (Mentha arvensis). Aquacult. Eng. 2016, 72, 53–57. [Google Scholar] [CrossRef]
- Espinosa Moya, E.A.; Angel Sahagún, C.A.; Mendoza Carrillo, J.M.; Albertos Alpuche, P.J.; Álvarez-González, C.A.; Martínez-Yáñez, R. Herbaceous plants as part of biological filter for aquaponics system. Aquacult. Res. 2016, 47, 1716–1726. [Google Scholar] [CrossRef]
- Diver, S. Aquaponics-Integration of Hydroponics with Aquaculture; Attra: Melbourne, Australia, 2000. [Google Scholar]
- Lennard, W. A New Look at NFT Aquaponics. Aquapon. J. 2010, 56, 4. [Google Scholar]
- Goddek, S.; Espinal, C.A.; Delaide, B.; Jijakli, M.H.; Schmautz, Z.; Wuertz, S.; Keesman, K.J. Navigating towards decoupled aquaponic systems: A system dynamics design approach. Water 2016, 8, 303. [Google Scholar] [CrossRef]
- Al-Hafedh, Y.S.; Alam, A.; Beltagi, M.S. Food production and water conservation in a recirculating aquaponic system in Saudi Arabia at different ratios of fish feed to plants. J. World Aquacult. Soc. 2008, 39, 510–520. [Google Scholar] [CrossRef]
- Bittsanszky, A.; Uzinger, N.; Gyulai, G.; Mathis, A.; Junge, R.; Kotzen, B.; Komives, T. Nutrient supply of plants in aquaponic systems. Ecocycles 2016, 2, 17–20. [Google Scholar] [CrossRef]
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; Kotzen, B. Towards commercial aquaponics: A review of systems, designs, scales and nomenclature. Aquacult. Int. 2018, 1, 30. [Google Scholar] [CrossRef]
- Goddek, S.; Keesman, K.J. The necessity of desalination technology for designing and sizing multi-loop aquaponics systems. Desalination 2018, 428, 76–85. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Gonnella, M.; Serio, F.; Conversa, G.; Santamaria, P. Yield and quality of lettuce grown in floating system using different sowing density and plant spatial arrangements. In Proceedings of the VI International Symposium on Protected Cultivation in Mild Winter Climate: Product and Process Innovation, 5–8 March 2002; Volume 614, pp. 687–692. [Google Scholar]
- Subhasree, B.; Baskar, R.; Keerthana, R.L.; Susan, R.L.; Rajasekaran, P. Evaluation of antioxidant potential in selected green leafy vegetables. Food Chem. 2009, 115, 1213–1220. [Google Scholar] [CrossRef]
- Saini, R.K.; Ko, E.Y.; Keum, Y.S. Minimally processed ready-to-eat baby-leaf vegetables: Production, processing, storage, microbial safety, and nutritional potential. Food Rev. Int. 2017, 33, 644–663. [Google Scholar] [CrossRef]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D. HydroBuddy: An Open Source Nutrient Calculator for Hydroponics and 464 General Agriculture, v1.5. 2013. Available online: http://scienceinhydroponics.com (accessed on 15 February 2017).
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bindraban, P.S.; Dimkpa, C.; Nagarajan, L.; Roy, A.; Rabbinge, R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol. Fert. Soils 2015, 51, 897–911. [Google Scholar] [CrossRef]
- Silberbush, M.; Ben-Asher, J. Simulation study of nutrient uptake by plants from soilless cultures as affected by salinity buildup and transpiration. Plant Soil 2001, 233, 59–69. [Google Scholar] [CrossRef]
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Jijakli, M.H. Lettuce (Lactuca sativa L. Var. Sucrine) growth performance in complemented aquaponic solution outperforms hydroponics. Water 2016, 8, 467. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen transformations in aquaponic systems: A review. Aquacult. Eng. 2017, 76, 9–19. [Google Scholar] [CrossRef]
- Cerozi, B.S.; Fitzsimmons, K. Phosphorus dynamics modeling and mass balance in an aquaponics system. Agric. Syst. 2017, 153, 94–100. [Google Scholar] [CrossRef]
- Roosta, H.R.; Hamidpour, M. Effects of foliar application of some macro-and micro-nutrients on tomato plants in aquaponic and hydroponic systems. Sci. Hortic. 2011, 129, 396–402. [Google Scholar] [CrossRef]
- Roosta, H.R. Effects of foliar spray of K on mint, radish, parsley and coriander plants in aquaponic system. J. Plant Nutr. 2014, 37, 2236–2254. [Google Scholar] [CrossRef]
- Pineda-Pineda, J.; Miranda-Velázquez, I.; Rodríguez-Pérez, J.E.; Ramírez-Arias, J.A.; Pérez-Gómez, E.A.; García-Antonio, I.N.; Morales-Parada, J.J. Nutrimental balance in aquaponic lettuce production. Acta Hortic. 2017, 1170, 1093–1100. [Google Scholar] [CrossRef]
- Urrestarazu, M.; del Carmen Salas, M.; Valera, D.; Gómez, A.; Mazuela, P.C. Effects of heating nutrient solution on water and mineral uptake and early yield of two cucurbits under soilless culture. J. Plant Nutr. 2008, 31, 527–538. [Google Scholar] [CrossRef]
- Amalfitano, C.; Del Vacchio, L.; Somma, S.; Cuciniello, A.; Caruso, G. Effects of cultural cycle and nutrient solution electrical conductivity on plant growth, yield and fruit quality of “Friariello” pepper grown in hydroponics. Hort. Sci. 2017, 44, 91–98. [Google Scholar] [CrossRef]
- Bergstrand, K.J.; Hultin, S. Development of strategies for hydroponic cultivation in vertical systems. Acta Hortic. 2014, 1034, 149–156. [Google Scholar] [CrossRef]
- D’Imperio, M.; Renna, M.; Cardinali, A.; Buttaro, D.; Serio, F.; Santamaria, P. Calcium biofortification and bioaccessibility in soilless “baby leaf” vegetable production. Food Chem. 2016, 213, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, P.; Elia, A.; Serio, F. Effect of solution nitrogen concentration on yield, leaf element content, and water and nitrogen use efficiency of three hydroponically-grown rocket salad genotypes. J. Plant Nutr. 2002, 25, 245–258. [Google Scholar] [CrossRef]
- Tuncay, Ö.; Eşiyok, D.; Yağmur, B.; Okur, B. The effect of nitrogen sources on yield and quality of salad rocket grown in different months of the year. J. Plant Nutr. 2011, 34, 477–491. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J. Agric. Food Chem. 2008, 56, 2330–2340. [Google Scholar] [CrossRef] [PubMed]
- Maucieri, C.; Nicoletto, C.; Schmautz, Z.; Sambo, P.; Komives, T.; Borin, M.; Junge, R. Vegetable Intercropping in a Small-Scale Aquaponic System. Agronomy 2017, 7, 63. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, Z.; Meng, H.; Ahmad, I.; Zhao, H. Growth, yield and quality of spring tomato and physicochemical properties of medium in a tomato/garlic intercropping system under plastic tunnel organic medium cultivation. Sci. Hortic. 2014, 170, 159–168. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Gonnella, M. Changes in nitrate accumulation and growth of endive plants during the light period as affected by nitrogen level and form. J. Plant Nutr. 1997, 20, 1255–1266. [Google Scholar] [CrossRef]
- Santamaria, P.; Gonnella, M.; Elia, A.; Parente, A.; Serio, F. Ways of reducing rocket salad nitrate content. Acta Hort. 2001, 548, 529–536. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Caruso, G.; Conti, S.; La Rocca, G. Influence of crop cycle and nitrogen fertilizer form on yield and nitrate content in different species of vegetables. Adv. Hort. Sci. 2011, 25, 81–89. [Google Scholar]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar] [CrossRef]
- Jakobsen, S.T. Interaction between plant nutrients: III. Antagonism between potassium, magnesium and calcium. Acta Agric. Scand. B Soil Plant Sci. 1993, 43, 1–5. [Google Scholar] [CrossRef]








| Nutrient Solution | N-NH4 | N-NO2 | N-NO3 | K | P-PO43 | Ca | S-SO42− | Mg | pH | EC |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg L−1) | (µS cm−1) | |||||||||
| HC | 0 | 0 | 65 | 120 | 25 | 66 | 23.4 | 20 | 7.87 | 1718 |
| APW | 0.075 | 0.023 | 63.5 | 0.078 | 1.55 | 66 | 27.6 | 21 | 7.79 | 824 |
| CAPW | 0.075 | 0.023 | 63.5 | 120 | 25 | 66 | 23.4 | 20 | 7.13 | 1680 |
| Vegetable Treatments | Plant Density | Abbreviation |
|---|---|---|
| Mizuna high density | 3000 plants m−2 | Mhd |
| Rocket salad high density | Rhd | |
| Intercropping high density | Mihd Rihd | |
| Intercropping low density | 1500 plants m−2 | Mild Rild |
| Treatments | Parameters | 1st Cycle | 2nd Cycle | p Value | Interaction Cycle × Water Treatment | Interaction Cycle × Vegetable Treatment |
|---|---|---|---|---|---|---|
| water | pH | ns | ns | 0.1928 | ns | ns |
| EC | ns | ns | 0.3516 | ns | ns | |
| NO3-N | ns | ns | 0.2968 | ns | ns | |
| NH4-N | ns | ns | 0.3862 | ns | ns | |
| pH | ns | ns | 0.2549 | ns | ns | |
| K | ns | ns | 0.4851 | ns | ns | |
| S | ns | ns | 0.4118 | ns | ns | |
| Ca | ns | ns | 0.3972 | ns | ns | |
| Mg | ns | ns | 0.3319 | ns | ns | |
| plant height | ns | ns | 0.4528 | ns | ns | |
| chlorophyll content | ns | ns | 0.2516 | ns | ns | |
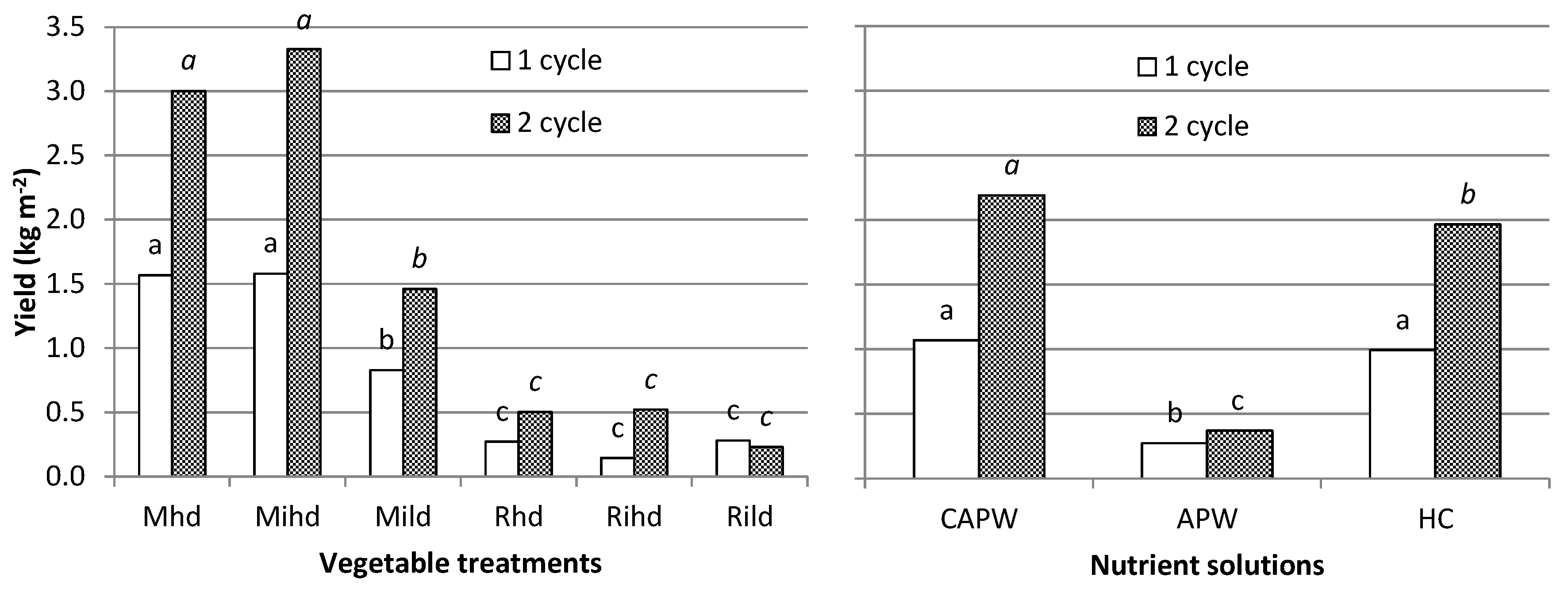
| yield | b | a | 0.0018 | ns | ns | |
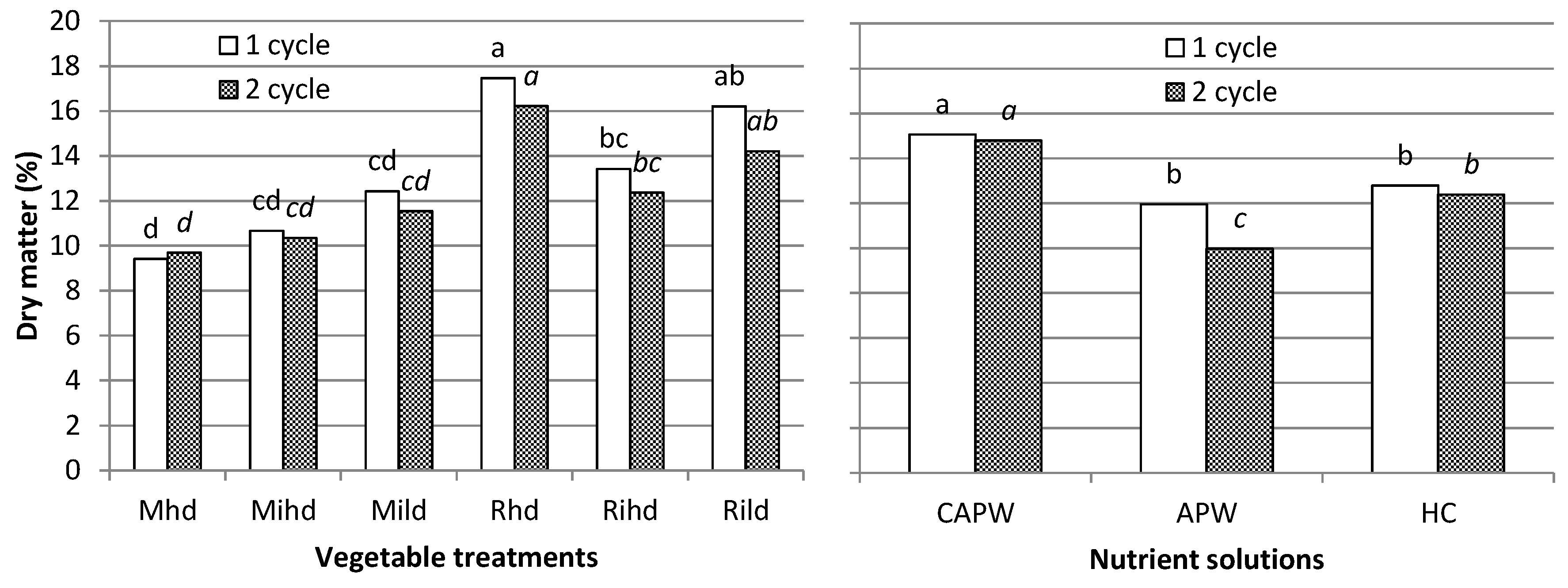
| dry matter | ns | ns | 0.1497 | ns | ns | |
| antiox compounds | ns | ns | 0.2637 | ns | ns | |
| ions concentration | ns | ns | 0.3794 | ns | ns | |
| vegetables | plant height | ns | ns | 0.2153 | ns | ns |
| chlorophyll content | ns | ns | 0.1845 | ns | ns | |
| yield | b | a | 0.0028 | ns | ns | |
| dry matter | ns | ns | 0.1738 | ns | ns | |
| antiox compounds | a | b | 0.0037 | ns | ns | |
| ions concentration | a | b | 0.0012 | ns | ns |
| Antioxidant Capacity (g Fe2+E kg−1 dw) | Total Phenols (g GAE kg−1 dw) | Vitamin C (g kg−1 dw) | |||||
|---|---|---|---|---|---|---|---|
| 1 Cycle | 2 Cycle | 1 Cycle | 2 Cycle | 1 Cycle | 2 Cycle | ||
| Nutrient solutions | |||||||
| HC | 50.4 ± 1.3 a | 45.2 ± 4.7 a | 5.12 ± 0.98 a | 4.90 ± 0.48 a | 3.31 ± 0.12 b | 3.13 ± 0.14 b | |
| APW | 49.6 ± 4.6 a | 46.2 ± 2.9 a | 5.93 ± 0.76 a | 5.55 ± 0.57 a | 4.15 ± 0.18 a | 3.84 ± 0.16 a | |
| CAPW | 39.0 ± 5.4 b | 35.6 ± 3.3 b | 4.34 ± 0.59 b | 4.20 ± 0.43 b | 3.69 ± 0.13 b | 3.25 ± 0.12 b | |
| Vegetable treatments | |||||||
| Mhd | 35.7 ± 3.2 c | 32.0 ± 4.5 b | 3.75 ± 0.27 c | 3.46 ± 0.22 b | 3.40 ± 0.17 c | 2.82 ± 0.09 c | |
| Mihd | 46.9 ± 1.6 a | 40.9 ± 2.2 a | 5.53 ± 0.36 ab | 4.57 ± 0.19 a | 3.35 ± 0.09 c | 2.53 ± 0.15 c | |
| Mild | 44.9 ± 2.5 a | 41.4 ± 3.5 a | 4.82 ± 0.49 b | 4.14 ± 0.31 ab | 3.24 ± 0.15 c | 2.38 ± 0.13 c | |
| Rhd | 49.8 ± 3.2 a | 38.8 ± 3.0 ab | 5.92 ± 0.83 a | 4.84 ± 0.51 a | 5.37 ± 0.18 a | 3.76 ± 0.28 a | |
| Rihd | 47.9 ± 2.8 a | 35.2 ± 2.3 b | 6.26 ± 0.61 a | 4.65 ± 0.12 a | 4.80 ± 0.10 b | 3.44 ± 0.21 b | |
| Rild | 41.4 ± 2.4 b | 33.3 ± 3.2 b | 6.20 ± 0.27 a | 4.47 ± 0.11 a | 3.43 ± 0.08 c | 2.37 ± 0.16 c | |
| WxV | Ns | ns | ns | ns | ns | ns | |
| Cl− | NO2− | NO3− | PO43− | SO42− | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1 dw) | ||||||||||
| Nutrient solutions | ||||||||||
| HC | 2985 b | 7.24 b | 4214 b | 7664 a | 9539 b | 1914 b | 327 b | 35,094 a | 6598 b | 12,136 b |
| APW | 5473 a | 130 a | 37,753 a | 4808 b | 31,774 a | 4336 a | 1821 a | 11,999 c | 8010 a | 46,363 a |
| CAPW | 3572 b | 145 a | 1819 b | 7998 a | 9468 b | 1961 b | 324 b | 24,719 b | 4662 c | 12,657 b |
| Vegetables treatments | ||||||||||
| Mhd | 4647 a | 40.2 b | 26,576 a | 6326 | 22,740 a | 3291 a | 896 | 28,039 a | 4982 | 22,044 |
| Mihd | 3402 b | 30.7 b | 20,440 ab | 5922 | 20,126 a | 2604 b | 944 | 23,529 ab | 4945 | 20,908 |
| Mild | 3218 b | 43.0 b | 22,008 ab | 4899 | 17,278 ab | 2732 b | 795 | 20,497 bc | 5108 | 21,949 |
| Rhd | 3950 ab | 200 a | 12,704 b | 4797 | 7232 c | 2503 b | 846 | 16,649 c | 4850 | 21,911 |
| Rihd | 4536 a | 101 a | 8893 b | 6708 | 10,573 bc | 2517 b | 697 | 25,132 ab | 5642 | 22,249 |
| Rild | 3987 ab | 207 a | 13,387 b | 5126 | 9211 c | 2319 b | 683 | 20,029 bc | 5558 | 25,638 |
| WxV | * | ns | * | * | * | * | * | ns | ns | ns |
| Cl− | NO2− | NO3− | PO43− | SO42− | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1 dw) | ||||||||||
| Nutrient solutions | ||||||||||
| HC | 3185 b | 4.98 b | 4014 b | 8010 a | 10,053 b | 2470 b | 375 b | 40,086 a | 7272 b | 14,152 b |
| APW | 5637 a | 107 a | 34,601 a | 5486 b | 35,070 a | 4674 a | 2391 a | 13,317 c | 8836 a | 51,625 a |
| CAPW | 3666 b | 119 a | 1357 c | 8228 a | 9996 b | 2259 b | 402 b | 26,381 b | 5718 c | 16,511 b |
| Vegetables treatments | ||||||||||
| Mhd | 4797 a | 78.2 b | 21,502 a | 8896 ab | 30,572 a | 4029 a | 1040 a | 33,935 a | 9184 | 29,450 |
| Mihd | 3602 b | 38.3 b | 17,582 b | 8256 ab | 26,978 a | 3102 ab | 1270 a | 29,919 ab | 8649 | 29,370 |
| Mild | 3652 b | 53.0 b | 14,650 b | 7893 b | 22,900 ab | 3314 ab | 1227 a | 26,301 b | 7900 | 27,693 |
| Rhd | 4146 ab | 254 b | 8778 c | 8103 ab | 13,854 b | 2993 b | 1132 a | 23,473 b | 8378 | 27,467 |
| Rihd | 4822 a | 163 a | 6239 c | 9554 a | 16,475 b | 3171 ab | 873 b | 30,006 ab | 8374 | 28,639 |
| Rild | 4279 ab | 256 a | 8325 c | 7908 b | 13,863 b | 2653 b | 875 b | 25,681 b | 8622 | 29,570 |
| WxV | * | ns | * | * | * | * | * | ns | ns | ns |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoletto, C.; Maucieri, C.; Mathis, A.; Schmautz, Z.; Komives, T.; Sambo, P.; Junge, R. Extension of Aquaponic Water Use for NFT Baby-Leaf Production: Mizuna and Rocket Salad. Agronomy 2018, 8, 75. https://doi.org/10.3390/agronomy8050075
Nicoletto C, Maucieri C, Mathis A, Schmautz Z, Komives T, Sambo P, Junge R. Extension of Aquaponic Water Use for NFT Baby-Leaf Production: Mizuna and Rocket Salad. Agronomy. 2018; 8(5):75. https://doi.org/10.3390/agronomy8050075
Chicago/Turabian StyleNicoletto, Carlo, Carmelo Maucieri, Alex Mathis, Zala Schmautz, Tamas Komives, Paolo Sambo, and Ranka Junge. 2018. "Extension of Aquaponic Water Use for NFT Baby-Leaf Production: Mizuna and Rocket Salad" Agronomy 8, no. 5: 75. https://doi.org/10.3390/agronomy8050075
APA StyleNicoletto, C., Maucieri, C., Mathis, A., Schmautz, Z., Komives, T., Sambo, P., & Junge, R. (2018). Extension of Aquaponic Water Use for NFT Baby-Leaf Production: Mizuna and Rocket Salad. Agronomy, 8(5), 75. https://doi.org/10.3390/agronomy8050075









