Response of Common Bean Genotypes Grown in Soil with Normal or Limited Moisture, with Special Reference to the Nutrient Phosphorus
Abstract
1. Introduction
Theoretical Perception of Drought
2. Materials and Methods
2.1. Data Collection
2.1.1. Climate Parameters
2.1.2. Atmospheric Temperatures
2.1.3. Drought Intensity Index (DII)
2.1.4. Determination of Plant Growth
2.1.5. Bean Plant Phenology
2.1.6. Bean Yield Components
2.2. Data Analysis
3. Results
3.1. Drought Intensity
3.2. Growth Parameters
3.2.1. Leaf Area Index (LAI)
3.2.2. Shoot Biomass
3.2.3. Harvest Index (HI)
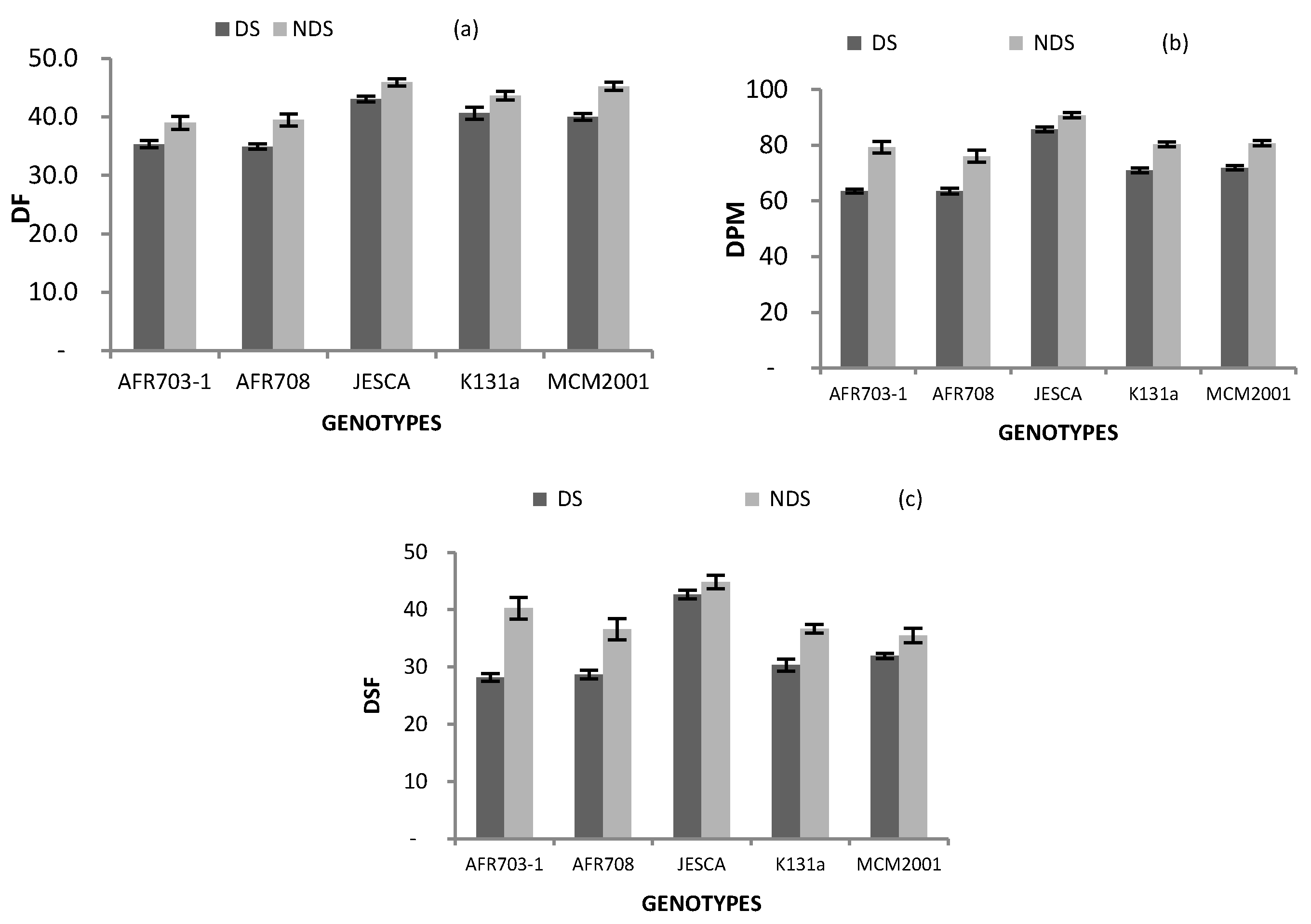
3.3. Plant Phenology
3.4. Yield and Its Components
3.5. Correlation of Grain Yield with Measured Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beebe, E.S.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for Adaptation to drought. Front. Physiol. 2013, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- CIAT. Annual Report 2008: Outcome Line SBA-1: Improved Beans for the Developing World; International Center for Tropical Agriculture (CIAT): Cali, Colombia, 2008. [Google Scholar]
- Buruchara, R.; Mukankusi, C.; Ampofo, K.O. Bean Desease and Pest Identification and Mangement. Handbooks for Small-Scale Seed Producers; International Centre for Tropical Agriculture (CIAT): Kampala, Uganda, 2011; Volume 371, p. 67. [Google Scholar]
- Wortmann, S.C.; Kirkby, A.R.; Eledu, AC.; Allen, J.D. (Eds.) Atlas of Common Bean (Phasealus vulgaris L.) Production in Africa; International Centre for Tropical Agriculture (CIAT): Cali, Colombia, 2004. [Google Scholar]
- Namugwanya, M.; Tenywa, J.S.; Otabbong, E.; Mubiru, D.N.; Masamba, T.A. Development of commom bean (Phaseolus vulgaris L.) production under low soil phosphorus and drought in Sub-Saharan Africa: A review. J. Sustain. Dev. 2014, 7, 128–139. [Google Scholar]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root Structure and Functioning for Efficient Acquisition of Phosphorus: Matching Morphological and Physiological Traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [PubMed]
- Suriyagoda, L.D.B.; Ryan, M.H.; Renton, M.; Lambers, H. Plant responses to limited moisture and phosphorus availability: A meta-analysis. Adv. Agron. 2014, 124, 143–200. [Google Scholar]
- Jin, J.; Lauricella, D.; Armstrong, R.; Sale, P.; Tang, C. Phosphorus application and elevated CO2 enhance drought tolerance in field pea grown in a phosphorus-deficient vertisol. Ann. Bot. 2014, 116, 975–985. [Google Scholar] [PubMed]
- CIAT. Annual Report 2006: Bean Improvement for the Tropics; International Center for Tropical Agriculture (CIAT): Cali, Colombia, 2006. [Google Scholar]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nut. 2012, 12, 547–562. [Google Scholar] [CrossRef]
- Kumar, P.V.; Bindi, M.; Crisci, A.; Maracchi, G. Detection of variations in precipitation at different time scales of twentieth century at three locations of Italy. Weath. Clim. Extrem. 2013, 2, 7–15. [Google Scholar] [CrossRef]
- Beebe, E.S.; Ramirez, J.; Jarvis, A.; Rao, M.I.; Mosquera, G.; Bueno, M.J.; Blair, W.M. Genetic improvement of common beans and the challenges of climate change. In Crop Adaptation to Climate Change, 1st ed.; Yadav, S.S., Redden, J.R., Hatfield, L.J., Lotze-Campen, H., Hall, E.A., Eds.; Blackwell Publishing Ltd.: Cali, Colombia, 2011; pp. 356–369. [Google Scholar]
- Keyantash, J.A.; Dracup, J.A. An aggregate drought index: Assessing drought severity based on fluctuations in the hydrologic cycle and surface water storage. Water Resour. Res. 2004, 40, 1–13. [Google Scholar] [CrossRef]
- Wilhelmi, V.O.; Hubbard, G.K.; Wiilhite, A.D. Spatial representation of agro-climatology in a study of agricultural drought. Int. J. Clim. 2002, 22, 1399–1414. [Google Scholar] [CrossRef]
- Sivakumar, V.K.M.; Motha, R.P.; Wilhite, D.A.; Wood, D.A. (Eds.) Agricultural Drought Indices. In Proceedings of the Expert Group Meeting, Murcia, Spain, 2–4 June 2010; World Meteorological Organization: Geneva, Switzerland, 2011; p. 197. [Google Scholar]
- Kisamba-Mugerwa, W. Rangelands management policy in Uganda. In Proceedings of the International Conference on Policy and Institutional Options for the Management of Rangelands in Dry Areas, Kampala, Uganda, 7–11 May 2001; pp. 6–11. [Google Scholar]
- Tabatabai, M.A. Agronomy Monograph 2. In Methods of Soil Analysis Part 2, 2nd ed.; Page, R., Miller, H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965; p. 577. [Google Scholar]
- Namugwanya, M.; Tenywa, J.S.; Otabbong, E.; Basamba, T.A. Root Architectural Development and Yield Sensitivity of Phosphorus Tolerant Common Bean under Low Soil Phosphorus and Drought. Int. J. Plant Soil Sci. 2016, 9, 1–10. [Google Scholar]
- Lunze, L.; Lunze, L.; Kimani, P.M.; Ngatoluwa, R.; Rabary, B.; Rachier, G.O.; Ugen, M.M.; Ruganza, V.; Awad elkarim, E.E. Bean improvement for low soil fertility adaptation in Eastern and Central Africa. In Advances in Integrated Soil Fertility Management in Sub-Saharan Africa: Challenges and Opportunities; Bationo, A., Waswa, B., Kihara, J., Kimetu, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 325–332. [Google Scholar]
- Brouwer, C.; Heibloem, M. Irrigation Water Management: Irrigation Water Needs; Food and Agriculture Organization of the United Nations: Rome, Italy, 1986. [Google Scholar]
- De Jesus, W.C.; do Vale, F.X.R.; Coelho, R.R.; Costa, L.C. Comparison of two methods for estimating leaf area index on common bean. Agron. J. 2001, 93, 989–991. [Google Scholar] [CrossRef]
- Acosta-Díaz, E.; Acosta-Gallegos, J.A.; Trejo-López, C.; Padilla-Ramírez, J.S.; Amador-Ramírez, M.D. Adaptation traits in dry bean cultivars grown under drought stress. Agric. Téc. Méx. 2009, 35, 416–425. [Google Scholar]
- Beebe, S.E.; Rao, I.M.; Cajiao, C.; Grajales, M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop Sci. 2008, 48, 582–592. [Google Scholar]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistics: A guide for non-statisticans. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.S. Some examples of statistical methods of research in agriculture and applied biology. Suppl. J. R. Statist. Soc. 1937, 4, 137–183. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS; SAGE Publication Inc.: Thiusands Oaks, CA, USA, 2009. [Google Scholar]
- Ott, L.R.; Longnecker, M. An Introduction to Statistical Methods and Data Analysis, 5th ed.; Duxburry Thomson Learning: Toronto, ON, Cadana, 2001. [Google Scholar]
- Salcedo, J.M. Regeneration guidelines: Common bean. In Crop Specific Regeneration Guidelines; Dulloo, M.E., Thormann, I., Jorge, M.A., Hanson, J., Eds.; CGIAR System-Wide Genetic Resource Programm: Rome, Italy, 2008. [Google Scholar]
- Klaedtke, S.M.; Cajiao, C.; Grajales, M.; Polanía, J.; Borrero, G.; Guerrero, A.; Rivera, M.; Rao, I.; Beebe, S.E.; Léon, J. Photosynthate remobilization capacity from drought-adapted common bean (Phaseolus vulgaris L.) lines can improve yield potential of interspecific populations within the secondary gene pool. J. Plant Breed. Crop Sci. 2012, 4, 49–61. [Google Scholar]




| Weather Parameters | Pooled Mean | Values * |
|---|---|---|
| DS | NDS | |
| Average daily mean min Temp (°C) | 18.2 | 17.6 |
| Average daily mean max Temp (°C) | 29.7 | 27.3 |
| Mean rainy days (#) | 25.5 | 43 |
| Total Rain received (mm season−1) (A) | 237 | 464 |
| Total water need (mm season−1) (B) | 393 | 380 |
| Difference (A-B) | (156) | 84 |
| Mean rainfall received (mm day−1) (C) | 2.37 | 4.65 |
| Mean water requirement (mm day−1) (D) | 3.93 | 3.80 |
| Difference (C-D) | (1.56) (0.0002) | 0.85 (0.15) |
| DII (%) = (C-D/D) × 100 | (40) | 22 † |
| Genotypes | DF | DPM | DSF |
|---|---|---|---|
| AFR703-1 | 37 §§§ | 71 §§§ | 34 |
| AFR708 | 37 §§§ | 70 §§§ | 33 |
| JESCA | 45 § | 88 §§§ | 44 §§§ |
| K131 a | 42 | 76 | 34 |
| MCM2001 | 43 | 76 | 34 |
| Drought Conition | |||
| DS | 39 | 71 | 33 |
| NDS | 43 | 81 | 39 |
| Difference | *** | *** | *** |
| (a) Statistical Correlation (r Values) of Grain Yield with Other Parameters Determined NDS Conditions in Uganda. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Grain Yield | Pods Plant−1 | Seeds Plant−1 | 100 Seeds Weight | LAI | Shoot Biomass | Yield Day−1 | HI |
| Grain yield | 1 | |||||||
| Pods plant−1 | 0.49 §§ | 1 | ||||||
| Seeds plant−1 | 0.09 | 0.27 § | 1 | |||||
| Weight of 100 seeds | 0.56 §§§ | −0.17 | −0.41 §§ | 1 | ||||
| LAI | 0.62 §§§ | 0.45 §§ | 0.05 | 0.62 §§§ | 1 | |||
| Shoot biomass | 0.36 § | 0.47 §§§ | 0.14 | 0.17 | 0.21 § | 1 | ||
| Yield day−1 | 0.99 §§§ | 0.45 §§ | 0.06 | 0.73 §§§ | 0.64 §§§ | 0.04 | ||
| HI | 0.72 §§§ | 0.22 § | 0.06 | 0.99 §§§ | 0.26 § | 0.62 §§§ | 0.69 §§§ | 1 |
| (b) Statistical Correlation (r Values) of Grain Yield with Other Parameters Determined in DS Conditions in Uganda. | ||||||||
| Parameters | Grain Yield | Pods Plant−1 | Seeds Plant−1 | 100 Seeds Weight | LAI | Shoot Biomass | Yield Day−1 | HI |
| Grain yield | - | |||||||
| Pods plant−1 | 0.51 *** | - | ||||||
| Seeds plant−1 | 0.31 * | 0.66 *** | - | |||||
| 100 seeds weight | 0.2 * | −0.51 *** | −0.31 * | - | ||||
| LAI | 0.16 | −0.38 ** | −0.56 *** | 0.51 *** | - | |||
| Shoot biomass | −0.01 | 0.15 | 0.16 | 0.17 | −0.31 * | - | ||
| Yield day−1 | 0.98 *** | 0.46 ** | 0.24 * | 0.24 * | 0.18 | −0.00 | - | |
| HI | 0.87 *** | 0.59 *** | 0.44 ** | 0.44 ** | 0.09 | −0.17 | 0.84 *** | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namugwanya, M.; Tenywa, J.S.; Otabbong, E. Response of Common Bean Genotypes Grown in Soil with Normal or Limited Moisture, with Special Reference to the Nutrient Phosphorus. Agronomy 2018, 8, 132. https://doi.org/10.3390/agronomy8080132
Namugwanya M, Tenywa JS, Otabbong E. Response of Common Bean Genotypes Grown in Soil with Normal or Limited Moisture, with Special Reference to the Nutrient Phosphorus. Agronomy. 2018; 8(8):132. https://doi.org/10.3390/agronomy8080132
Chicago/Turabian StyleNamugwanya, Margaret, John Stephen Tenywa, and Erasmus Otabbong. 2018. "Response of Common Bean Genotypes Grown in Soil with Normal or Limited Moisture, with Special Reference to the Nutrient Phosphorus" Agronomy 8, no. 8: 132. https://doi.org/10.3390/agronomy8080132
APA StyleNamugwanya, M., Tenywa, J. S., & Otabbong, E. (2018). Response of Common Bean Genotypes Grown in Soil with Normal or Limited Moisture, with Special Reference to the Nutrient Phosphorus. Agronomy, 8(8), 132. https://doi.org/10.3390/agronomy8080132




