Agronomic Advancement in Tillage, Crop Rotation, Soil Health, and Genetic Gain in Durum Wheat Cultivation: A 17-Year Canadian Story
Abstract
1. Introduction
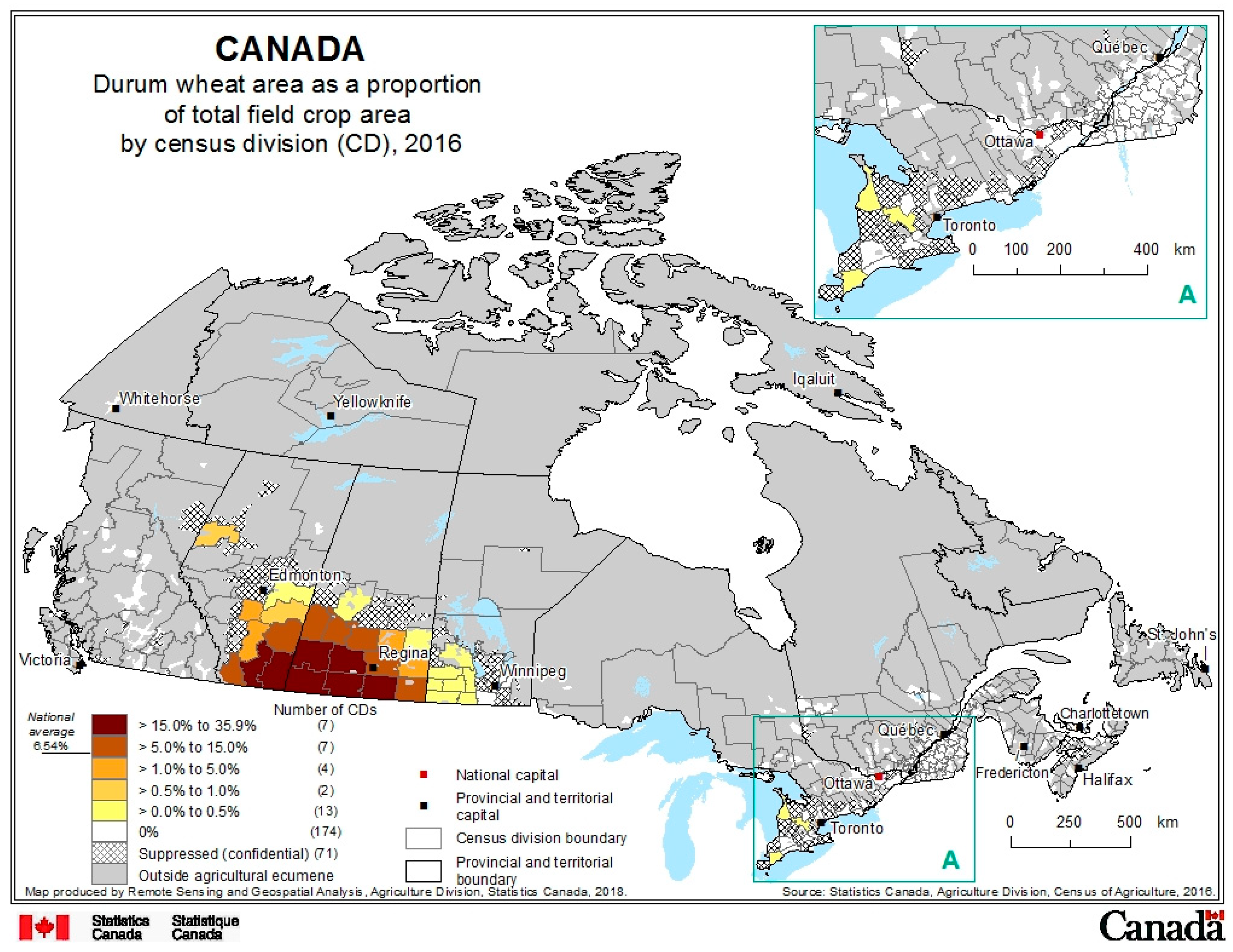
2. Production Background
3. Agronomic Advancement
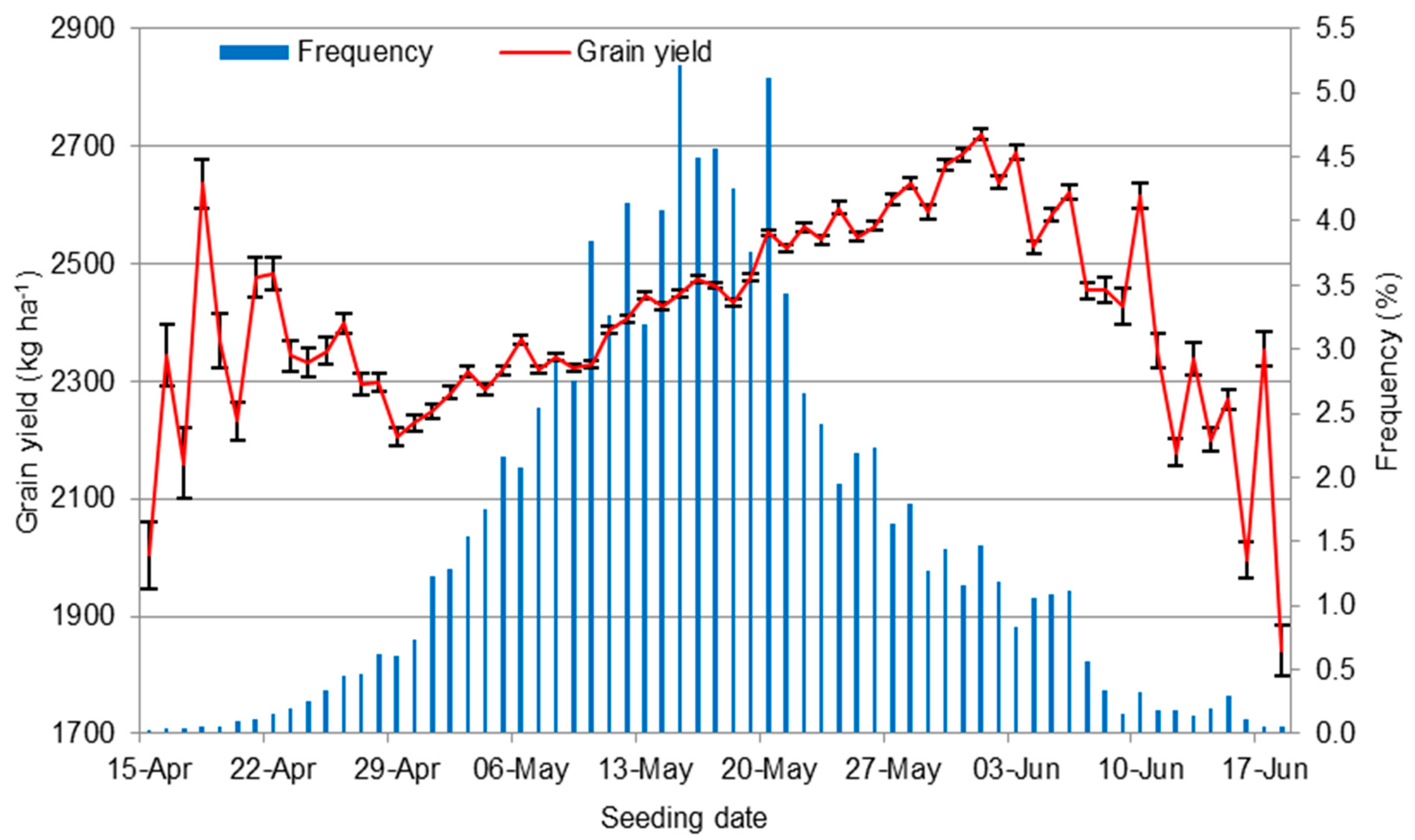
3.1. Decisions on Seeding Date and Seeding Rate
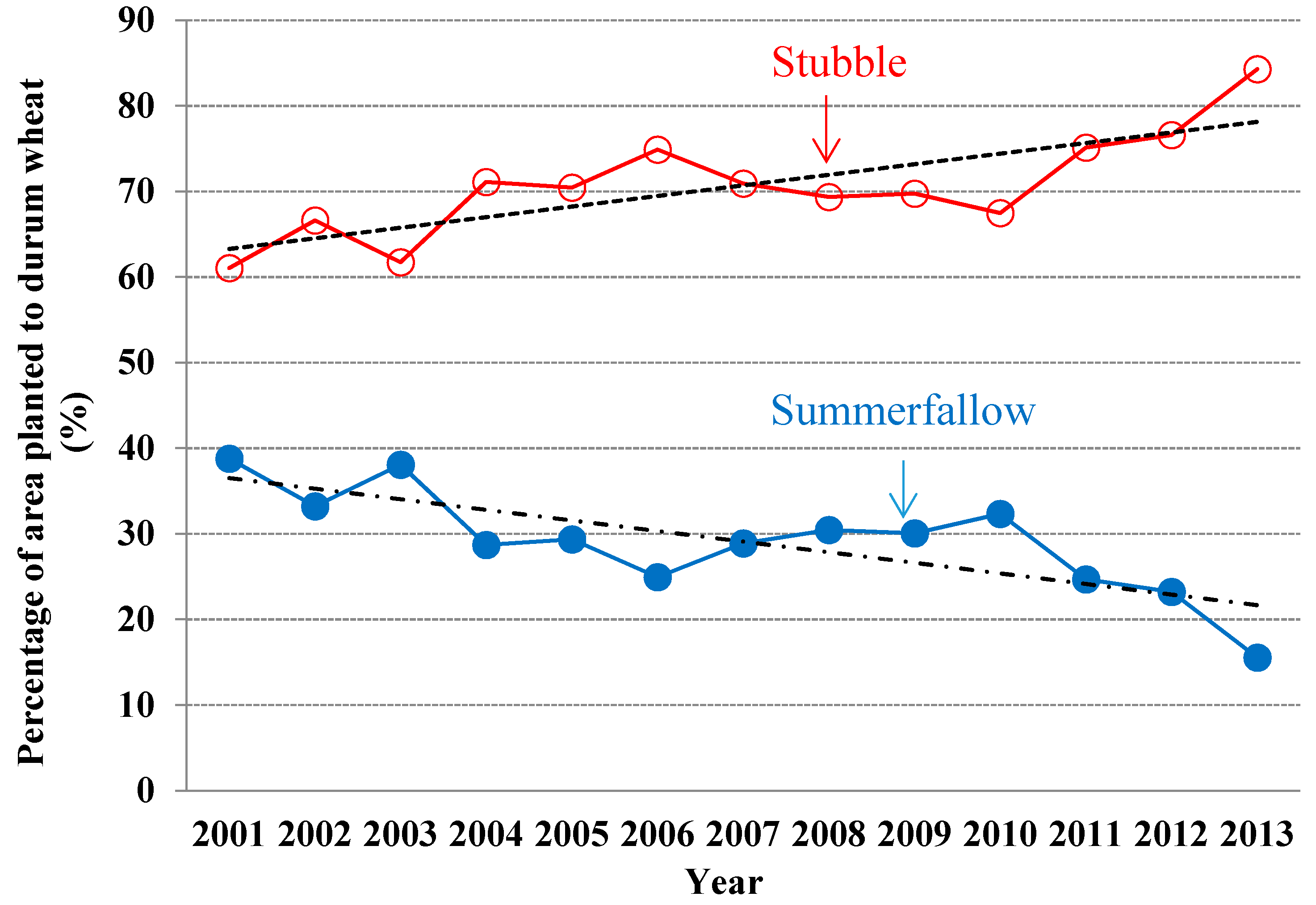
3.2. Selection of Land and Tillage
3.3. Diversification of Crop Rotations
3.3.1. Soil Water
3.3.2. Soil Nutrients
3.3.3. Soil Microbiome
3.4. Management of Soil Fertility
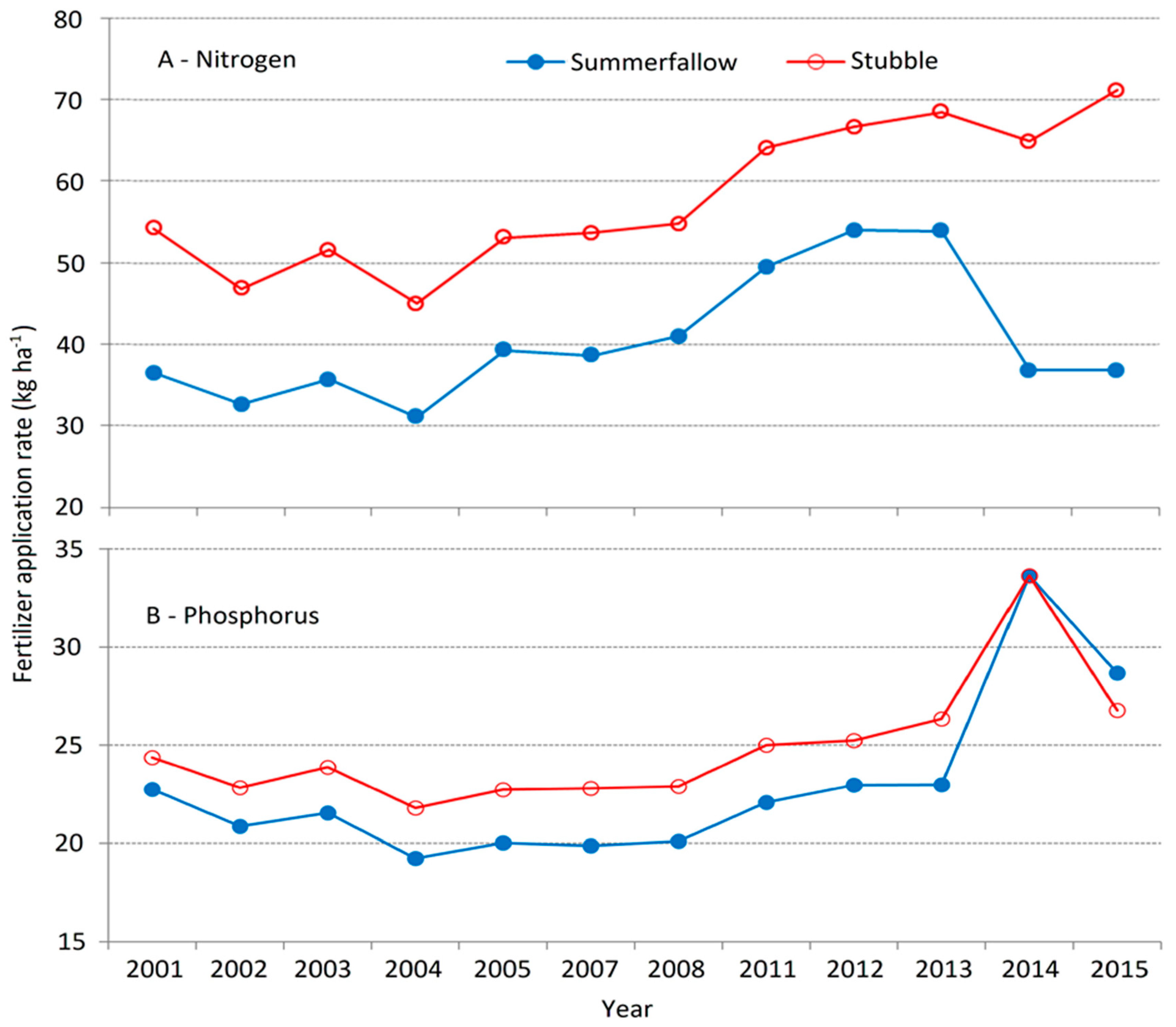
3.4.1. Nitrogen and Phosphorus
3.4.2. Fertilizer Management and Environmental Footprint
3.5. Management to Minimize Grain Cadmium
3.6. Optimizing Feedback Benefits from Soil Microbiomes
3.6.1. Crop Species and Genotypes
3.6.2. Pulse Termination
3.6.3. Soil N Effect
3.6.4. Pesticide Use
4. Identification of Constraints to Durum Wheat Production
5. Genetic Enhancement
5.1. Genetic Gain
5.2. Historical Change of Durum Wheat Cultivars
5.3. Breeding for Resistance to Abiotic Stresses
5.4. Breeding for Resistance to Biotic Stresses
5.5. Breeding for Quality Traits
6. Near-Term Agronomic Research Priorities
- (i)
- Define the association of durum wheat productivity and arbuscular mycorrhizal (AM) fungi in the soil. The AM fungi are a normal component of the Canadian prairie ecosystems where they assist plants for nutrient uptake and, thus, increase crop productivity. Research is needed to define whether the colonization of AM in increasing durum wheat yield is dependent on genotype and soil fertility, or whether increasing grain yield of durum wheat can be accomplished through direct manipulation of soil microbiome, or whether there are significant interactions between year/site and preceding crops, and the structure of AM fungal community colonizing wheat roots. The factors affecting soil AM fungal resources in Canadian prairie need to be clearly identified before undertaking any further directions;
- (ii)
- Assess whether breeding efforts can be taken to improve soil biological attributes through genotypes. Genetic variation exists in soil microbial community interactions among Canadian durum wheat genotypes and, more importantly, there is genetic variation in the influence of plant genotype on the soil microbial community on the performance of crop plants in rotation. However, it is unknown how this information may be used to build a strategy of selecting both the right preceding crop genotypes to engineer the beneficial soil microbial environments for subsequent durum wheat in a rotation. To our best knowledge, no direct selection for plant interaction with microbial community to improve durum wheat performance has been conducted on the Canadian prairie. To optimize the legendary effects of cropping systems rotation through managing soil microbial resources, we need multidisciplinary collaboration of durum breeders, agronomists, and soil biologists. The goals of this effort are to enhance the positive interaction between plant roots and soil microbial community, promote an effective feedback to the crop, and improve soil nutrient use efficiency through the improved genotypes;
- (iii)
- Search for effective approaches to enhance NUE in durum wheat cultivation. Crop diversification in rotations on the semiarid Canadian prairie offers some significant benefits to the agroecosystems, including increased systems productivity and enhanced resource use efficiency. This practice has been shown to provide farmers with an alternative practice to conserve soil moisture, replace traditional summerfallow, and increase long-term sustainability. However, studies investigating the direct effect of diversified crop rotations on the NUE of durum wheat are little to none. We suggest multidisciplinary research, including breeding efforts, agronomic practices, and in soil biology, is needed to find solutions to enhance NUE in durum wheat production; and finally
- (iv)
- Develop cultivar-specific cultivation systems for durum wheat production. There have been 23 cultivars released since 2010, introducing new traits such as solid stem, antibiosis to midge, imidazolinone tolerance, and a range of agronomic traits. There is a need to develop cultivar-specific management practices to optimize the productivity (yield, quality, biomass, and the impacts on soil and environment) for a cluster of cultivars with similar attributes. Positive outcomes can be achieved through a combination of mechanistic and systems-based modelling approaches using weather data, crop growth and development, soil and environmental conditions, and crop management practices.
7. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | arbuscular mycorrhizal fungi |
| DON | trichothecene deoxynivalenol |
| FHB | Fusarium head blight |
| GHG | greenhouse gas |
| OWBM | orange wheat blossom midge |
| NUE | nitrogen use efficiency |
| PHS | preharvest sprouting |
| VB | varietal blend |
| WUE | water use efficiency |
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.M.; Fischhoff, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D.; et al. Radically rethinking agriculture for the 21st century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C.; et al. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- FAOSTAT. FAO Statistical Yearbooks—World Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- International-Grain-Council. 2018. Available online: https://www.igc.int/en/default.aspx (accessed on 25 April 2018).
- Statistics-Canada. Durum Wheat Area as a Proportion of Total Field Crop Area by Census Division (CD), 2016; Statistics Canada: Ottawa, ON, Canada, 2018. Available online: http://www.statcan.gc.ca/pub/95-634-x/2017001/article/54904/catm-ctra-045-eng.htm (accessed on 25 April 2018).
- Statistics-Canada. Estimated Areas, Yield, Production, Average Farm Price and Total Farm Value of Principal Field Crops, in Metric and Imperial Units; Statistics Canada: Ottawa, ON, USA, 2018. Available online: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=10017 (accessed on 25 April 2018).
- Statistics-Canada. Principal Field Crop Areas; Statistics Canada: Ottawa, ON, USA, 2017. Available online: https://www.statcan.gc.ca (accessed on 25 April 2018).
- Gan, Y.T.; Miller, P.R.; McConkey, B.G.; Zentner, R.P.; Stevenson, F.C.; McDonald, C.L. Influence of diverse cropping sequences on durum wheat yield and protein in the semiarid northern Great Plains. Agron. J. 2003, 95, 245–252. [Google Scholar] [CrossRef]
- Cutforth, H.W.; Jones, K.; Lang, T.-A. Agroclimate of the Brown Soil Zone of Southwestern Saskatchewan; Publication 379MOO88; Semi-Arid Prairie Agricultural Research Centre, Agriculture and Agri-Food Canada, Swift Current: Indian Head, SK, Canada, 1993.
- Masud, M.B.; Khaliq, M.N.; Wheater, H.S. Projected changes to short- and long-duration precipitation extremes over the Canadian Prairie Provinces. Clim. Dyn. 2017, 49, 1597–1616. [Google Scholar] [CrossRef]
- Asong, Z.E.; Khaliq, M.N.; Wheater, H.S. Multisite multivariate modeling of daily precipitation and temperature in the Canadian Prairie Provinces using generalized linear models. Clim. Dyn. 2016, 47, 2901–2921. [Google Scholar] [CrossRef]
- Mooleki, S.P.; Gan, Y.; Lemke, R.L.; Zentner, R.P.; Hamel, C. Effect of green manure crops, termination method, stubble crops, and fallow on soil water, available N, and exchangeable P. Can. J. Plant Sci. 2016, 96, 867–886. [Google Scholar] [CrossRef]
- Zentner, R.P.; Campbell, C.A.; Biederbeck, V.O.; Selles, F.; Lemke, R.; Jefferson, P.G.; Gan, Y. Long-term assessment of management of an annual legume green manure crop for fallow replacement in the Brown soil zone. Can. J. Plant Sci. 2004, 84, 11–22. [Google Scholar] [CrossRef]
- Schnitzer, M.; McArthur, D.F.E.; Schulten, H.R.; Kozak, L.M.; Huang, P.M. Long-term cultivation effects on the quantity and quality of organic matter in selected Canadian prairie soils. Geoderma 2006, 130, 141–156. [Google Scholar] [CrossRef]
- Paré, M.C.; Lafond, J.; Pageau, D. Best management practices in Northern agriculture: A twelve-year rotation and soil tillage study in Saguenay-Lac-Saint-Jean. Soil Tillage Res. 2015, 150, 83–92. [Google Scholar] [CrossRef]
- Schillinger, W.F.; Young, D.L. Best Management Practices for Summer Fallow in the World’s Driest Rainfed Wheat Region. Soil Sci. Soc. Am. J. 2014, 78, 1707–1715. [Google Scholar] [CrossRef]
- Zavattaro, L.; Costamagna, C.; Grignani, C.; Bechini, L.; Spiegel, A.; Lehtinen, T.; Guzmán, G.; Krüger, J.; D’Hose, T.; Pecio, A.; et al. Long-term effects of best management practices on crop yield and nitrogen surplus. Ital. J. Agron. 2015, 10, 47–50. [Google Scholar] [CrossRef]
- Asgedom, H.; Kebreab, E. Beneficial management practices and mitigation of greenhouse gas emissions in the agriculture of the Canadian Prairie: A review. Agron. Sustain. Dev. 2011, 31, 433–451. [Google Scholar] [CrossRef]
- Baird, J.; Jollineau, M.; Plummer, R.; Valenti, J. Exploring agricultural advice networks, beneficial management practices and water quality on the landscape: A geospatial social-ecological systems analysis. Land Use Policy 2016, 51, 236–243. [Google Scholar] [CrossRef]
- Li, S.; Elliott, J.A.; Tiessen, K.H.D.; Yarotski, J.; Lobb, D.A.; Flaten, D.N. The effects of multiple beneficial management practices on hydrology and nutrient losses in a small watershed in the Canadian Prairies. J. Environ. Qual. 2011, 40, 1627–1642. [Google Scholar] [CrossRef] [PubMed]
- Lychuk, T.E.; Moulin, A.P.; Izaurralde, R.C.; Lemke, R.L.; Johnson, E.N.; Olfert, O.O.; Brandt, S.A. Climate change, agricultural inputs, cropping diversity, and environmental covariates in multivariate analysis of future wheat, barley, and canola yields in Canadian Prairies: A case study. Can. J. Soil Sci. 2017, 97, 300–318. [Google Scholar] [CrossRef]
- Cutforth, H.W.; McGinn, S.M.; McPhee, K.E.; Miller, P.R. Adaptation of Pulse Crops to the Changing Climate of the Northern Great Plains. Agron. J. 2007, 99, 1684–1699. [Google Scholar] [CrossRef]
- McKenzie, R.H.; Bremer, E.; Middleton, A.B.; Pfiffner, P.G.; Woods, S.A. Optimum seeding date and rate for irrigated cereal and oilseed crops in southern Alberta. Can. J. Plant Sci. 2011, 91, 293–303. [Google Scholar] [CrossRef]
- Hwang, S.F.; Gossen, B.D.; Turnbull, G.D.; Chang, K.F.; Howard, R.J.; Thomas, A.G. Effect of temperature, seeding date, fungicide seed treatment and inoculation with Fusarium avenaceum on seedling survival, root rot severity and yield of lentil. Can. J. Plant Sci. 2000, 80, 899–907. [Google Scholar] [CrossRef]
- He, Y.; Wang, H.; Qian, B.; McConkey, B.; DePauw, R. How early can the seeding dates of spring wheat be under current and future climate in Saskatchewan, Canada? PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- McCaig, T.N.; Gan, Y.T.; Clarke, P.; Clarke, J.M.; DePauw, R.M. Kernel colour changes associated with field weathering of spring wheat. Can. J. Plant Sci. 2006, 86, 371–377. [Google Scholar] [CrossRef]
- Steel, R.; Torrie, J.; Dickey, D. Principles and procedures of statistics: A biometrical approach. J. Food Sci. 1997, 51, 574–576. [Google Scholar]
- May, W.E.; Lafond, G.P.; Gan, Y.T.; Hucl, P.; Holzapfel, C.B.; Johnston, A.M.; Stevenson, C. Yield variability in Phalaris canariensis L. due to seeding date, seeding rate and nitrogen fertilizer. Can. J. Plant Sci. 2012, 92, 651–669. [Google Scholar] [CrossRef]
- Gusta, L.V.; Johnson, E.N.; Nesbitt, N.T.; Kirkland, K.J. Effect of seeding date on canola seed quality and seed vigour. Can. J. Plant Sci. 2004, 84, 463–471. [Google Scholar] [CrossRef]
- Johnson, E.N.; Miller, P.R.; Blackshaw, R.E.; Gan, Y.; Harker, K.N.; Clayton, G.W.; Kephart, K.D.; Wichman, D.M.; Topinka, K.; Kirkland, K.J. Seeding date and polymer seed coating effects on plant establishment and yield of fall-seeded canola in the Northern Great Plains. Can. J. Plant Sci. 2004, 84, 955–963. [Google Scholar] [CrossRef]
- Gao, X.; Lukow, O.M.; Grant, C.A. Grain concentrations of protein, iron and zinc and bread making quality in spring wheat as affected by seeding date and nitrogen fertilizer management. J. Geochem. Explor. 2012, 121, 36–44. [Google Scholar] [CrossRef]
- Beres, B.L.; McKenzie, R.H.; Cárcamo, H.A.; Dosdall, L.M.; Evenden, M.L.; Yang, R.C.; Spaner, D.M. Influence of seeding rate, Nitrogen management, and micronutrient blend applications on pith expression in solid-stemmed spring wheat. Crop Sci. 2012, 52, 1316–1329. [Google Scholar] [CrossRef]
- Isidro-Sánchez, J.; Perry, B.; Singh, A.K.; Wang, H.; DePauw, R.M.; Pozniak, C.J.; Beres, B.L.; Johnson, E.N.; Cuthbert, R.D. Effects of seeding rate on durum crop production and physiological responses. Agron. J. 2017, 109, 1981–1990. [Google Scholar] [CrossRef]
- Clarke, J.M.; McLeod, J.G.; McCaig, T.N.; DePauw, R.M.; Knox, R.E.; Fernandez, M.R. AC Avonlea durum wheat. Can. J. Plant Sci. 1998, 78, 621–623. [Google Scholar] [CrossRef]
- Beres, B.L.; Cárcamo, H.A.; Yang, R.C.; Spaner, D.M. Integrating spring wheat sowing density with variety selection to manage wheat stem sawfly. Agron. J. 2011, 103, 1755–1764. [Google Scholar] [CrossRef]
- Nilsen, K.T.; Clarke, J.M.; Beres, B.L.; Pozniak, C.J. Sowing density and cultivar effects on pith expression in solid-stemmed durum wheat. Agron. J. 2016, 108, 219–228. [Google Scholar] [CrossRef]
- Campbell, C.A.; Janzen, H.H.; Paustian, K.; Gregorich, E.G.; Sherrod, L.; Liang, B.C.; Zentner, R.P. Carbon storage in soils of the North American Great Plains: Effect of cropping frequency. Agron. J. 2005, 97, 349–363. [Google Scholar] [CrossRef]
- Gan, Y.; Mooleki, S.P.; Lemke, R.L.; Zentner, R.P.; Ruan, Y. Durum wheat productivity in response to soil water and soil residual nitrogen associated with previous crop management. Agron. J. 2016, 108, 1468–1478. [Google Scholar] [CrossRef]
- Campbell, C.A.; Zentner, R.P.; Basnyat, P.; DeJong, R.; Lemke, R.; Desjardins, R.; Reiter, M. Nitrogen mineralization under summer fallow and continuous wheat in the semiarid Canadian prairie. Can. J. Soil Sci. 2008, 88, 681–696. [Google Scholar] [CrossRef]
- Myers, R.J.K.; Campbell, C.A.; Weier, K.L. Quantitative relationship between net nitrogen mineralization and moisture content of soils. Can. J. Soil Sci. 1982, 62, 111–124. [Google Scholar] [CrossRef]
- McConkey, B.G.; Curtin, D.; Campbell, C.A.; Brandt, S.A.; Selles, F. Crop and soil nitrogen status of tilled and no-tillage systems in semiarid regions of Saskatchewan. Can. J. Soil Sci. 2002, 82, 489–498. [Google Scholar] [CrossRef]
- Gan, Y.; Hamel, C.; Kutcher, H.R.; Poppy, L. Lentil enhances agroecosystem productivity with increased residual soil water and nitrogen. Renew. Agric. Food Syst. 2017, 32, 319–330. [Google Scholar] [CrossRef]
- Lemke, R.L.; Vandenbygaart, A.J.; Campbell, C.A.; Lafond, G.P.; McConkey, B.G.; Grant, B. Long-term effects of crop rotations and fertilization on soil C and N in a thin Black Chernozem in southeastern Saskatchewan. Can. J. Soil Sci. 2012, 92, 449–461. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Rennie, D.A.; Paul, E.A. Gaseous nitrogen losses from cropped and summer-fallowed soils. Can. J. Soil Sci. 1982, 62, 187–196. [Google Scholar] [CrossRef]
- Selles, F.; Clarke, J.M.; DePauw, R.M. Quantification of the yield and protein response to N and water availability by two wheat classes in the semiarid prairies. Can. J. Plant Sci. 2006, 86, 981–993. [Google Scholar] [CrossRef]
- Clarke, J.M.; DePauw, R.M. Residue production of semidwarf and conventional wheat genotypes. Can. J. Plant Sci. 1993, 73, 769–776. [Google Scholar] [CrossRef]
- Le Roy, D.G.; Smith, E.G.; MacCallum, P.J.; Janzen, H.H. Will summer fallow re-emerge in the dark brown soil zone of the Canadian prairie as a response to net return risk? Can. J. Plant Sci. 2017, 97, 214–249. [Google Scholar]
- Dyer, J.A.; Desjardins, R.L. Analysis of trends in CO2 Emissions from Fossil Fuel use for farm fieldwork related to harvesting annual crops and hay, changing tillage practices and reduced summerfallow in Canada. J. Sustain. Agric. 2005, 25, 141–155. [Google Scholar] [CrossRef]
- Liu, C.; Cutforth, H.; Chai, Q.; Gan, Y. Farming tactics to reduce the carbon footprint of crop cultivation in semiarid areas. A review. Agron. Sustain. Dev. 2016, 36, 69. [Google Scholar] [CrossRef]
- Shrestha, B.M.; McConkey, B.G.; Smith, W.N.; Desjardins, R.L.; Campbell, C.A.; Grant, B.B.; Miller, P.R. Effects of crop rotation, crop type and tillage on soil organic carbon in a semiarid climate. Can. J. Soil Sci. 2013, 93, 137–146. [Google Scholar] [CrossRef]
- Dyer, J.A.; Desjardins, R.L. A review and evaluation of fossil energy and carbon dioxide emissions in Canadian agriculture. J. Sustain. Agric. 2009, 33, 210–228. [Google Scholar] [CrossRef]
- Janzen, H.H.; Angers, D.A.; Boehm, M.; Bolinder, M.; Desjardins, R.L.; Dyer, J.A.; Ellert, B.H.; Gibb, D.J.; Gregorich, E.G.; Helgason, B.L.; et al. A proposed approach to estimate and reduce net greenhouse gas emissions from whole farms. Can. J. Soil Sci. 2006, 86, 401–418. [Google Scholar] [CrossRef]
- Gan, Y.; Liang, C.; Campbell, C.A.; Zentner, R.P.; Lemke, R.L.; Wang, H.; Yang, C. Carbon footprint of spring wheat in response to fallow frequency and soil carbon changes over 25 years on the semiarid Canadian prairie. Eur. J. Agron. 2012, 43, 175–184. [Google Scholar] [CrossRef]
- Curtin, D.; Wang, H.; Selles, F.; Zentner, R.P.; Biederbeck, V.O.; Campbell, C.A. Legume green manure as partial fallow replacement in semiarid Saskatchewan: Effect on carbon fluxes. Can. J. Soil Sci. 2000, 80, 499–505. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Ryan, M.H. Magnitude and mechanisms of persistent crop sequence effects on wheat. Field Crops Res. 2014, 164, 154–165. [Google Scholar] [CrossRef]
- Gan, Y.; Hamel, C.; O’Donovan, J.T.; Cutforth, H.; Zentner, R.P.; Campbell, C.A.; Niu, Y.; Poppy, L. Diversifying crop rotations with pulses enhances system productivity. Sci. Rep. 2015, 5, 14625. [Google Scholar] [CrossRef] [PubMed]
- Harker, K.N.; O’Donovan, J.T.; Turkington, T.K.; Blackshaw, R.E.; Lupwayi, N.Z.; Smith, E.G.; Klein-Gebbinck, H.; Dosdall, L.M.; Hall, L.M.; Willenborg, C.J.; et al. High-yield no-till canola production on the Canadian prairies. Can. J. Plant Sci. 2012, 92, 221–233. [Google Scholar] [CrossRef]
- Campbell, C.A.; Zentner, R.P.; Gameda, S.; Blomert, B.; Wall, D.D. Production of annual crops on the Canadian prairies: Trends during 1976–1998. Can. J. Soil Sci. 2002, 82, 45–57. [Google Scholar] [CrossRef]
- Miller, P.; Gan, Y.; McConkey, B.; McDonald, C. Pulse crops for the northern Great Plains. II: Cropping sequence effects on cereal, oilseed, and pulse crops. Agron. J. 2003, 95, 980–986. [Google Scholar] [CrossRef]
- Miller, P.R.; Gan, Y.; McConkey, B.G.; McDonald, C.L. Pulse crops in the Northern Great Plains. I. Grain productivity and residual effects on soil water and nitrogen. Agron. J. 2003, 95, 972–979. [Google Scholar] [CrossRef]
- Hossain, Z.; Wang, X.; Hamel, C.; Diane Knight, J.; Morrison, M.J.; Gan, Y. Biological nitrogen fixation by pulse crops on semiarid Canadian prairies. Can. J. Plant Sci. 2016, 97, 119–131. [Google Scholar]
- Hossain, Z.; Wang, X.; Hamel, C.; Gan, Y. Nodulation and nitrogen accumulation in pulses grown on the Canadian Prairie. Can. J. Plant Sci. 2018, 98, 527–545. [Google Scholar] [CrossRef]
- Hunt, J.R.; Browne, C.; McBeath, T.M.; Verburg, K.; Craig, S.; Whitbread, A.M. Summer fallow weed control and residue management impacts on winter crop yield though soil water and N accumulation in a winter-dominant, low rainfall region of southern Australia. Crop Pasture Sci. 2013, 64, 922–934. [Google Scholar] [CrossRef]
- De Jong, R.; Campbell, C.A.; Zentner, R.P.; Basnyat, P.; Cutforth, H.; Desjardins, R. Quantifying soil water conservation in the semiarid region of Saskatchewan, Canada: Effect of fallow frequency and N fertilizer. Can. J. Soil Sci. 2008, 88, 461–475. [Google Scholar] [CrossRef]
- Liu, L.; Gan, Y.; Bueckert, R.; Van Rees, K. Rooting systems of oilseed and pulse crops. II: Vertical distribution patterns across the soil profile. Field Crops Res. 2011, 122, 248–255. [Google Scholar] [CrossRef]
- Liu, L.; Gan, Y.; Bueckert, R.; Van Rees, K. Rooting systems of oilseed and pulse crops I: Temporal growth patterns across the plant developmental periods. Field Crops Res. 2011, 122, 256–263. [Google Scholar] [CrossRef]
- Wang, X.; Gan, Y.; Hamel, C.; Lemke, R.; McDonald, C. Water use profiles across the rooting zones of various pulse crops. Field Crops Res. 2012, 134, 130–137. [Google Scholar] [CrossRef]
- Niu, Y.; Bainard, L.; Bandara, M.S.; Hamel, C.; Gan, Y. Soil residual water and nutrients explain about 30% of the rotational effect in 4-year pulse-intensified rotation systems. Can. J. Plant Sci. 2017, 97, 852–864. [Google Scholar] [CrossRef]
- Campbell, C.A.; McConkey, B.G.; Zentner, R.P.; Selles, F.; Dyck, F.B. Benefits of wheat stubble strips for conserving snow in southwestern Saskatchewan. J. Soil Water Conserv. 1992, 47, 112–115. [Google Scholar]
- Steppuhn, H. Snow cover retention capacities for direct combined wheat and barley stubble in windy environments. Can. J. Agric. Eng. 1994, 36, 215–223. [Google Scholar]
- Cutforth, H.W.; Jefferson, P.G.; Campbell, C.A. Lower limit of available water for three plant species grown on a medium-textured soil in southwestern Saskatchewan. Can. J. Soil Sci. 1991, 71, 247–252. [Google Scholar] [CrossRef]
- He, Y.; Wang, H.; Qian, B.; McConkey, B.; Cutforth, H.; Lemke, R.; DePauw, R.M.; Brandt, K.; Hu, K.L.; Hoogenboom, G. Effects of climate change on killing frost in the Canadian prairies. Clim. Res. 2012, 54, 221–231. [Google Scholar] [CrossRef]
- DePauw, R.M.; Knox, R.E.; Humphreys, D.G.; Thomas, J.B.; Fox, S.L.; Brown, P.D.; Singh, A.K.; Pozniak, C.; Randhawa, H.S.; Fowler, D.B.; et al. New breeding tools impact Canadian commercial farmer fields. Czech J. Genet. Plant Breed. 2011, 47, 28–34. [Google Scholar] [CrossRef]
- Gan, Y.T.; Campbell, C.A.; Janzen, H.H.; Lemke, R.; Liu, L.P.; Basnyat, P.; McDonald, C.L. Root mass for oilseed and pulse crops: Growth and distribution in the soil profile. Can. J. Plant Sci. 2009, 89, 883–893. [Google Scholar] [CrossRef]
- Campbell, C.A.; Selles, F.; Zentner, R.P.; De Jong, R.; Lemke, R.; Hamel, C. Nitrate leaching in the semiarid prairie: Effect of cropping frequency, crop type, and fertilizer after 37 years. Can. J. Soil Sci. 2006, 86, 701–710. [Google Scholar] [CrossRef]
- Kröbel, R.; Campbell, C.A.; Zentner, R.P.; Lemke, R.; Steppuhn, H.; Desjardins, R.L.; De Jong, R. Nitrogen and phosphorus effects on water use efficiency of spring wheat grown in a semi-arid region of the Canadian prairies. Can. J. Soil Sci. 2012, 92, 573–587. [Google Scholar] [CrossRef]
- St. Luce, M.; Grant, C.A.; Ziadi, N.; Zebarth, B.J.; O’Donovan, J.T.; Blackshaw, R.E.; Harker, K.N.; Johnson, E.N.; Gan, Y.; Lafond, G.P.; et al. Preceding crops and nitrogen fertilization influence soil nitrogen cycling in no-till canola and wheat cropping systems. Field Crops Res. 2016, 191, 20–32. [Google Scholar] [CrossRef]
- Borrell, A.N.; Shi, Y.; Gan, Y.; Bainard, L.D.; Germida, J.J.; Hamel, C. Fungal diversity associated with pulses and its influence on the subsequent wheat crop in the Canadian prairies. Plant Soil 2017, 414, 13–31. [Google Scholar] [CrossRef]
- Yang, C.; Hamel, C.; Gan, Y.; Vujanovic, V. Bacterial endophytes mediate positive feedback effects of early legume termination times on the yield of subsequent durum wheat crops. Can. J. Microbiol. 2012, 58, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hamel, C.; Gan, Y.; Vujanovic, V. Pyrosequencing reveals how pulses influence rhizobacterial communities with feedback on wheat growth in the semiarid Prairie. Plant Soil 2013, 367, 493–505. [Google Scholar] [CrossRef]
- Bainard, L.D.; Navarro-Borrell, A.; Hamel, C.; Braun, K.; Hanson, K.; Gan, Y. Increasing the frequency of pulses in crop rotations reduces soil fungal diversity and increases the proportion of fungal pathotrophs in a semiarid agroecosystem. Agric. Ecosyst. Environ. 2017, 240, 206–214. [Google Scholar] [CrossRef]
- Hamel, C.; Gan, Y.; Sokolski, S.; Bainard, L.D. High frequency cropping of pulses modifies soil nitrogen level and the rhizosphere bacterial microbiome in 4-year rotation systems of the semiarid prairie. Appl. Soil Ecol. 2018, 126, 47–56. [Google Scholar] [CrossRef]
- Khakbazan, M.; Grant, C.A.; Huang, J.; Berry, N.J.; Smith, E.G.; O’Donovan, J.T.; Blackshaw, R.E.; Harker, K.N.; Lafond, G.P.; Johnson, E.N.; et al. Preceding crops and nitrogen effects on crop energy use efficiency in canola and barley. Agron. J. 2016, 108, 1079–1088. [Google Scholar] [CrossRef]
- St. Luce, M.; Grant, C.A.; Zebarth, B.J.; Ziadi, N.; O’Donovan, J.T.; Blackshaw, R.E.; Harker, K.N.; Johnson, E.N.; Gan, Y.; Lafond, G.P.; et al. Legumes can reduce economic optimum nitrogen rates and increase yields in a wheat-canola cropping sequence in western Canada. Field Crops Res. 2015, 179, 12–25. [Google Scholar] [CrossRef]
- Gan, Y.; Liang, C.; Wang, X.; McConkey, B.G. Lowering carbon footprint of durum wheat by diversifying cropping systems. Field Crops Res. 2011, 122, 199–206. [Google Scholar] [CrossRef]
- Bazghaleh, N.; Hamel, C.; Gan, Y.; Knight, J.D.; Vujanovic, V.; Cruz, A.F.; Ishii, T. Phytochemicals induced in chickpea roots selectively and non-selectively stimulate and suppress fungal endophytes and pathogens. Plant Soil 2016, 409, 479–493. [Google Scholar] [CrossRef]
- Ellouze, W.; Hamel, C.; Cruz, A.F.; Ishii, T.; Gan, Y.; Bouzid, S.; St-Arnaud, M. Phytochemicals and spore germination: At the root of AMF host preference? Appl. Soil Ecol. 2012, 60, 98–104. [Google Scholar] [CrossRef]
- Niu, Y.; Bainard, L.D.; May, W.E.; Hossain, Z.; Hamel, C.; Gan, Y. Intensified pulse rotations buildup pea rhizosphere pathogens in cereal and pulse based cropping systems. Front. Microbiol. 2018, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Grant, C.A. Cadmium and zinc concentration in grain of durum wheat in relation to phosphorus fertilization, crop sequence and tillage management. Appl. Environ. Soil Sci. 2012, 2012, 817107. [Google Scholar] [CrossRef]
- Gulden, R.H.; Tenuta, M.; Mitchell, S.; Langarica Fuentes, A.; Daniell, T.J. Preceding crop and weed management history affect denitrification and denitrifier community structure throughout the development of durum wheat. Agric. Ecosyst. Environ. 2015, 212, 49–63. [Google Scholar] [CrossRef]
- Kubota, H.; Iqbal, M.; Dyck, M.; Quideau, S.; Yang, R.C.; Spaner, D. Investigating genetic progress and variation for nitrogen use efficiency in spring wheat. Crop Sci. 2018, 58, 1542–1557. [Google Scholar] [CrossRef]
- Mansour, E.; Merwad, A.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; El-Sobky, E.E.A.; Oraby, H.F. Nitrogen use efficiency in spring wheat: Genotypic variation and grain yield response under sandy soil conditions. J. Agric. Sci. 2017, 155, 1407–1423. [Google Scholar] [CrossRef]
- Han, M.; Okamoto, M.; Beatty, P.H.; Rothstein, S.J.; Good, A.G. The genetics of nitrogen use efficiency in crop plants. Annu. Rev. Genet. 2015, 49, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Chaplot, P.C. Effect of balanced fertilization, organic manures and bioregulator on nutrient uptake by late sown wheat and soil nutrient status. Ann. Biol. 2014, 30, 627–630. [Google Scholar]
- Kröbel, R.; Campbell, C.A.; Zentner, R.P.; Lemke, R.; Desjardins, R.L.; Karimi-Zindashty, Y. Effect of N, P and cropping frequency on nitrogen use efficiencies of spring wheat in the Canadian semi-arid prairie. Can. J. Plant Sci. 2012, 92, 141–154. [Google Scholar] [CrossRef]
- Grant, C.A.; O’Donovan, J.T.; Blackshaw, R.E.; Harker, K.N.; Johnson, E.N.; Gan, Y.; Lafond, G.P.; May, W.E.; Turkington, T.K.; Lupwayi, N.Z.; et al. Residual effects of preceding crops and nitrogen fertilizer on yield and crop and soil N dynamics of spring wheat and canola in varying environments on the Canadian prairies. Field Crops Res. 2016, 192, 86–102. [Google Scholar] [CrossRef]
- Malhi, S.S.; Oliver, E.; Mayerle, G.; Kruger, G.; Gill, K.S. Improving effectiveness of seedrow-placed urea with urease inhibitor and polymer coating for durum wheat and canola. Commun. Soil Sci. Plant Anal. 2003, 34, 1709–1727. [Google Scholar] [CrossRef]
- Bremner, J.M. Recent research on problems in the use of urea as a nitrogen fertilizer. Fertil. Res. 1995, 42, 321–329. [Google Scholar] [CrossRef]
- Christianson, C.B.; Baethgen, W.E.; Carmona, G.; Howard, R.G. Microsite reactions of urea-nBTPT fertilizer on the soil surface. Soil Biol. Biochem. 1993, 25, 1107–1117. [Google Scholar] [CrossRef]
- Selles, F.; Clarke, J.M.; Zentner, R.P.; Campbell, C.A. Effects of source and placement of phosphorus on concentration of cadmium in the grain of two durum wheat cultivars. Can. J. Plant Sci. 2003, 83, 475–482. [Google Scholar] [CrossRef]
- Beres, B.L.; Bremer, E.; Sadasivaiah, R.S.; Clarke, J.M.; Graf, R.J.; McKenzie, R.H.; Dyck, R.J. Post-emergence application of N fertilizer to improve grain yield and quality of irrigated durum and bread wheat. Can. J. Plant Sci. 2008, 88, 509–512. [Google Scholar] [CrossRef]
- May, W.E.; Fernandez, M.R.; Selles, F.; Lafond, G.P. Agronomic practices to reduce leaf spotting and Fusarium kernel infections in durum wheat on the Canadian prairies. Can. J. Plant Sci. 2014, 94, 141–152. [Google Scholar] [CrossRef]
- McNeal, F.H.; Berg, M.A.; Brown, P.L.; McGuire, C.F. Productivity and Quality Response of Five Spring Wheat Genotypes, Triticum aestivum, L., to Nitrogen Fertilizer1. Agron. J. 1971, 63, 908–910. [Google Scholar] [CrossRef]
- Knapp, J.S.; Harms, C.L. Nitrogen Fertilization and Plant Growth Regulator Effects on Yield and Quality of Four Wheat Cultivars. J. Prod. Agric. 1988, 1, 94–98. [Google Scholar] [CrossRef]
- Selles, F.; Campbell, C.A.; Zentner, R.P.; Curtin, D.; James, D.C.; Basnyat, P. Phosphorus use efficiency and long-term trends in soil available phosphorus in wheat production systems with and without nitrogen fertilizer. Can. J. Soil Sci. 2011, 91, 39–52. [Google Scholar] [CrossRef]
- Campbell, C.A.; Zentner, R.P.; Selles, F.; Jefferson, P.G.; McConkey, B.G.; Lemke, R.; Blomert, B.J. Long-term effect of cropping system and nitrogen and phosphorus fertilizer on production and nitrogen economy of grain crops in a Brown Chernozem. Can. J. Plant Sci. 2005, 85, 81–93. [Google Scholar] [CrossRef]
- Karamanos, R.E.; Harapiak, J.T.; Flore, N.A. Application of seed-row potash to barley (Hordeum vulgare L.) grown on soils with high “available” potassium levels. Can. J. Plant Sci. 2003, 83, 291–303. [Google Scholar] [CrossRef]
- May, W.E.; Fernandez, M.R.; Holzapfel, C.B.; Lafond, G.P. Influence of phosphorus, nitrogen, and potassium chloride placement and rate on durum wheat yield and quality. Agron. J. 2008, 100, 1173–1179. [Google Scholar] [CrossRef]
- Bailey, L.; Grant, C.; Flore, N.; Harapiak, J. Effect of phosphate source, rate and cadmium content and use of Penicillium bilaii on phosphorus, zinc and cadmium concentration in durum wheat grain. J. Sci. Food Agric. 2002, 82, 301–308. [Google Scholar]
- Jiao, Y.; Grant, C.A.; Bailey, L.D. Growth and nutrient response of flax and durum wheat to phosphorus and zinc fertilizers. Can. J. Plant Sci. 2007, 87, 461–470. [Google Scholar] [CrossRef]
- Campbell, C.A.; Selles, F.; Lafond, G.P.; Biederbeck, V.O.; Zentner, R.P. Tillage—Fertilizer changes: Effect on some soil quality attributes under long-term crop rotations in a thin Black Chernozem. Can. J. Soil Sci. 2001, 81, 157–165. [Google Scholar] [CrossRef]
- Campbell, C.A.; Selles, F.; Lafond, G.P.; Zentner, R.P. Adopting zero tillage management: Impact on soil C and N under long-term crop rotations in a thin Black Chernozem. Can. J. Soil Sci. 2001, 81, 139–148. [Google Scholar] [CrossRef]
- Chai, Q.; Qin, A.Z.; Gan, Y.T.; Yu, A.Z. Higher yield and lower carbon emission by intercropping maize with rape, pea, and wheat in arid irrigation areas. Agron. Sustain. Dev. 2014, 34, 535–543. [Google Scholar] [CrossRef]
- Statistics-Canada. Fertilizer and Pesticide Management in Canada, Catalogue No. 21-021-MIE No. 003; Statistics Canada: Ottawa, ON, Canada, 2004.
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef]
- Bronson, K.F.; White, J.W.; Conley, M.M.; Hunsaker, D.J.; Thorp, K.R.; French, A.N.; Mackey, B.E.; Holland, K.H. Active optical sensors in irrigated durum wheat: Nitrogen and water effects. Agron. J. 2017, 109, 1060–1071. [Google Scholar] [CrossRef]
- Raghuvanshi, R.; Chaudhari, A.; Kumar, G.N. Amelioration of cadmium- and mercury-induced liver and kidney damage in rats by genetically engineered probiotic Escherichia coli Nissle 1917 producing pyrroloquinoline quinone with oral supplementation of citric acid. Nutrition 2016, 32, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Büyükkiliç Yanardağ, A.; Mermut, A.R.; Cano, Á.F.; Garces, D.M.C.; Yanardağ, İ.H. Cadmium contents of soils and durum and bread wheats on Harran Plain, southeast Turkey. Turk. J. Agric. For. 2016, 40, 772–782. [Google Scholar] [CrossRef]
- Harris, N.S.; Taylor, G.J. Cadmium uptake and partitioning in durum wheat during grain filling. BMC Plant Biol. 2013, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.J.; Mohammad, A.; Macfie, S.M. Accumulation of cadmium in near-isogenic lines of durum wheat (Triticum turgidum L. var durum): The role of transpiration. Physiol. Mol. Biol. Plants 2011, 17, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Grant, C.A.; Bailey, L.D. Effects of phosphorus and zinc fertilizer on cadmium uptake and distribution in flax and durum wheat. J. Sci. Food Agric. 2004, 84, 777–785. [Google Scholar] [CrossRef]
- Gao, X.; Flaten, D.N.; Tenuta, M.; Grimmett, M.G.; Gawalko, E.J.; Grant, C.A. Soil solution dynamics and plant uptake of cadmium and zinc by durum wheat following phosphate fertilization. Plant Soil 2011, 338, 423–434. [Google Scholar] [CrossRef]
- Gao, X.; Brown, K.R.; Racz, G.J.; Grant, C.A. Concentration of cadmium in durum wheat as affected by time, source and placement of nitrogen fertilization under reduced and conventional-tillage management. Plant Soil 2010, 337, 341–354. [Google Scholar] [CrossRef]
- Knox, R.E.; Pozniak, C.J.; Clarke, F.R.; Clarke, J.M.; Houshmand, S.; Singh, A.K. Chromosomal location of the cadmium uptake gene (Cdu1) in durum wheat. Genome 2009, 52, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Garrett, R.G.; MacLaurin, A.I.; Gawalko, E.J.; Tkachuk, R.; Hall, G.E.M. A prediction model for estimating the cadmium content of durum wheat from soil chemistry. J. Geochem. Explor. 1998, 64, 101–110. [Google Scholar] [CrossRef]
- Esmaeili Taheri, A.; Hamel, C.; Gan, Y. Cropping practices impact fungal endophytes and pathogens in durum wheat roots. Appl. Soil Ecol. 2016, 100, 104–111. [Google Scholar] [CrossRef]
- Ellouze, W.; Hamel, C.; Vujanovic, V.; Gan, Y.; Bouzid, S.; St-Arnaud, M. Chickpea genotypes shape the soil microbiome and affect the establishment of the subsequent durum wheat crop in the semiarid North American Great Plains. Soil Biol. Biochem. 2013, 63, 129–141. [Google Scholar] [CrossRef]
- Ellouze, W.; Hamel, C.; Singh, A.K.; Mishra, V.; DePauw, R.; Knox, R. Abundance of the arbuscular mycorrhizal fungal taxa associated with the roots and rhizosphere soil of different durum wheat cultivars in the Canadian prairies. Can. J. Microbiol. 2018, 64, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Bazghaleh, N.; Hamel, C.; Gan, Y.; Tar’an, B.; Knight, J.D. Genotype-specific variation in the structure of root fungal communities is related to chickpea plant productivity. Appl. Environ. Microbiol. 2015, 81, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hamel, C.; Gan, Y.; Vujanovic, V. Tag-encoded pyrosequencing analysis of the effects of fungicide application and plant genotype on rhizobacterial communities. Appl. Soil Ecol. 2012, 60, 92–97. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Harker, K.N.; O’Donovan, J.T.; Turkington, T.K.; Blackshaw, R.E.; Hall, L.M.; Willenborg, C.J.; Gan, Y.; Lafond, G.P.; May, W.E.; et al. Relating soil microbial properties to yields of no-till canola on the Canadian prairies. Eur. J. Agron. 2015, 62, 110–119. [Google Scholar] [CrossRef]
- Cruz, A.F.; Hamel, C.; Yang, C.; Matsubara, T.; Gan, Y.; Singh, A.K.; Kuwada, K.; Ishii, T. Phytochemicals to suppress Fusarium head blight in wheat-chickpea rotation. Phytochemistry 2012, 78, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Ellouze, W.; Hamel, C.; DePauw, R.M.; Knox, R.E.; Cuthbert, R.D.; Singh, A.K. Potential to breed for mycorrhizal association in durum wheat. Can. J. Microbiol. 2015, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Ellouze, W.; Esmaeili Taheri, A.; Bainard, L.D.; Yang, C.; Bazghaleh, N.; Navarro-Borrell, A.; Hanson, K.; Hamel, C. Soil Fungal Resources in Annual Cropping Systems and Their Potential for Management. BioMed Res. Int. 2014, 2014, 531824. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Sanders, I.R. The role of community and population ecology in applying mycorrhizal fungi for improved food security. ISME J. 2015, 9, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili Taheri, A.; Hamel, C.; Gan, Y. Pyrosequencing reveals the impact of foliar fungicide application to chickpea on root fungal communities of durum wheat in subsequent year. Fungal Ecol. 2015, 15, 73–81. [Google Scholar] [CrossRef]
- Yang, C.; Hamel, C.; Gan, Y. Incongruous variation of denitrifying bacterial communities as soil N level rises in Canadian canola fields. Appl. Soil Ecol. 2015, 89, 93–101. [Google Scholar] [CrossRef]
- Banniza, S.; Armstrong-Cho, C.L.; Gan, Y.; Chongo, G. Evaluation of fungicide efficacy and application frequency for the control of ascochyta blight in chickpea. Can. J. Plant Pathol. 2011, 33, 135–149. [Google Scholar] [CrossRef]
- Yang, C.; Hamel, C.; Vujanovic, V.; Gan, Y. Nontarget effects of foliar fungicide application on the rhizosphere: Diversity of nifH gene and nodulation in chickpea field. J. Appl. Microbiol. 2012, 112, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.L.; Gossen, B.D.; Gugel, R.K.; Morrall, R.A.A. Diseases of Field Crops in Canada, 3rd ed.; Canadian Phytopathological Society, University Extension Press, University of Saskatchewan: Saskatoon, SK, Canada, 2003. [Google Scholar]
- DePauw, R.M.; Malhi, S.S.; Bullock, P.R.; Gan, Y.T.; McKenzie, R.H.; Larney, F.J.; Janzen, H.H.; Cutforth, H.W.; Wang, H. Wheat Production in North High Latitudes—Canadian Example; Angus, W., Bonjean, A., van Ginkel, M., Eds.; World Wheat Book 2; A History of Wheat Breeding; Lavoisier Tech et Doc: Paris, France, 2011; Volume 2, pp. 607–651. [Google Scholar]
- Langevin, F.; Eudes, F.; Comeau, A. Effect of trichothecenes produced by Fusarium graminearum during Fusarium head blight development in six cereal species. Eur. J. Plant Pathol. 2004, 110, 735–746. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Clarke, F.R.; Knox, R.E.; Clarke, J.M.; Singh, A.K. Quantification of effects of leaf spotting diseases on grain yield and market quality of durum wheat using near-isogenic lines. Can. J. Plant Pathol. 2010, 32, 177–187. [Google Scholar] [CrossRef]
- Fernandez, M.R.; May, W.E.; Chalmers, S.; Savard, M.E.; Singh, A.K. Are early foliar fungicide applications on durum wheat grown in southeast Saskatchewan beneficial in increasing grain productivity? Can. J. Plant Sci. 2014, 94, 891–903. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Stevenson, C.F.; Hodge, K.; Dokken-Bouchard, F.; Pearse, P.G.; Waelchli, F.; Brown, A.; Peluola, C. Assessing effects of climatic change, region and agronomic practices on leaf spotting of bread and durum wheat in the western Canadian Prairies, from 2001 to 2012. Agron. J. 2016, 108, 1180–1195. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Clarke, J.M.; DePauw, R.M. The effect of plant height on tan spot on durum wheat in southern Saskatchewan. Crop Sci. 2002, 42, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fernandez, M.R.; Clarke, F.R.; DePauw, R.M.; Clarke, J.M. Effect of leaf spotting diseases on grain yield and seed traits of wheat in southern Saskatchewan. Can. J. Plant Sci. 2002, 82, 507–512. [Google Scholar] [CrossRef]
- Wang, H.; Fernandez, M.R.; Clarke, F.R.; DePauw, R.M.; Clarke, J.M. Effects of foliar fungicides on kernel black point of wheat in southern Saskatchewan. Can. J. Plant Pathol. 2002, 24, 287–293. [Google Scholar] [CrossRef]
- Beres, B.L.; Dosdall, L.M.; Weaver, D.K.; Cárcamo, H.A.; Spaner, D.M. Biology and integrated management of wheat stem sawfly and the need for continuing research. Can. Entomol. 2011, 143, 105–125. [Google Scholar] [CrossRef]
- Lamb, R.J.; Wise, I.L.; Olfert, O.O.; Gavloski, J.; Barker, P.S. Distribution and seasonal abundance of Sitodiplosis mosellana (Diptera: Cecidomyiidae) in spring wheat. Can. Entomol. 1999, 131, 387–397. [Google Scholar] [CrossRef]
- Smith, M.A.H.; Lamb, R.J. Factors influencing oviposition by Sitodiplosis mosellana (Diptera: Cecidomyiidae) on wheat spikes (Gramineae). Can. Entomol. 2001, 133, 533–548. [Google Scholar] [CrossRef]
- Bokore, F.E.; Knox, R.E.; Cuthbert, R.D.; Ruan, Y.; DePauw, R.M. Effects of media supplements on doubled haploid production in durum wheat. Can. J. Plant Sci. 2016, 97, 65–71. [Google Scholar] [CrossRef]
- Clarke, J.M.; Clarke, F.R.; Pozniak, C.J. Forty-six years of genetic improvement in Canadian durum wheat cultivars. Can. J. Plant Sci. 2010, 90, 791–801. [Google Scholar] [CrossRef]
- McCaig, T.N.; Clarke, J.M. Breeding durum wheat in western Ganada: Historical trends in yield and related variables. Can. J. Plant Sci. 1995, 75, 55–60. [Google Scholar] [CrossRef]
- Singh, A.K.; Clarke, J.M.; Knox, R.E.; DePauw, R.M.; McCaig, T.N.; Fernandez, M.R.; Clarke, F.R. Transcend Durum wheat. Can. J. Plant Sci. 2012, 92, 809–813. [Google Scholar] [CrossRef]
- DePauw, R.M.; Knox, R.E.; Clarke, F.R.; Wang, H.; Fernandez, M.R.; Clarke, J.M.; McCaig, T.N. Shifting undesirable correlations. Euphytica 2007, 157, 409–415. [Google Scholar] [CrossRef]
- McCallum, B.D.; DePauw, R.M. A review of wheat cultivars grown in the Canadian prairies. Can. J. Plant Sci. 2008, 88, 649–677. [Google Scholar] [CrossRef]
- Townley-Smith, T.F.; Patterson, L.A.; DePauw, R.M.; Lendrum, C.W.B.; McCrystal, G.E. Kyle Durum Wheat. Can. J. Plant Sci. 1987, 67, 225–227. [Google Scholar] [CrossRef]
- Clarke, J.M.; Knox, R.E.; DePauw, R.M.; Clarke, F.R.; Fernandez, M.R.; McCaig, T.N.; Singh, A.K. Brigade durum wheat. Can. J. Plant Sci. 2009, 89, 505–509. [Google Scholar] [CrossRef]
- Pozniak, C.J. CDC Desire durum wheat. Can. J. Plant Sci. 2013, 93, 1265–1270. [Google Scholar] [CrossRef]
- Pozniak, C.J. CDC Vivid durum wheat. Can. J. Plant Sci. 2013, 93, 137–141. [Google Scholar] [CrossRef]
- Singh, A.K.; Clarke, J.M.; DePauw, R.M.; Knox, R.E.; McCaig, T.N.; Cuthbert, R.D. AAC current durum wheat. Can. J. Plant Sci. 2015, 95, 589–594. [Google Scholar] [CrossRef]
- Singh, A.K.; Clarke, J.M.; Knox, R.E.; DePauw, R.M.; McCaig, T.N.; Cuthbert, R.D.; Clarke, F.R.; Fernandez, M.R. AAC Raymore durum wheat. Can. J. Plant Sci. 2014, 94, 1289–1296. [Google Scholar] [CrossRef]
- Pozniak, C.J.; Nilsen, K.; Clarke, J.M.; Beres, B.L. CDC Fortitude durum wheat. Can. J. Plant Sci. 2015, 95, 1013–1019. [Google Scholar] [CrossRef]
- Singh, A.K.; DePauw, R.M.; Knox, R.E.; Clarke, J.M.; McCaig, T.N.; Cuthbert, R.D.; Ruan, Y. AAC Durafield durum wheat. Can. J. Plant Sci. 2016, 96, 719–725. [Google Scholar] [CrossRef]
- Singh, A.K.; Clarke, J.M.; Knox, R.E.; DePauw, R.M.; Wise, I.; Thomas, J.; McCaig, T.N.; Cuthbert, R.D.; Clarke, F.R.; Fernandez, M.R.; et al. AAC Marchwell durum wheat. Can. J. Plant Sci. 2015, 95, 189–195. [Google Scholar] [CrossRef]
- Pozniak, C.J.; Clarke, J.M. CDC carbide durum wheat. Can. J. Plant Sci. 2015, 95, 1007–1012. [Google Scholar] [CrossRef]
- Singh, A.K.; DePauw, R.M.; Knox, R.E.; Clarke, J.M.; McCaig, T.N.; Cuthbert, R.D.; Ruan, Y. AAC Cabri durum wheat. Can. J. Plant Sci. 2016, 97, 135–143. [Google Scholar] [CrossRef]
- Singh, A.K.; DePauw, R.M.; Knox, R.E.; Clarke, J.M.; McCaig, T.N.; Cuthbert, R.D.; Ruan, Y. AAC Spitfire durum wheat. Can. J. Plant Sci. 2016, 97, 157–164. [Google Scholar] [CrossRef]
- Pozniak, C.J.; Clarke, J.M. CDC precision durum wheat. Can. J. Plant Sci. 2017, 97, 344–348. [Google Scholar] [CrossRef]
- Pozniak, C.J.; Clarke, J.M. CDC dynamic durum wheat. Can. J. Plant Sci. 2017, 97, 380–384. [Google Scholar] [CrossRef]
- Pozniak, C.J.; Clarke, J.M. CDC alloy durum wheat. Can. J. Plant Sci. 2017, 97, 385–389. [Google Scholar] [CrossRef]
- Ruan, Y.; Singh, A.K.; DePauw, R.M.; Knox, R.E.; McCaig, T.N.; Cuthbert, R.D.; McCallum, B.; Fetch, T.; Beres, B. AAC Congress durum wheat. Can. J. Plant Sci. 2017, 98, 483–491. [Google Scholar] [CrossRef]
- Clarke, F.R.; Clarke, J.M.; DePauw, R.M.; Fernandez, M.R.; Fox, S.; Gilbert, J.; Humphreys, G.; Knox, R.E.; McCaig, T.N.; Procunier, D.; et al. Strategic approach to mitigating weather induced defects of wheat quality. Euphytica 2005, 143, 285–290. [Google Scholar] [CrossRef]
- Knox, R.E.; Clarke, F.R.; Clarke, J.M.; Fox, S.L.; DePauw, R.M.; Singh, A.K. Enhancing the identification of genetic loci and transgressive segregants for preharvest sprouting resistance in a durum wheat population. Euphytica 2012, 186, 193–206. [Google Scholar] [CrossRef]
- DePauw, R.M.; Knox, R.E.; Singh, A.K.; Fox, S.L.; Humphreys, D.G.; Hucl, P. Developing standardized methods for breeding preharvest sprouting resistant wheat, challenges and successes in Canadian wheat. Euphytica 2012, 188, 7–14. [Google Scholar] [CrossRef]
- Singh, A.K.; Knox, R.E.; Clarke, J.M.; Clarke, F.R.; Singh, A.; DePauw, R.M.; Cuthbert, R.D. Genetics of pre-harvest sprouting resistance in a cross of Canadian adapted durum wheat genotypes. Mol. Breed. 2014, 33, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.; Elias, E.; Benscher, D.; Ishikawa, G.; Huang, Y.F.; Saito, M.; Nakamura, T.; Xu, S.; Faris, J.; Sorrells, M. Genetic mapping of major-effect seed dormancy quantitative trait loci on chromosome 2B using recombinant substitution lines in tetraploid wheat. Crop Sci. 2016, 56, 59–72. [Google Scholar] [CrossRef]
- Codex-Alimentarius-Commission. Report of the 36th Session of the Codex Committee on Food Additives and Contaminants; FAO/WHO: Rome, Italy, 2004. [Google Scholar]
- Wiebe, K.; Harris, N.S.; Faris, J.D.; Clarke, J.M.; Knox, R.E.; Taylor, G.J.; Pozniak, C.J. Targeted mapping of Cdu1, a major locus regulating grain cadmium concentration in durum wheat (Triticum turgidum, L. var durum). Theor. Appl. Genet. 2010, 121, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Varieties of Grain Crops; Saskatchewan Ministry of Agriculture: Regina, SK, Canada, 2018; p. 32. [Google Scholar]
- Anderson, J.A. Marker-assisted selection for Fusarium head blight resistance in wheat. Int. J. Food Microbiol. 2007, 119, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Somers, D.J.; Fedak, G.; Clarke, J.; Cao, W. Mapping of FHB resistance QTLs in tetraploid wheat. Genome 2006, 49, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Comeau, A.; Langevin, F.; Hucl, P.; Clarke, J.M.; Brule-Babel, A.; Pozniak, C.J. Identification of novel QTL for resistance to Fusarium head blight in a tetraploid wheat population. Genome 2012, 55, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Barker, P.S.; McKenzie, R.I.H. Possible sources of resistance to the wheat midge in wheat. Can. J. Plant Sci. 1996, 76, 689–695. [Google Scholar] [CrossRef]
- Thomas, J.; Fineberg, N.; Penner, G.; McCartney, C.; Aung, T.; Wise, I.; McCallum, B. Chromosome location and markers of Sm1: A gene of wheat that conditions antibiotic resistance to orange wheat blossom midge. Mol. Breed. 2005, 15, 183–192. [Google Scholar] [CrossRef]
- Smith, M.A.H.; Lamb, R.J.; Wise, I.L.; Olfert, O.O. An interspersed refuge for Sitodiplosis mosellana (Diptera: Cecidomyiidae) and a biocontrol agent Macroglenes penetrans (Hymenoptera: Pteromalidae) to manage crop resistance in wheat. Bull. Entomol. Res. 2004, 94, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.H.; Wise, I.L.; Lamb, R.J. Survival of Sitodiplosis mosellana (Diptera: Cecidomyiidae) on wheat (Poaceae) with antibiosis resistance: Implication for the evolution of virulence. Can. Entomol. 2007, 139, 133–140. [Google Scholar] [CrossRef]
- Clarke, F.R.; Clarke, J.M.; Knox, R.E. Inheritance of stem solidness in eight durum wheat crosses. Can. J. Plant Sci. 2002, 82, 661–664. [Google Scholar] [CrossRef]
- Nilsen, K.T.; N’Diaye, A.; MacLachlan, P.R.; Clarke, J.M.; Ruan, Y.; Cuthbert, R.D.; Knox, R.E.; Wiebe, K.; Cory, A.T.; Walkowiak, S.; et al. High density mapping and haplotype analysis of the major stem-solidness locus SSt1 in durum and common wheat. PLoS ONE 2017, 12, e0175285. [Google Scholar] [CrossRef] [PubMed]








| Name | Grain Yield % Increase over Strongfield 1 | Protein Dev Strongfield 1 | Key Traits | Released | Reference |
|---|---|---|---|---|---|
| Transcend | 3.1 | −0.3 | Improved FHB resistance MS * | 2010 | [156] |
| CDC Desire | 1.0 | −0.2 | High grain pigment | 2012 | [161] |
| CDC Vivid | 3.0 | −0.3 | High grain pigment, strong straw | 2012 | [162] |
| AAC Current | 1.0 | 0.0 | High test weight | 2012 | [163] |
| AAC Raymore | −5.0 | 0.2 | Solid stem, resistant to sawfly | 2012 | [164] |
| CDC Fortitude | 4.0 | −0.2 | Solid stem, resistant to sawfly | 2013 | [165] |
| AAC Durafield | 2.0 | −0.2 | Semolina yield | 2013 | [166] |
| AAC Marchwell VB | −1.0 | −0.1 | Midge tolerant | 2013 | [167] |
| CDC Carbide VB | 7.0 | −0.2 | Midge tolerant | 2014 | [168] |
| AAC Cabri | 5.0 | −0.3 | Solid stem, resistant to sawfly | 2014 | [169] |
| AAC Spitfire | 9.0 | −0.5 | High pigment, strong straw | 2014 | [170] |
| CDC Precision | 10.0 | −0.6 | High test weight | 2015 | [171] |
| CDC Dynamic | 7.0 | 0.0 | High test weight | 2015 | [172] |
| CDC Alloy | 10.0 | −0.4 | High test weight | 2015 | [173] |
| AAC Congress | 9.0 | −0.5 | Semolina yield | 2015 | [174] |
| CDC Credence | 6.0 | −0.7 | Improved FHB resistance (MS *) | 2016 | N/A |
| AAC Stronghold | 4.0 | −0.4 | Very strong straw, solid stem, resistant to sawfly | 2016 | N/A |
| DT587 | 8.0 | −0.5 | N/A | 2017 | N/A |
| AAC Succeed VB | 4.0 | −0.1 | Midge tolerant | 2017 | N/A |
| DT591 | 6.1 | −0.2 | Imidazolinone tolerance | 2018 | N/A |
| DT878 | 9.5 | −0.2 | Solid stem, resistant to sawfly | 2018 | N/A |
| DT881 | 9.9 | −0.3 | Strong straw | 2018 | N/A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Niu, Y.; Ruan, Y.; DePauw, R.M.; Singh, A.K.; Gan, Y. Agronomic Advancement in Tillage, Crop Rotation, Soil Health, and Genetic Gain in Durum Wheat Cultivation: A 17-Year Canadian Story. Agronomy 2018, 8, 193. https://doi.org/10.3390/agronomy8090193
Li L, Niu Y, Ruan Y, DePauw RM, Singh AK, Gan Y. Agronomic Advancement in Tillage, Crop Rotation, Soil Health, and Genetic Gain in Durum Wheat Cultivation: A 17-Year Canadian Story. Agronomy. 2018; 8(9):193. https://doi.org/10.3390/agronomy8090193
Chicago/Turabian StyleLi, Lin, Yining Niu, Yuefeng Ruan, Ron M. DePauw, Asheesh K. Singh, and Yantai Gan. 2018. "Agronomic Advancement in Tillage, Crop Rotation, Soil Health, and Genetic Gain in Durum Wheat Cultivation: A 17-Year Canadian Story" Agronomy 8, no. 9: 193. https://doi.org/10.3390/agronomy8090193
APA StyleLi, L., Niu, Y., Ruan, Y., DePauw, R. M., Singh, A. K., & Gan, Y. (2018). Agronomic Advancement in Tillage, Crop Rotation, Soil Health, and Genetic Gain in Durum Wheat Cultivation: A 17-Year Canadian Story. Agronomy, 8(9), 193. https://doi.org/10.3390/agronomy8090193





