Kura Clover Living Mulch: Spring Management Effects on Nitrogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Soil N
2.3. Soil-Atmosphere Gas Exchange
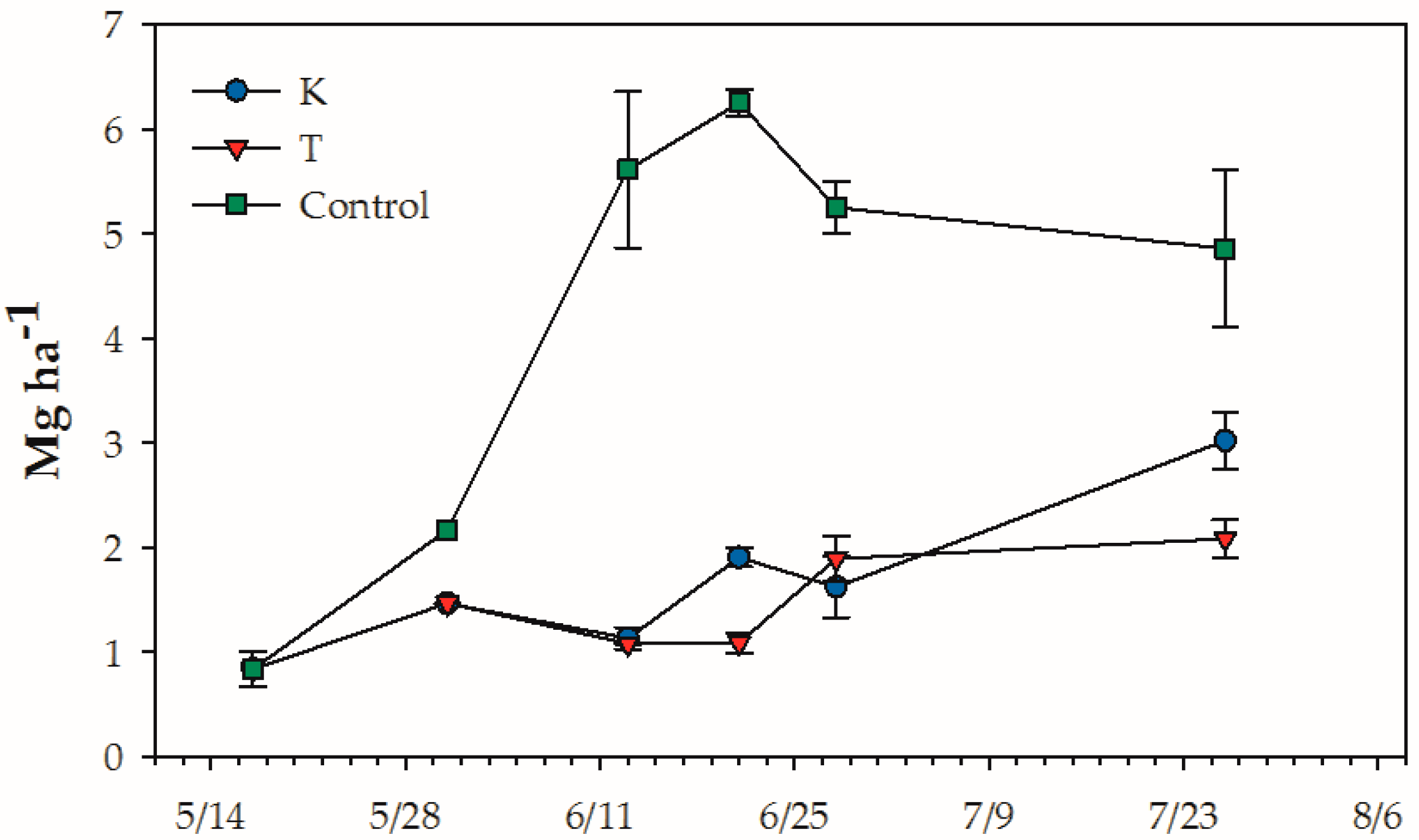
2.4. Clover Sampling
2.5. Environmental Conditions
2.6. Data Analysis
3. Results
3.1. Environmental Conditions
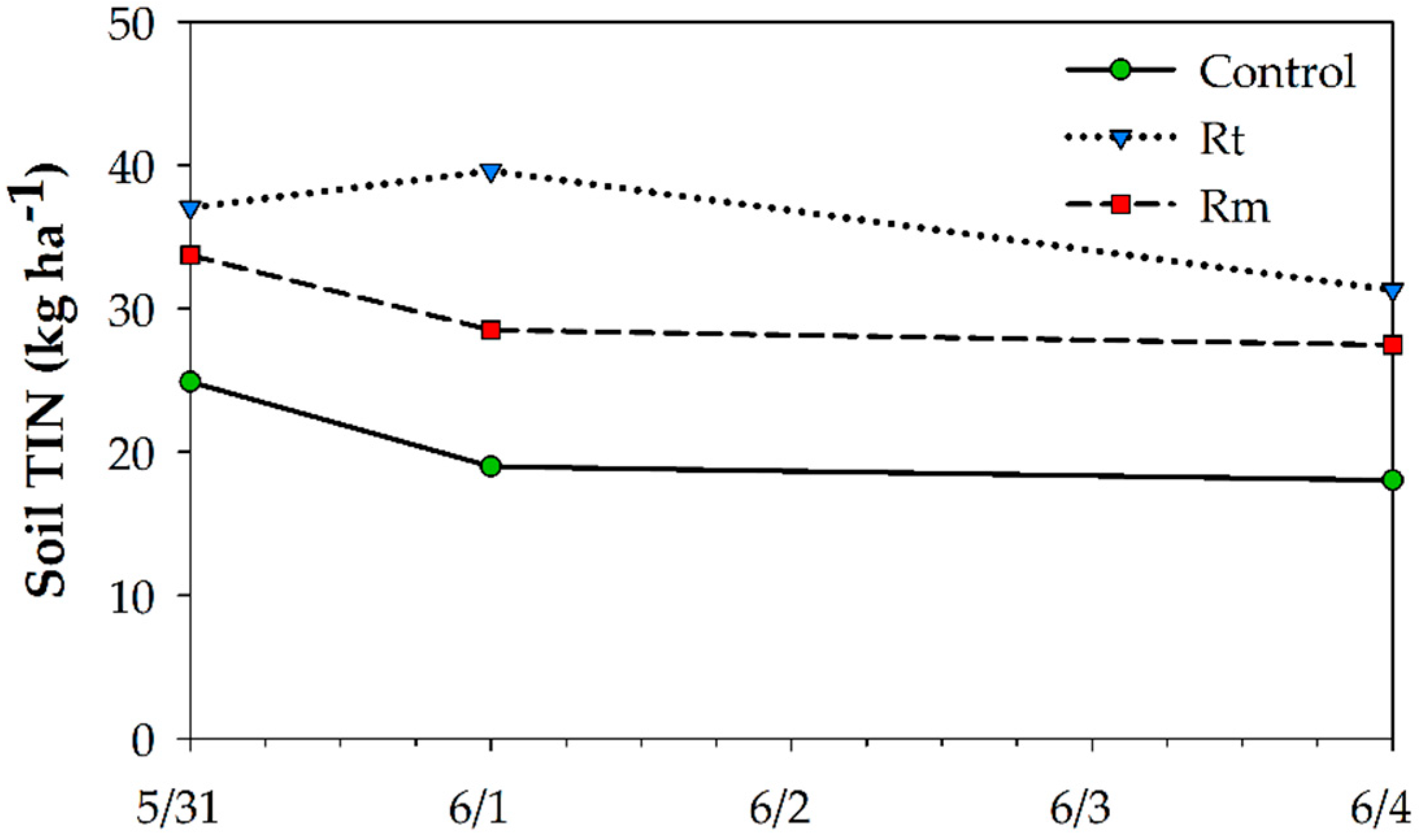
3.2. Short-Term Soil N Response to Residue Management
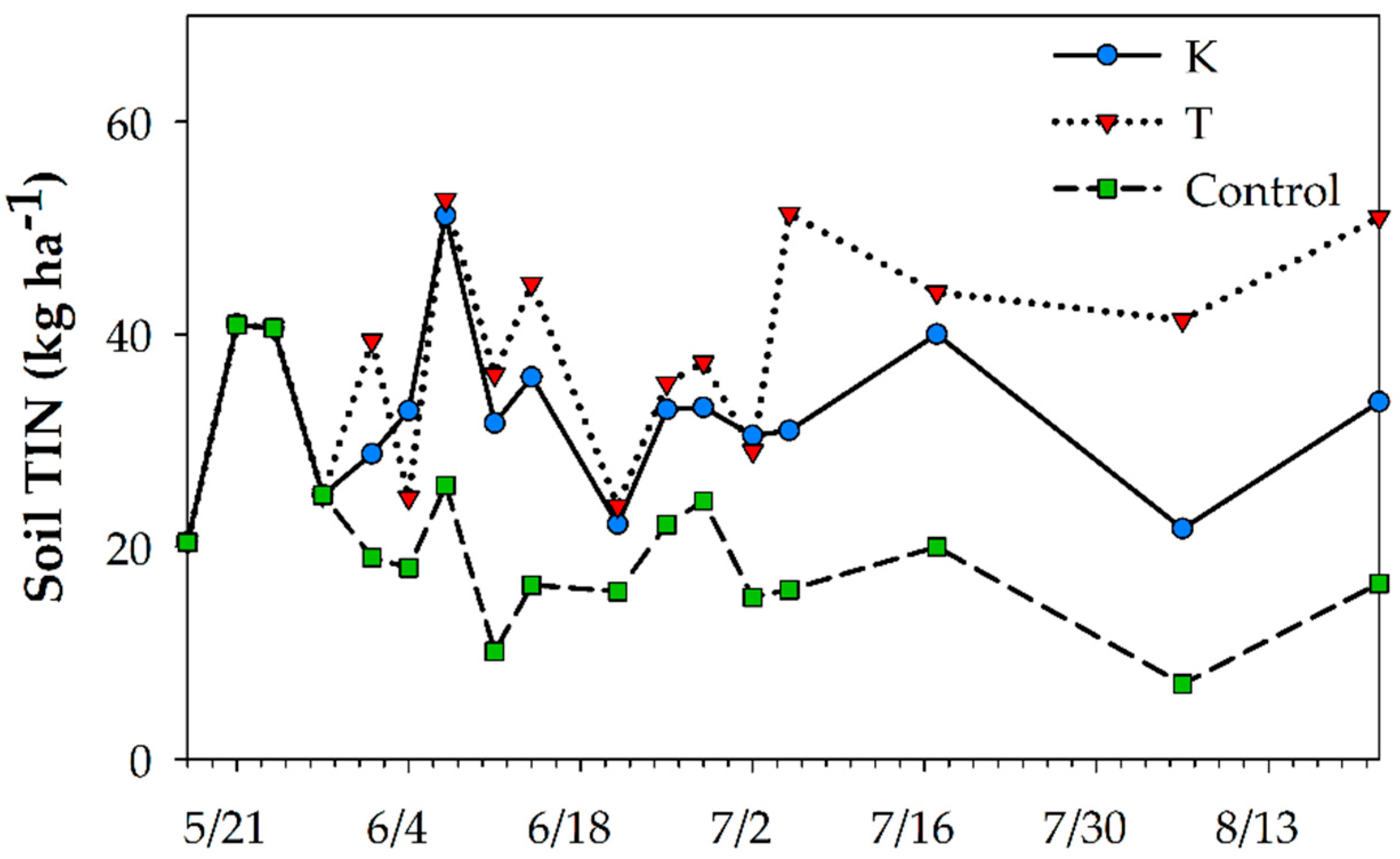
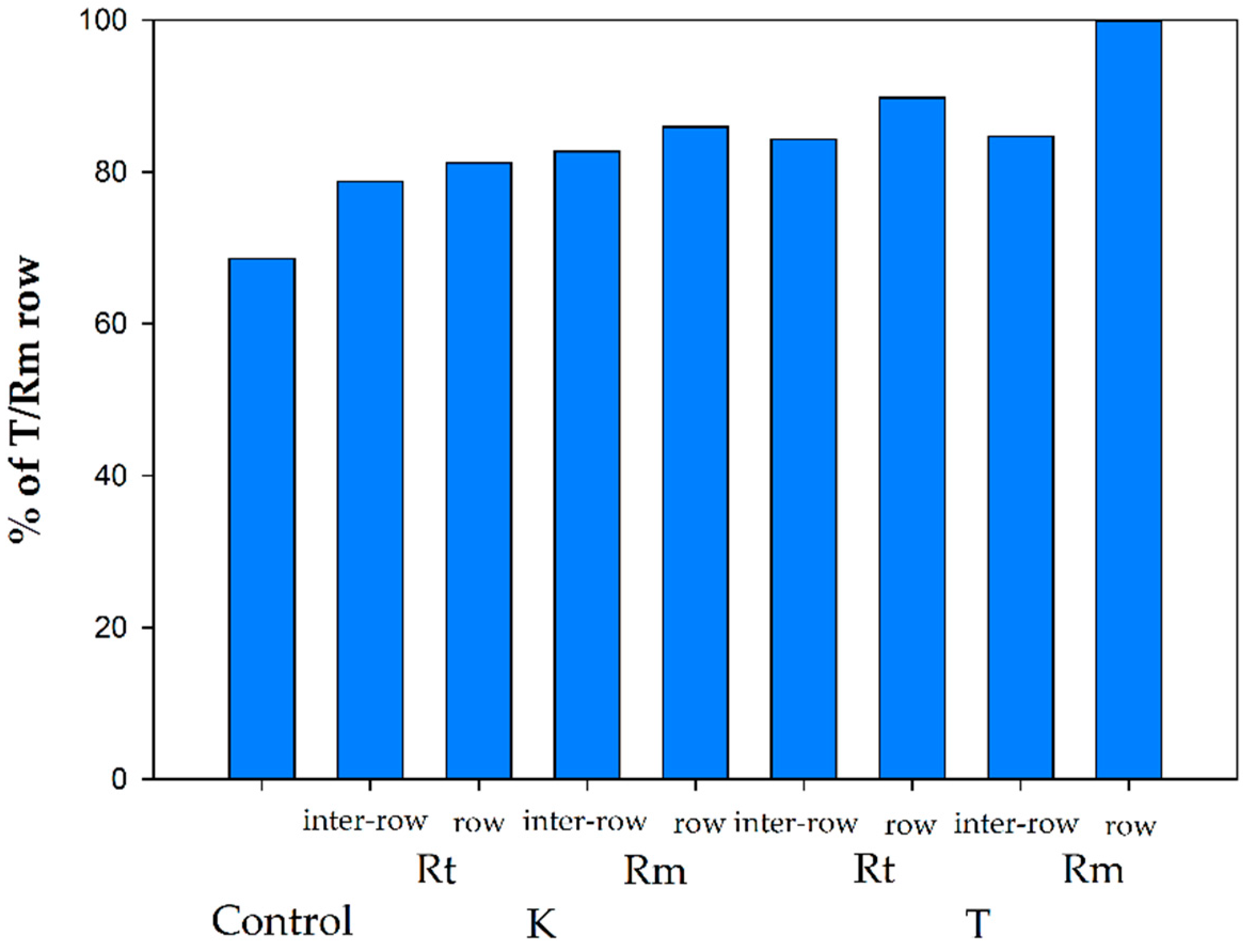
3.3. Zone Differentiated N Response to Residue Management and Row Establishment
3.4. Zone Weighted N Response to Residue and Row Management
3.5. Cumulative N Gas Emissions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Von Bieberstein, M. Flora Taurico-Caucasica Exhibens Stirpes Phaenogamas; Volume 2; Imperatorskii Khar’kovskii Universitet ptr: Kharkov, Ukraine, 1808. [Google Scholar]
- Peterson, P.R.; Sheaffer, C.C.; Jordan, R.M.; Christians, C.J. Responses of kura clover to sheep grazing and clipping: I.Yield and forage quality. Agron. J. 1994, 86, 655–660. [Google Scholar] [CrossRef]
- Speer, G.S.; Allinson, D.W. Kura Clover (Trifolium ambiguum): Legume for Forage and Soil Conservation. Econ. Bot. 1985, 39, 165–176. [Google Scholar] [CrossRef]
- Zemenchik, R.A.; Albrecht, K.A.; Boerboom, C.M.; Lauer, J.G. Corn production with kura clover as a living mulch. Agron. J. 2000, 92, 698–705. [Google Scholar] [CrossRef]
- Siller, A.R.S.; Albrecht, K.A.; Jokela, W.E. Soil erosion and nutrient runoff in corn silage production with Kura clover living mulch and winter rye. Agron. J. 2016, 108, 989–999. [Google Scholar] [CrossRef]
- Ochsner, T.E.; Schumacher, T.W.; Venterea, R.T.; Feyereisen, G.W.; Baker, J.M. Soil Water Dynamics and Nitrate Leaching Under Corn–Soybean Rotation, Continuous Corn, and Kura Clover. Vadose Zo. J. 2017. [Google Scholar] [CrossRef]
- Ochsner, T.E.; Albrecht, K.A.; Schumacher, T.W.; Baker, J.M.; Berkevich, R.J. Water balance and nitrate leaching under corn in kura clover living mulch. Agron. J. 2010, 102, 1169–1178. [Google Scholar] [CrossRef]
- Affeldt, R.P.; Albrecht, K.A.; Boerboom, C.M.; Bures, E.J. Integrating Herbicide-Resistant Corn Technology in a Kura Clover Living Mulch System. Agron. J. 2004, 96, 247–251. [Google Scholar] [CrossRef]
- Pedersen, P.; Bures, E.J.; Albrecht, K.A. Soybean production in a kura clover living mulch system. Agron. J. 2009, 101, 653–656. [Google Scholar] [CrossRef]
- Grabber, J.H.; Jokela, W.E.; Lauer, J.G. Soil nitrogen and forage yields of corn grown with clover or grass companion crops and manure. Agron. J. 2014, 106, 952–961. [Google Scholar] [CrossRef]
- Sawyer, J.E.; Pedersen, P.; Barker, D.W.; Ruiz Diaz, D.A.; Albrecht, K. Intercropping corn and kura clover: Response to nitrogen fertilization. Agron. J. 2010, 102, 568–574. [Google Scholar] [CrossRef]
- Turner, P.A.; Baker, J.M.; Griffis, T.J.; Venterea, R.T. Impact of Kura Clover Living Mulch on Nitrous Oxide Emissions in a Corn–Soybean System. J. Environ. Qual. 2016, 45, 1782. [Google Scholar] [CrossRef]
- Pearson, C.H.; Brummer, J.E.; Beahm, A.T.; Hansen, N.C. Kura clover living mulch for furrow-irrigated corn in the intermountain west. Agron. J. 2014, 106, 1324–1328. [Google Scholar] [CrossRef]
- Singer, J. Legume living mulches in corn and soybean. Iowa State Univ. Univ. Ext. 2005, PM 2006. [Google Scholar]
- Blackshaw, R.E.; Molnar, L.J.; Moyer, J.R. Sweet clover termination effects on weeds, soil water, soil nitrogen, and succeeding wheat yield. Agron. J. 2010, 102, 634–641. [Google Scholar] [CrossRef]
- Liebman, A.M.; Grossman, J.; Brown, M.; Wells, M.S.; Reberg-Horton, S.C.; Shi, W. Legume cover crops and tillage impact nitrogen dynamics in organic corn production. Agron. J. 2018, 110, 1046–1057. [Google Scholar] [CrossRef]
- Alexander, J.R.; Baker, J.M.; Venterea, R.T.; Coulter, J.A. High Yielding Corn with Reduced Nitrogen Inputs: Production in Kura Clover Living Mulch. In Proceedings of the ASA, CSSA, and CSA International Annual Meeting, Baltimore, MD, USA, 4–7 November 2018. [Google Scholar]
- Baker, J.M. Vegetative propagation of kura clover: A field-scale test. Can. J. Plant Sci. 2012, 92, 1245–1251. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen—Inorganic forms. In Methods of Soil Analysis; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Venterea, R.T.; Coulter, J.A.; Dolan, M.S. Evaluation of Intensive “4R” Strategies for Decreasing Nitrous Oxide Emissions and Nitrogen Surplus in Rainfed Corn. J. Environ. Qual. 2016, 45, 1186. [Google Scholar] [CrossRef] [Green Version]
- Reicosky, D.C.; Archer, D.W. Moldboard plow tillage depth and short-term carbon dioxide release. Soil Tillage Res. 2007, 94, 109–121. [Google Scholar] [CrossRef]
- Reicosky, D.C.; Lindstrom, M.J. Fall tillage method: Effect on short-term carbon dioxide flux from soil. Agron. J. 1993, 85, 1237–1243. [Google Scholar] [CrossRef]
- Collier, S.M.; Dean, A.P.; Oates, L.G.; Ruark, M.D.; Jackson, R.D. Does Plant Biomass Manipulation in Static Chambers Affect Nitrous Oxide Emissions from Soils? J. Environ. Qual. 2016, 45, 751. [Google Scholar] [CrossRef] [Green Version]
- Parkin, T.B.; Venterea, R.T.; Hargreaves, S.K. Calculating the Detection Limits of Chamber-based Soil Greenhouse Gas Flux Measurements. J. Environ. Qual. 2012, 41, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigaki, F.; Dell, C.J. Comparison of low-cost methods for measuring ammonia volatilization. Agron. J. 2015, 107, 1392–1400. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen—Inorganic Forms. In Methods of Soil Analysis Part 3: Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis; Sparks, D., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 1085–1089. [Google Scholar]
- Pietikäinen, J.; Pettersson, M.; Bååth, E. Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiol. Ecol. 2005, 52, 49–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolardot, B.; Fauvet, G.; Cheneby, D. Carbon and nitrogen cycling through soil microbial biomass at various temperatures. Soil Biol. Biochem. 1994, 26, 253–261. [Google Scholar] [CrossRef]
- Maharjan, B.; Venterea, R.T. Nitrite intensity explains N management effects on N2O emissions in maize. Soil Biol. Biochem. 2013, 66, 229–238. [Google Scholar] [CrossRef]
- Engel, R.; Liang, D.L.; Wallander, R.; Bembenek, A. Influence of Urea Fertilizer Placement on Nitrous Oxide Production from a Silt Loam Soil. J. Environ. Qual. 2010, 39, 115. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.L.; Li, X.; Grant, C.A. Influence of fertilizer nitrogen source and management practice on N2O emissions from two Black Chernozemic soils. Can. J. Soil Sci. 2008, 88, 219–227. [Google Scholar] [CrossRef]
- Venterea, R.T.; Clough, T.J.; Coulter, J.A.; Breuillin-Sessoms, F.; Wang, P.; Sadowsky, M.J. Ammonium sorption and ammonia inhibition of nitrite-oxidizing bacteria explain contrasting soil N2O production. Sci. Rep. 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Loska, K.; Wiechuła, D.; Pelczar, J. Application of enrichment factor to assessment of zinc enrichment/depletion in farming soils. Commun. Soil Sci. Plant Anal. 2005, 36, 1117–1128. [Google Scholar] [CrossRef]
- Mariotti, A.; Germon, J.C.; Hubert, P.; Kaiser, P.; Letolle, R.; Tardieux, A.; Tardieux, P. Experimental Determination of Nitrogen Kinetic Isotope Fractionation: Some Principles; Illistration for the Denitrification and Nitrification Processes. Plant Soil 1981, 62, 413–430. [Google Scholar] [CrossRef]
- Brimhall, G.H.; Lewis, C.J.; Ague, J.J.; Dietrich, W.E.; Hampel, J.; Teague, T.; Rix, P. Metal enrichment in bauxites by deposition of chemically mature aeolian dust. Nature 1988, 336, 403–405. [Google Scholar] [CrossRef]
- Kutner, M.H.; Nachtsheim, C.J.; Neter, J. Applied Linear Regression Models; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Cassman, K.G.; Dobermann, A.R.; Walters, D.T. Agroecosystems, Nitrogen-use Efficiency, and Nitrogen Management. Agron. Hortic. 2002, 31, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Follett, R.F.; Hatfield, J.L. Nitrogen in the Environment: Sources, Problems, and Management. Sci. World J. 2001, 1, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Bertram, T.H.; Heckel, A.; Richter, A.; Burrows, J.P.; Cohen, R.C. Satellite measurements of daily variations in soil NOx emissions. Geophys. Res. Lett. 2005, 32, 3–6. [Google Scholar] [CrossRef]
- Harper, L.A.; Hendrix, P.F.; Langdale, G.W.; Coleman, D.C. Clover management to provide optimum nitrogen and soil water conservation. Crop Sci. 1995, 35, 176–182. [Google Scholar] [CrossRef]
- Quemada, M.; Cabrera, M.L. Carbon and Nitrogen Mineralized from Leaves and Stems of Four Cover Crops. Soil Sci. Soc. Am. J. 1995, 59, 471–477. [Google Scholar] [CrossRef]
- Dabney, S.M.; Bouldin, D.R. Fluxes of Ammonia Over an Alfalfa Field. Agron. J. 1985, 77, 572–578. [Google Scholar] [CrossRef]
- US EPA. 2014 National Emissions Inventory, Version 2; Technical Support Document; US EPA: Washington, DC, USA, 2018.
- Kirchmann, H.; Witter, E. Ammonia volatilization during aerobic and anaerobic manure decomposition. Plant Soil 1989, 115, 35–41. [Google Scholar] [CrossRef]
- Duan, Y.-F.; Kong, X.-W.; Schramm, A.; Labouriau, R.; Eriksen, J.; Petersen, S.O. Microbial N Transformations and N2O Emission after Simulated Grassland Cultivation: Effects of the Nitrification Inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP). Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Licht, M.A.; Al-kaisi, M. Strip-tillage effect on seedbed soil temperature and other soil physical properties. Soil Tillage Res. 2005, 80, 233–249. [Google Scholar] [CrossRef]
- Van Den Bossche, A.; De Bolle, S.; De Neve, S.; Hofman, G. Effect of tillage intensity on N mineralization of different crop residues in a temperate climate. Soil Tillage Res. 2009, 103, 316–324. [Google Scholar] [CrossRef]
- Pattey, E.; Savoie, P.; Dube, P.A. The effect of a hay tedder on the field drying rate. Can. Agric. Eng. 1988, 30, 43–50. [Google Scholar]
- Buckmaster, D.R. Value of alfalfa losses on dairy farms. Am. Soc. Agric. Biol. Eng. 1990, 33. [Google Scholar] [CrossRef]
- Rotz, C.A.; Pitt, R.E.; Muck, R.E.; Allen, M.S.; Buckmaster, D.R. Direct-cut harvest and storage of alfalfa on the dairy farm. Am. Soc. Agric. Biol. Eng. 1993, 36, 621–628. [Google Scholar] [CrossRef]

| Treatment | Mowed | Residue | Row Management | Chemical Suppression |
|---|---|---|---|---|
| Date | 29 May | 31 May | 4 June | 22 June |
| Control | No | - | None | No |
| K/Rm | Yes | Removed | Band-kill | Yes |
| T/Rm | Zone-till | |||
| K/Rt | Returned | Band-kill | ||
| T/Rt | Zone-till |
| Factor | Time Integration | * EFs | ||||
|---|---|---|---|---|---|---|
| NO3–N | NH4–N | TIN | NO3–N | NH4–N | TIN | |
| ----g N m−2 * d---- | --------EF, %-------- | |||||
| System | ||||||
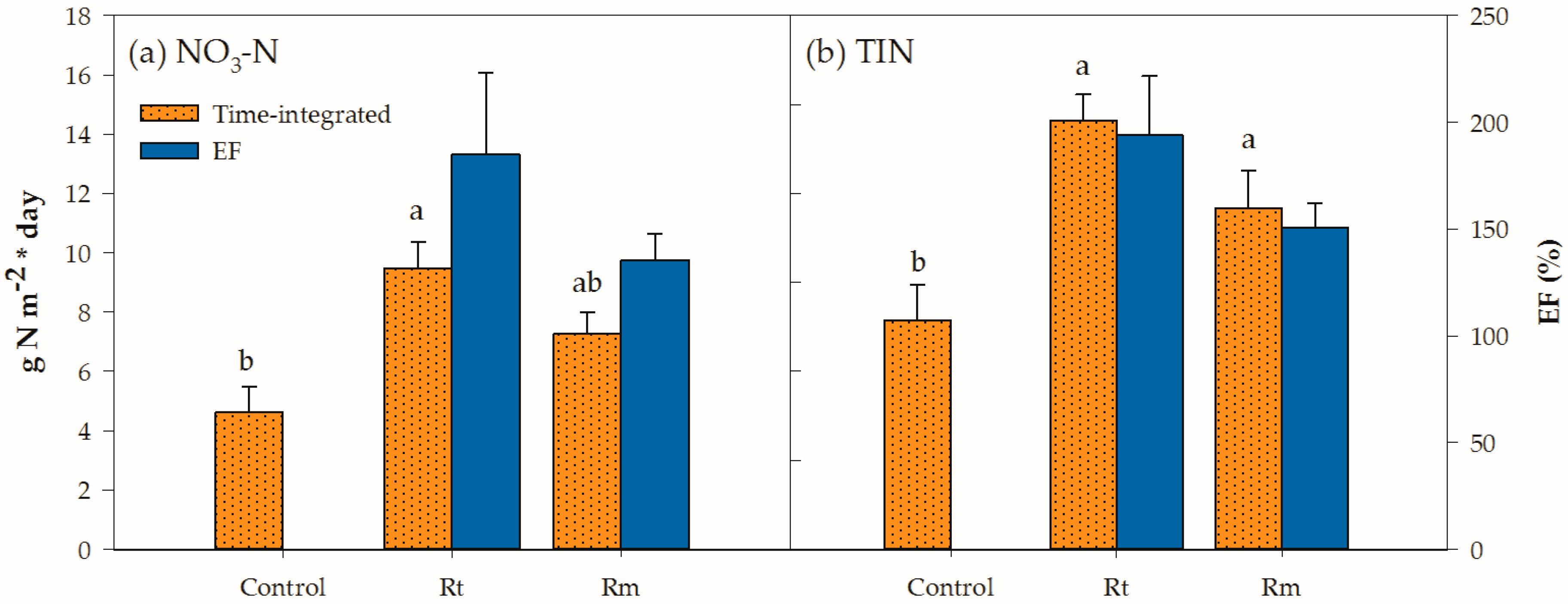
| ‡ Control | 4.6b† | 3.1 | 7.7b | |||
| § Rt | 9.5a | 5.0 | 14.5a | 222 | 161 | 194 |
| || Rm | 7.3ab | 4.3 | 11.5a | 162 | 135 | 151 |
| Significance | p-value | |||||
| 0.038 | 0.129 | 0.015 | 0.249 | 0.380 | 0.259 | |
| Fixed Effect | NO3–N | NH4–N | TIN | NH3–N |
|---|---|---|---|---|
| --------* EF, %-------- | ||||
| Row management (Rw) | ||||
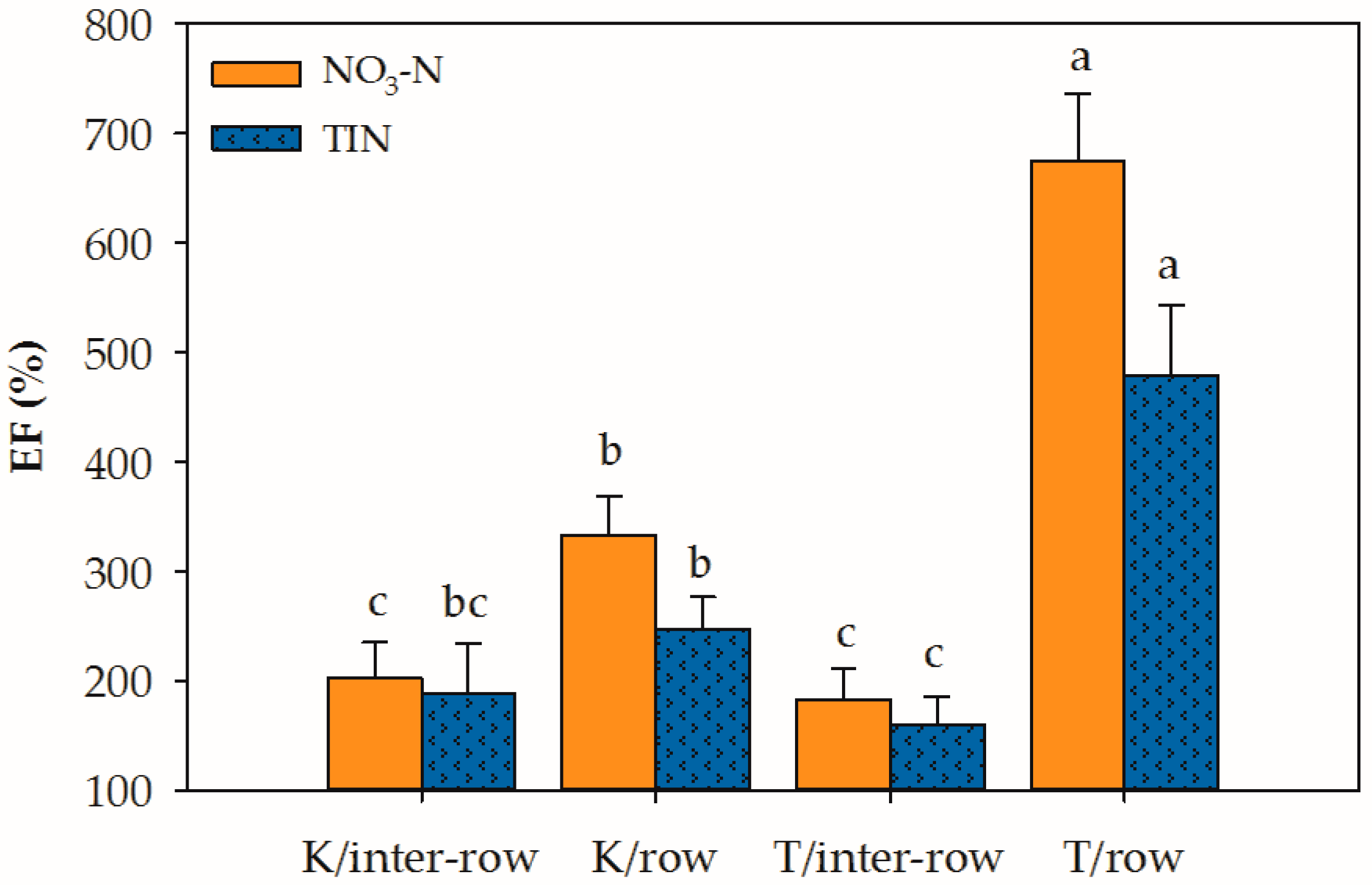
| ‡ K | 268b† | 125 | 218b | 146 |
| § T | 429a | 103 | 320a | 144 |
| Residue management (Rs) | ||||
| │ Rt | 357 | 98 | 270 | 151 |
| || Rm | 340 | 130 | 268 | 138 |
| Zone (Z) | ||||
| Interrow | 193b | 140 | 175b | 120b |
| Row | 504a | 88 | 363a | 170a |
| Significance | p-value | |||
| Rw | 0.001 | 0.535 | 0.005 | 0.881 |
| Rs | 0.578 | 0.377 | 0.954 | 0.369 |
| Rw × Rs | 0.144 | 0.787 | 0.218 | 0.140 |
| Z | <0.001 | 0.161 | <0.001 | 0.006 |
| Rw × Z | <0.001 | 0.340 | <0.001 | 0.476 |
| Rs × Z | 0.452 | 0.384 | 0.378 | 0.280 |
| Rw × Rs × Z | 0.938 | 0.649 | 0.749 | 0.475 |
| Fixed Effect | N2O–N | NH3–N | NO3–N | NH4–N | TIN | Biomass | Biomass-N |
|---|---|---|---|---|---|---|---|
| --------* EF, %-------- | Mg ha−1 | kg N ha−1 | |||||
| Row management (Rw) | |||||||
| ‡ K | 423 | 144 | 220b† | 116 | 179b | 1.6 | 47.3 |
| § T | 376 | 151 | 304a | 110 | 229a | 1.9 | 45.3 |
| Residue management (Rs) | |||||||
| │ Rt | 349 | 156 | 268 | 105 | 205 | 1.9 | 47.7 |
| || Rm | 450 | 139 | 256 | 121 | 203 | 1.6 | 45.0 |
| Significance | p-value | ||||||
| Rw | 0.594 | 0.507 | 0.002 | 0.765 | 0.003 | 0.339 | 0.789 |
| Rs | 0.272 | 0.149 | 0.495 | 0.486 | 0.850 | 0.305 | 0.724 |
| Rw × Rs | 0.810 | 0.102 | 0.100 | 0.690 | 0.082 | 0.852 | 0.887 |
| Fixed Effect | N2O–N | NH3–N |
|---|---|---|
| kg ha–1 | ||
| System | ||
| † Control | 0.65 | 0.64 |
| ‡ K/ │Rt | 2.12 | 0.81 |
| K/ || Rm | 2.59 | 0.86 |
| § T/Rt | 1.76 | 0.97 |
| T/Rm | 2.09 | 0.75 |
| Significance | p-value | |
| System | 0.060 | 0.274 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexander, J.R.; Venterea, R.T.; Baker, J.M.; Coulter, J.A. Kura Clover Living Mulch: Spring Management Effects on Nitrogen. Agronomy 2019, 9, 69. https://doi.org/10.3390/agronomy9020069
Alexander JR, Venterea RT, Baker JM, Coulter JA. Kura Clover Living Mulch: Spring Management Effects on Nitrogen. Agronomy. 2019; 9(2):69. https://doi.org/10.3390/agronomy9020069
Chicago/Turabian StyleAlexander, Jonathan R., Rodney T. Venterea, John M. Baker, and Jeffrey A. Coulter. 2019. "Kura Clover Living Mulch: Spring Management Effects on Nitrogen" Agronomy 9, no. 2: 69. https://doi.org/10.3390/agronomy9020069






