Land–Use Changes Influencing C Sequestration and Quality in Topsoil and Subsoil
Abstract
:1. Introduction
2. Materials and Methods
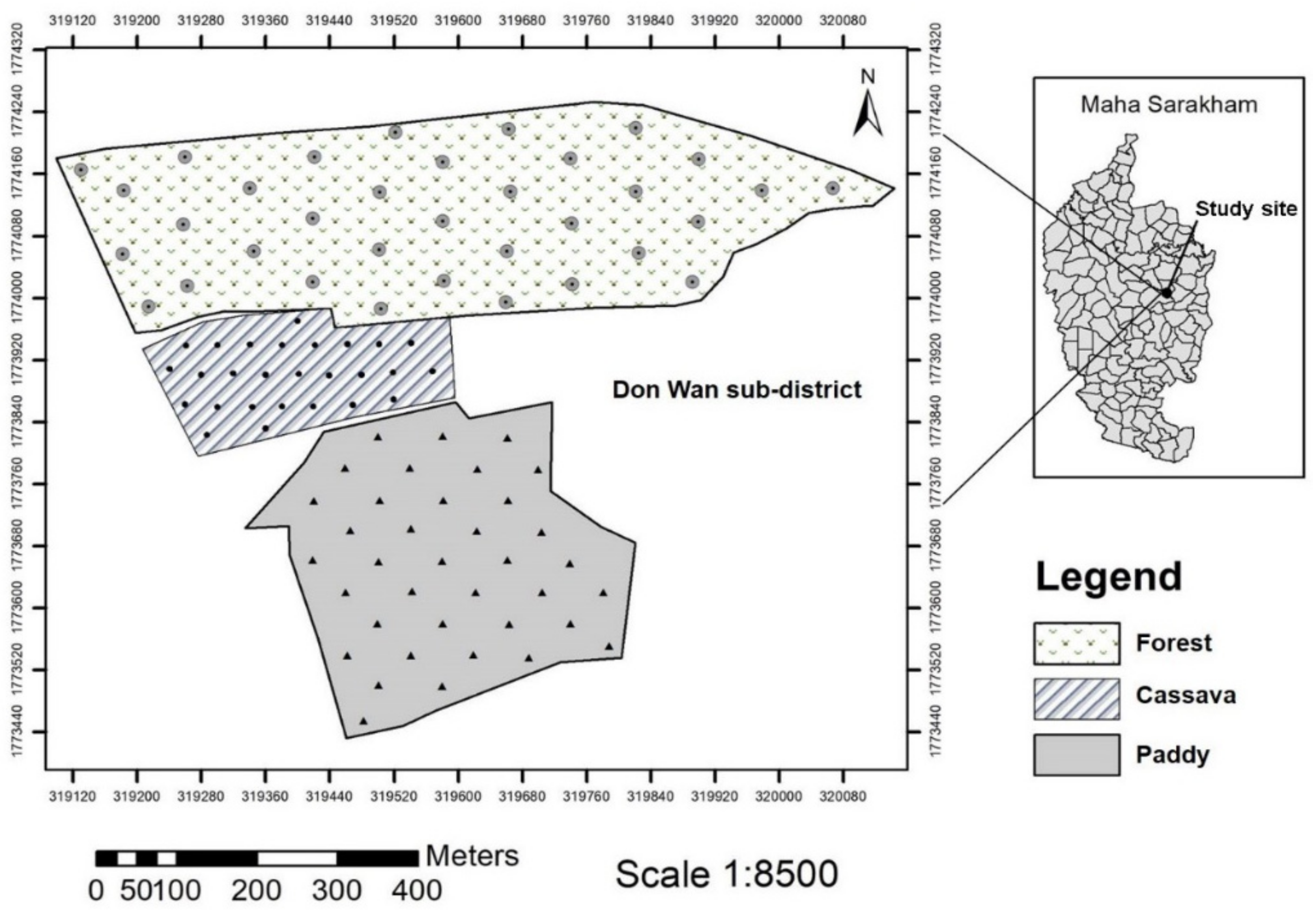
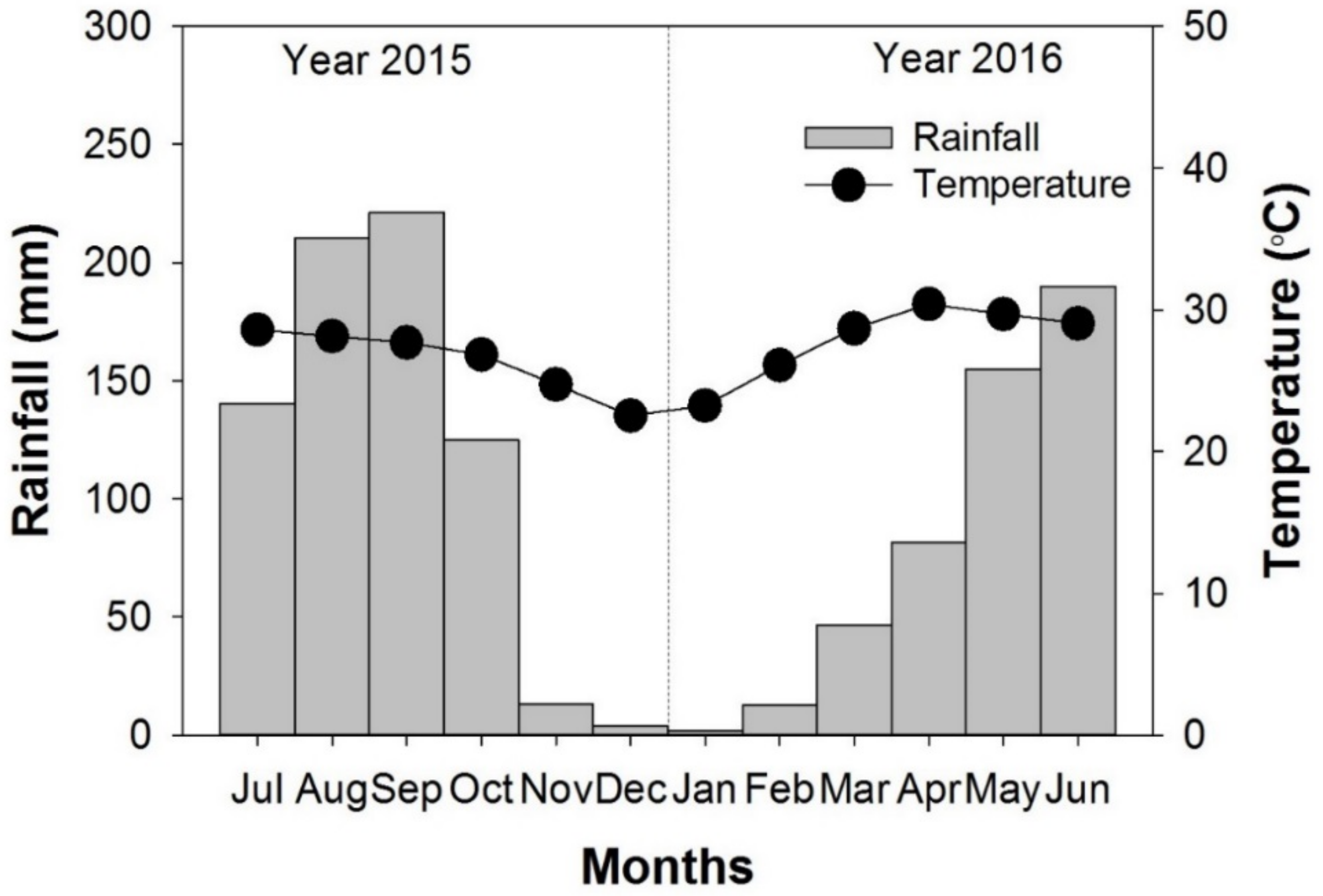
2.1. Study Site and Soil Sampling
2.2. Soil Physical Properties under Different Land Uses
2.3. Soil Analysis
2.4. Statistical Analysis
3. Results and Discussion
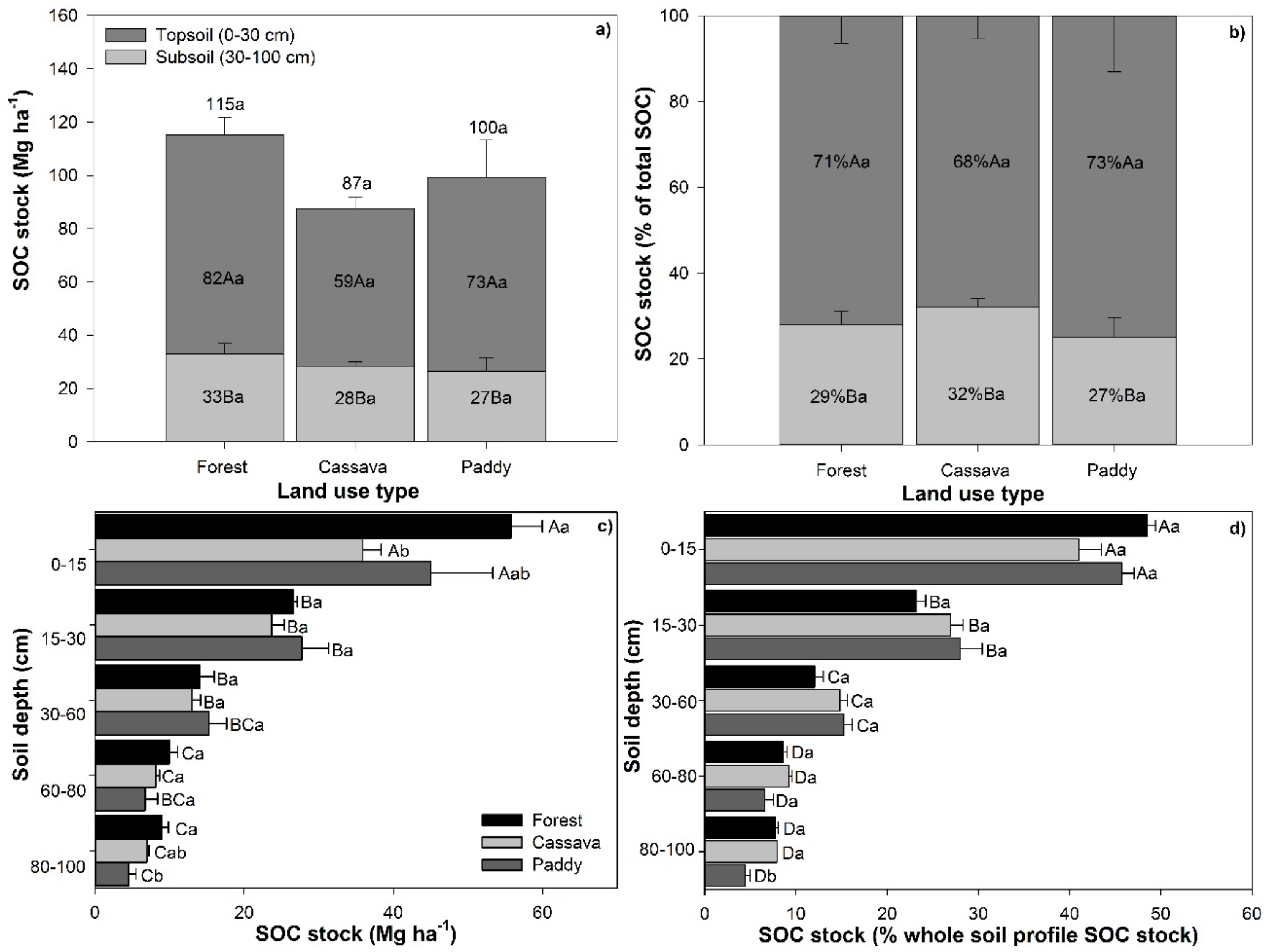
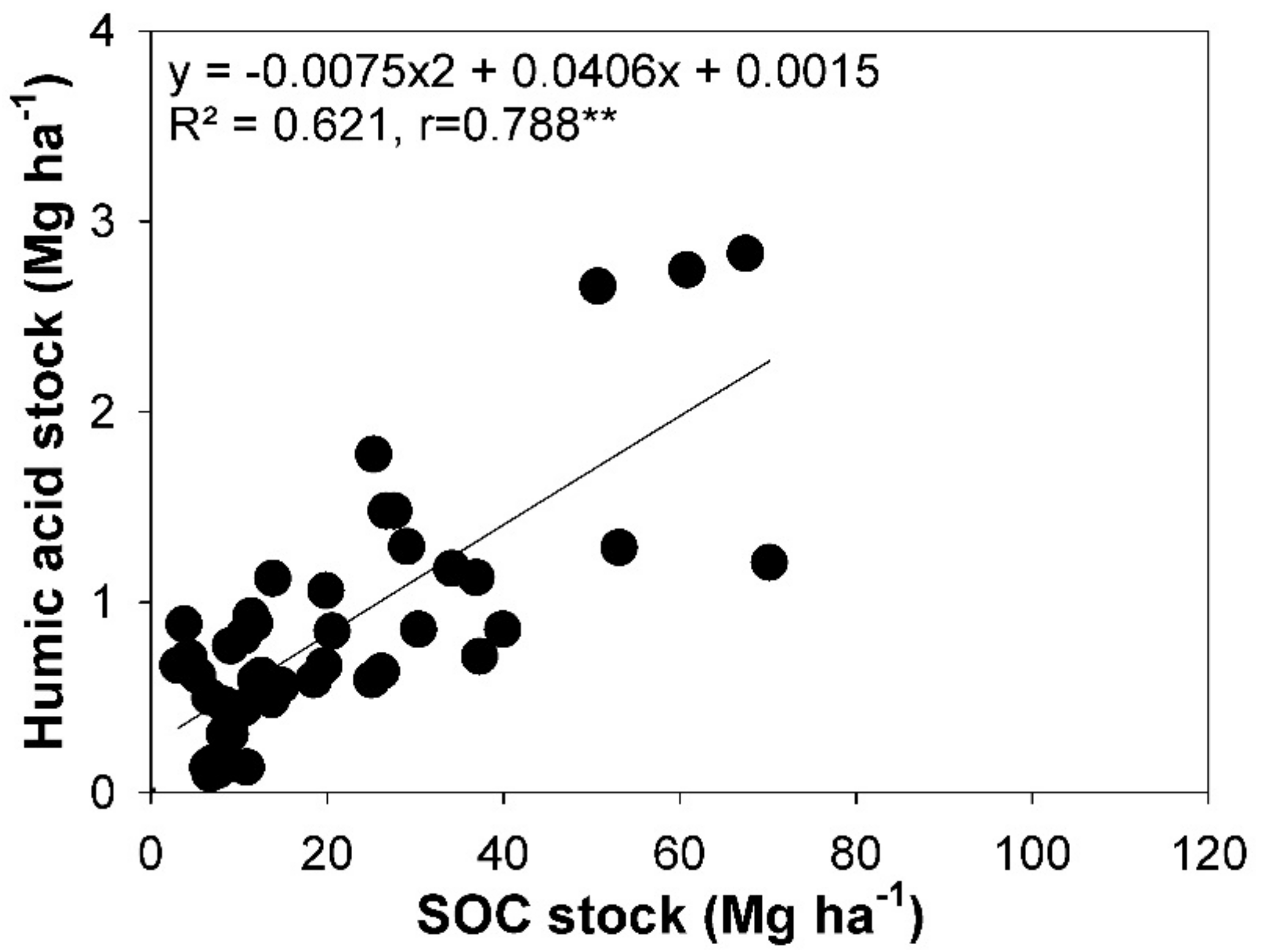
3.1. Carbon Sequestration in Topsoil and Subsoil
3.2. Carbon Loss Due to Land Use Change
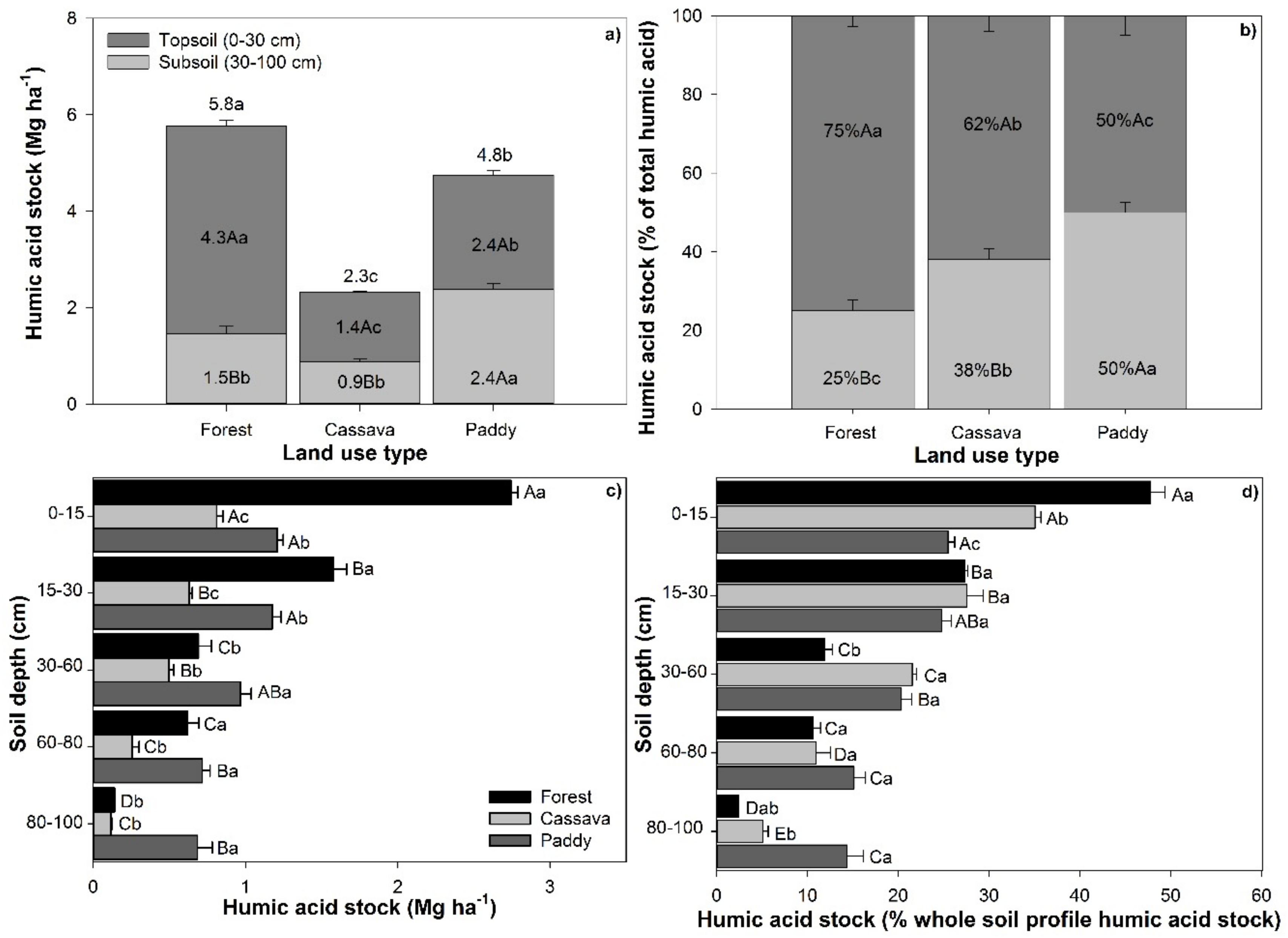
3.3. Chemical Composition of Carbon in Topsoils and Subsoils
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torn, M.S.; Swanston, C.W.; Castanha, C.; Trumbore, S.E. Storage and turnover of organic matter in soil. In Biophysico–Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; pp. 219–272. [Google Scholar]
- IPCC. IPCC Special Report on Land Use, Land–Use Change and Forestry; Cambridge University Press: Cambridge, UK, 2007; p. 375. [Google Scholar]
- Van der Werf, G.R.; Morton, D.C.; De Fries, R.S.; Olivier, J.G.J.; Kasibhatla, P.S.; Jackson, R.B.; Collatzg, G.J.; Randerson, J.T. CO2 emission from forest loss. Nat. Geosci. 2009, 2, 737–738. [Google Scholar] [CrossRef]
- FAO. FAO Forestry Paper; FAO: Rome, Italy, 2006; p. 147. [Google Scholar]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land–use change on soil organic carbon stocks—a meta–analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Barančíková, G.; Makovníková, J.; Halas, J. Effect of land use change on soil organic carbon. Agriculture 2016, 62, 10–18. [Google Scholar] [CrossRef]
- Aalde, H.; Gonzalez, P.; Gytarsky, M.; Krug, T.; Kurz, W.A.; Lasco, R.D.; Martino, D.L.; McConkey, B.G.; Ogle, S.; Paustian, K.; et al. Generic methodologies applicable to multiple land–use categories. In IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Agriculture, Forestry and Other Land Use; IGES: Kanagawa, Japan, 2006; Volume 4. [Google Scholar]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Rittl, T.F.; Oliveira, D.; Cerri, C.E.P. Soil carbon stock changes under different land uses in the Amazon. Geoderma Reg. 2017, 10, 138–143. [Google Scholar] [CrossRef]
- Bonfatti, B.R.; Hartemink, A.E.; Giasson, E.; Tornquist, C.G.; Adhikari, K. Digital mapping of soil carbon in a viticultural region of Southern Brazil. Geoderma 2016, 261, 204–221. [Google Scholar] [CrossRef]
- Callesen, I.; Harrison, R.; Stupak, I.; Hatten, J.; Raulund–Rasmussen, K.; Boyle, J.; Clarke, N.; Zabowski, D. Carbon storage and nutrient mobilization from soil minerals by deep roots and rhizospheres. Forest Ecol. Manag. 2016, 359, 322–331. [Google Scholar] [CrossRef]
- Lal, R. Digging deeper: A holistic perspective of factors affecting soil organic carbon sequestration in agroecosystems. Glob. Chang. Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef]
- Guo, L.; Gifford, R. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Murty, D.; Kirschbaum, M.U.; McMurtrie, R.E.; McGilvray, H. Does conversion of forest to agricultural land change soil carbon and nitrogen? A review of the literature. Glob. Chang. Biol. 2002, 8, 105–123. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci. Rep. 2014, 4, 4062. [Google Scholar] [CrossRef]
- Gregory, A.S.; Dungait, J.A.J.; Watts, C.W.; Bol, R.; Dixon, E.R.; White, R.P.; Whitmore, A.P. Long-term management changes topsoil and subsoil organic carbon and nitrogen dynamics in a temperate agricultural system. Eur. J. Soil Sci. 2016, 67, 421–430. [Google Scholar] [CrossRef]
- Strey, S.; Boy, J.; Strey, R.; Weber, O.; Guggenberger, G. Response of soil organic carbon to land-use change in central Brazil: A large-scale comparison of Ferralsols and Acrisols. Plant Soil 2016, 408, 327–342. [Google Scholar] [CrossRef]
- Buringh, P. Organic carbon in soils of the world. In The Role of Terrestrial Vegetation in the Global Carbon Cycle: Measurement by Remote Sensing; Woodwell, G.M., Ed.; John Wiley and Sons: New York, NY, USA, 1984; pp. 91–109. [Google Scholar]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Krull, E.S.; Baldock, J.A.; Skjemstad, J.O. Importance of mechanisms and processes of the stabilisation of soil organic matter for modelling carbon turnover. Funct. Plant Biol. 2003, 30, 207–222. [Google Scholar] [CrossRef]
- Feller, C.; Beare, M.H. Physical control of soil organic matter dynamics in the Tropics. Geoderma 1997, 79, 69–116. [Google Scholar] [CrossRef]
- Plante, A.F.; Conant, R.T.; Stewart, C.E.; Paustian, K.; Six, J. Impact of soil texture on the distribution of soil organic matter in physical and chemical fractions. Soil Sci. Soc. Am. J. 2006, 70, 287–296. [Google Scholar] [CrossRef]
- Kunlanit, B. Decomposition of Biochemically Contrasting Organic Residues Regulating Dissolved Organic Carbon Dynamics and Soil Organic Carbon Composition in a Sandy Soil; Graduate School, Khon Kaen University: Khon Kaen, Thailand, 2014; p. 166. [Google Scholar]
- Vicente, L.C.; Gama-Rodrigues, E.F.; Gama–Rodrigues, A.C. Soil carbon stocks of Ultisols under different land use in the Atlantic rainforest zone of Brazil. Geoderma Reg. 2016, 7, 330–337. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley: New York, NY, USA, 1994; p. 512. [Google Scholar]
- Huang, P.M. Soil mineral-organic matter microorganisms interactions: Fundamentals and impacts. Adv. Agron. 2004, 82, 391–472. [Google Scholar]
- Aranda, V.; Ayora-Cañada, M.J.; Domínguez-Vidal, A.; Martín-García, J.M.; Calero, J.; Delgado, R.; Verdejo, T.; González-Vila, F.J. Effect of soil type and management (organic vs. conventional) on soil organic matter quality in olive groves in a semi-arid environment in Sierra Mágina Natural Park (S Spain). Geoderma 2011, 164, 54–63. [Google Scholar] [CrossRef]
- Senesi, N.; D’Orazio, V.; Ricca, G. Humic acids in the first generation of Eurosoils. Geoderma 2003, 116, 325–344. [Google Scholar] [CrossRef]
- Spaccini, R.; Piccolo, A.; Haberhauer, G.; Gerzabek, M. Transformation of organic matter from maize residues into labile and humic fractions of three European soils as revealed by 13C distribution and CPMAS-NMR spectra. Eur. J. Soil Sci. 2006, 51, 583–594. [Google Scholar] [CrossRef]
- González-Pérez, M.; Milori, D.M.B.P.; Colnago, L.A.; Martin-Neto, L.; Melo, W.J.A. Laser-induced fluorescence spectroscopic study of organic matter in a Brazilian Oxisol under different tillage systems. Geoderma 2007, 138, 20–24. [Google Scholar] [CrossRef]
- Guimaraes, D.V.; Isidoria, M.; Gonzaga, S.; da Silva, T.O.; da Silva, T.L.; da Silva Dias, N.; Silva Matias, M.I. Soil organic matter pools and carbon fractions in soil under different land uses. Soil Tillage Res. 2013, 126, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Post, W.M.; Pastor, J.; Zinke, P.J.; Stangenberger, A.G. Global patterns of soil nitrogen storage. Nature 1985, 317, 613–616. [Google Scholar] [CrossRef]
- Yang, Y.H.; Fang, J.Y.; Guo, D.L.; Ji, C.J.; Ma, W.H. Vertical patterns of soil carbon, nitrogen and carbon: Nitrogen stoichiometry in Tibetan grasslands. Biogeosci. Discuss. 2010, 7, 1–24. [Google Scholar] [CrossRef]
- Lawrence, C.R.; Harden, J.W.; Xu, X.; Schulz, M.S.; Trumbore, S.E. Long–term controls on soil organic carbon with depth and time: A case study from the Cowlitz River Chronosequence, WA USA. Geoderma 2015, 247, 73–87. [Google Scholar] [CrossRef]
- Chandler, R.D. Soil Organic Carbon Distribution with Depth: Implications for Ecosystem Services. Master’s Thesis, Graduate School, Clemson University, Clemson, SC, USA, 2016; p. 48. [Google Scholar]
- Marty, C.; Houle, D.; Gagnon, C.; Courchesne, F. The relationships of soil total nitrogen concentrations, pools and C:N ratios with climate, vegetation types and nitrate deposition in temperate and boreal forests of eastern Canada. Catena 2017, 152, 163–172. [Google Scholar] [CrossRef]
- Tangtrakarnpong, S.; Vityakon, P. Land use and soil organic matter in Northeast Thailand: Microbial biomass, humic acid and mineral N. Symposium 5. In Transactions of the 17th World Congress of Soil Science; IUSS: Bangkok, Thailand, 2002; pp. 1–15. [Google Scholar]
- Lichaikul, N.; Chidthaisong, A.; Havey, N.W.; Wachrinra, C. Carbon stock and net CO2 emission in tropical upland soils under different land use. Kasetsart J. (Nat. Sci.) 2006, 40, 382–394. [Google Scholar]
- Bridhikitti, A. Soil and biomass carbon stocks in forest and agricultural lands in tropical climates. Songklanakarin J. Sci. Technol. 2017, 39, 697–707. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA–Natural Resources Conservation Service: Washington, DC, USA, 2014; p. 372.
- Chen, D.; Chen, H.W. Using the Köppen classification to quantify climate variation and change: An example for 1901–2010. Environ. Dev. 2013, 6, 69–79. [Google Scholar] [CrossRef]
- Dewis, J.; Freitas, F. Physical and Chemical Methods of Soil and Water Analysis; FAO Soils Bulletin 10; FAO: Rome, Italy, 1970; p. 275. [Google Scholar]
- Blake, G.R. Bulk Density in Methods of Soil Analysis; Black, C.A., Ed.; Agronomy, No. 9, Part 11; American Society of Agronomy: Madison, WI, USA, 1965; pp. 374–390. [Google Scholar]
- Black, C.A. Methods of Soil Analysis: Part I Physical and Mineralogical Properties; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Rayment, G.E.; Higginson, F.R. Australian Soil and Land Survey Handbook, Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press: Melbourne, Australia, 1992; pp. 38–41. [Google Scholar]
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Johnston, C.T., Sumner, M.E., Eds.; Soil Sci. Soc. Am. Book Series: 5; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 1018–1020. [Google Scholar]
- Tan, K.H. Humic Matter in Soil and the Environment, Principles and Controversies; Marcel Dekker: Madison, WI, USA, 2003; pp. 1018–1020. [Google Scholar]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information provided on humic substances by E4/E6 ratios. Soil Sci. Soc. Am. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Ellert, B.H.; Bettany, J.R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Nizami, S.M. Carbon stocks of different land uses in the Kumrat valley, Hindu Kush Region of Pakistan. J. For. Res. 2015, 26, 57–64. [Google Scholar] [CrossRef]
- Strey, S.; Boy, J.; Strey, R.; Welpelo, A.; Schönenberg, R.; Schumann, C.; Guggenberger, G. Digging deeper: The value of deep soil carbon for potential REDD+ projects in tropical forest communities in Amazonia. Erdkunde 2017, 71, 231–239. [Google Scholar] [CrossRef]
- Lal, R. Carbon sequestration in soils of central Asia. Land Degrad. Dev. 2004, 15, 563–572. [Google Scholar] [CrossRef]
- Emiru, N.; Gebrekidan, H. Effect of land use changes and soil depth on soil organic matter, total nitrogen and available phosphorus contents of soils in Senbat watershed, Western Ethiopia. ARPN J. Agric. Biol. Sci. 2013, 8, 206–212. [Google Scholar]
- Han, X.; Gao, G.; Chang, R.; Li, Z.; Ma, Y.; Wang, S.; Wang, C.; Lü, Y.; Fu, B. Changes in soil organic and inorganic carbon stocks in deep profiles following cropland abandonment along a precipitation gradient across the Loess Plateau of China. Agric. Ecosyst. Environ. 2018, 258, 1–13. [Google Scholar] [CrossRef]
- Thantrakarnpong, S. Changes of Different Pools of Soil Organic Matter under Different Land Use Systems in Undulating Terrain of Northeast Thailand. Master’s Thesis, Graduate School, Khon Kaen University, Khon Kaen, Thailand, 2002; p. 171, (In Thai with English Abstract). [Google Scholar]
- Dhakal, S.; Koirala, M.; Sharma, E.; Subedi, N.J. Effect of land use change on soil organic carbon stock in Balkhu Khola watershed southwestern part of Kathmandu valley, central Nepal. World Acad. Sci. Eng. Technol. 2010, 66, 581–591. [Google Scholar]
- Gebremariam, M.; Kebede, F. Land use change effect on soil carbon stock, aboveground biomass, aggregate stability and soil crust: A case from Tahtay Adyabo, north western Tigray, northern Ethiopia. J. Drylands 2010, 2, 220–225. [Google Scholar]
- Solomon, D.; Fritzsche, F.; Tekalign, M.; Lehmann, J.; Zech, W. Soil organic matter composition in the subhumid Ethiopian highlands as influenced by deforestation and agricultural management. Soil Sci. Soc. Am. J. 2002, 66, 68–82. [Google Scholar] [CrossRef]
- Xu, H.; Sieverding, H.; Kwon, H.; Clay, D.; Stewart, C.; Johnson, J.M.F.; Qin, Z.; Karlen, D.L.; Wang, M. A global meta-analysis of soil organic carbon response to corn stover removal. GCB Bioenergy 2019. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Soil organic matter stratification ratio as an indicator of soil quality. Soil Tillage Res. 2002, 66, 95–106. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R.; Shipitalo, M.J. Chemical stabilization of organic carbon pools in particle size fractions in no–till and meadow soils. Biol. Fertil. Soils 2008, 44, 1043–1051. [Google Scholar] [CrossRef]
- Meurer, K.H.E.; Haddaway, N.R.; Bolinder, M.A.; Kätterer, T. Tillage intensity affects total SOC stocks in boreo–temperate regions only in the topsoil—A systematic review using an ESM approach. Earth Sci. Rev. 2018, 177, 613–622. [Google Scholar] [CrossRef]
- Katoh, M.; Murase, J.; Sugimoto, A.; Kimura, M. Effect of rice straw amendment on dissolved organic and inorganic carbon and cationic nutrients in percolating water from a flooded paddy soil: A microcosm experiment using 13C–enriched rice straw. Org. Geochem. 2005, 362, 803–811. [Google Scholar] [CrossRef]
- Puttaso, A.; Vityakon, P.; Saenjan, P.; Trelo-Ges, V.; Cadisch, G. Relationship between residue quality, decomposition patterns, and soil organic matter accumulation in a tropical sandy soil after 13 years. Nutr. Cycl. Agroecosyst. 2011, 89, 159–174. [Google Scholar] [CrossRef]
- Kimura, M.; Miura, Y.; Watanabe, A.; Murase, J.; Kuwatsuka, S. Methane production and its fate in paddy fields. J. Soil Sci. Plant Nutr. 1992, 38, 665–672. [Google Scholar] [CrossRef]
- Kunlanit, B.; Vityakon, P.; Puttaso, A.; Cadisch, G.; Rasche, F. Mechanisms controlling soil organic carbon composition pertaining to microbial decomposition of biochemically contrasting organic residues: Evidence from midDRIFTS peak area analysis. Soil Biol. Biochem. 2014, 76, 100–108. [Google Scholar] [CrossRef]
- Reddy, H.; Nagaraja, M.S.; Punith Raj, T.S.; Police Patil, A.S.; Dhumgond, P. Elemental analysis, E4/E6 ratio and total acidity of soil humic and fulvic acids from different land use systems. Ann. Plant Soil Res. 2014, 16, 89–92. [Google Scholar]
- Martin, D.; Srivastava, P.C.; Ghosh, D.; Zech, W. Characteristics of humic substances in cultivated and natural forest soils of Sikkim. Geoderma 1998, 84, 345–362. [Google Scholar] [CrossRef]
- Szajdak, L.; Brandyk, T.; Szatylowicz, J. Chemical properties of different peat–marsh soils from the Biebrza River Valley. Agron. Res. 2007, 5, 165–174. [Google Scholar]
- Ding, F.; Hu, Y.L.; Li, L.J.; Li, A.; Shi, S.; Lian, P.Y.; Zeng, D.H. Changes in soil organic carbon and total nitrogen stocks after conversion of meadow to cropland in Northeast China. Plant Soil 2013, 373, 659–672. [Google Scholar] [CrossRef]
- Xu, X.; Li, D.; Cheng, X.; Ruan, H.; Luo, Y. Carbon: Nitrogen stoichiometry following afforestation: A global synthesis. Sci. Rep. 2016, 6, 19117. [Google Scholar] [CrossRef]
- Gao, Y.; He, N.; Yu, G.; Chen, W.; Wang, Q. Long–term effects of different land use types on C, N, and P stoichiometry and storage in subtropical ecosystems: A case study in China. Ecol. Eng. 2014, 67, 171–181. [Google Scholar] [CrossRef]
| Land Use Type | Soil Depth (cm) | Sand | Silt | Clay | Textures | Bulk Density (g cm−3) | Moisture Content (%) |
|---|---|---|---|---|---|---|---|
| (%) | |||||||
| Forest | 0–15 | 82.6 | 8.0 | 9.4 | Loamy sand | 1.43 | 0.81 |
| 15–30 | 84.0 | 9.0 | 7.0 | Loamy sand | 1.48 | 0.87 | |
| 30–60 | 84.0 | 7.9 | 8.1 | Loamy sand | 1.48 | 0.85 | |
| 60–80 | 76.5 | 11.8 | 11.7 | Sandy loam | 1.54 | 1.53 | |
| 80–100 | 65.0 | 10.9 | 24.1 | Sandy clay loam | 1.67 | 3.23 | |
| Cassava | 0–15 | 84.8 | 9.4 | 5.7 | Loamy sand | 1.41 | 1.50 |
| 15–30 | 82.7 | 9.6 | 7.7 | Loamy sand | 1.65 | 2.00 | |
| 30–60 | 84.2 | 9.9 | 5.9 | Loamy sand | 1.50 | 1.76 | |
| 60–80 | 81.9 | 10.2 | 7.9 | Loamy sand | 1.57 | 1.78 | |
| 80–100 | 74.0 | 9.9 | 16.1 | Sandy loam | 1.61 | 1.93 | |
| Paddy | 0–15 | 79.1 | 14.1 | 6.8 | Loamy sand | 1.49 | 11.66 |
| 15–30 | 79.9 | 13.1 | 7.0 | Loamy sand | 1.58 | 9.37 | |
| 30–60 | 80.8 | 12.9 | 6.3 | Loamy sand | 1.58 | 8.55 | |
| 60–80 | 87.4 | 7.2 | 5.4 | Loamy sand | 1.59 | 11.04 | |
| 80–100 | 93.6 | 2.0 | 4.5 | Sand | 1.55 | 11.84 | |
| Soil Depth (cm) | Land Use Type | ||||
|---|---|---|---|---|---|
| Forest | Cassava | Paddy | F–Test | CV (%) | |
| 0–15 | 3.04 a | 3.49 a | 3.91 a | ns | 11.34 |
| 15–30 | 2.56 a B | 2.85 b A B | 3.37a b A | ** | 6.64 |
| 30–60 | 1.95 b | 2.35 b | 2.34 b c | ns | 20.36 |
| 60–80 | 1.95 b | 1.83 c | 2.07 c | ns | 9.64 |
| 80–100 | 1.78 b | 1.61 c | 3.00 a b c | ns | 30.93 |
| F–test | ** | ** | * | ||
| CV (%) | 9.72 | 8.04 | 22.96 | ||
| Soil Depth (cm) | Land Use Type | ||||
|---|---|---|---|---|---|
| Forest | Cassava | Paddy | F–Test | CV (%) | |
| 0–15 | 15.20 a | 14.90 | 12.30 | ns | 12.00 |
| 15–30 | 13.80 a | 14.60 | 11.80 | ns | 12.60 |
| 30–60 | 11.70 a | 11.40 | 12.70 | ns | 28.50 |
| 60–80 | 6.50 b | 8.10 | 8.60 | ns | 29.00 |
| 80–100 | 5.10 b | 9.50 | 7.00 | ns | 30.00 |
| F–test | ** | ns | ns | ||
| CV (%) | 19.76 | 30.50 | 27.20 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunlanit, B.; Butnan, S.; Vityakon, P. Land–Use Changes Influencing C Sequestration and Quality in Topsoil and Subsoil. Agronomy 2019, 9, 520. https://doi.org/10.3390/agronomy9090520
Kunlanit B, Butnan S, Vityakon P. Land–Use Changes Influencing C Sequestration and Quality in Topsoil and Subsoil. Agronomy. 2019; 9(9):520. https://doi.org/10.3390/agronomy9090520
Chicago/Turabian StyleKunlanit, Benjapon, Somchai Butnan, and Patma Vityakon. 2019. "Land–Use Changes Influencing C Sequestration and Quality in Topsoil and Subsoil" Agronomy 9, no. 9: 520. https://doi.org/10.3390/agronomy9090520





