Serial Measurements of Apoptotic Cell Numbers Provide Better Acceptance Criterion for PBMC Quality than a Single Measurement Prior to the T Cell Assay
Abstract
:1. Introduction
2. Experimental Section
2.1. Thawing and Handling of PBMC
2.2. Antigens
2.3. Human Interferon-γ ELISPOT Assay
2.4. Viability and Apoptosis Detection
2.5. B cell Separation and Apoptosis Induction
3. Results and Discussion
3.1. Detecting Live, Dead and Apoptotic Cells
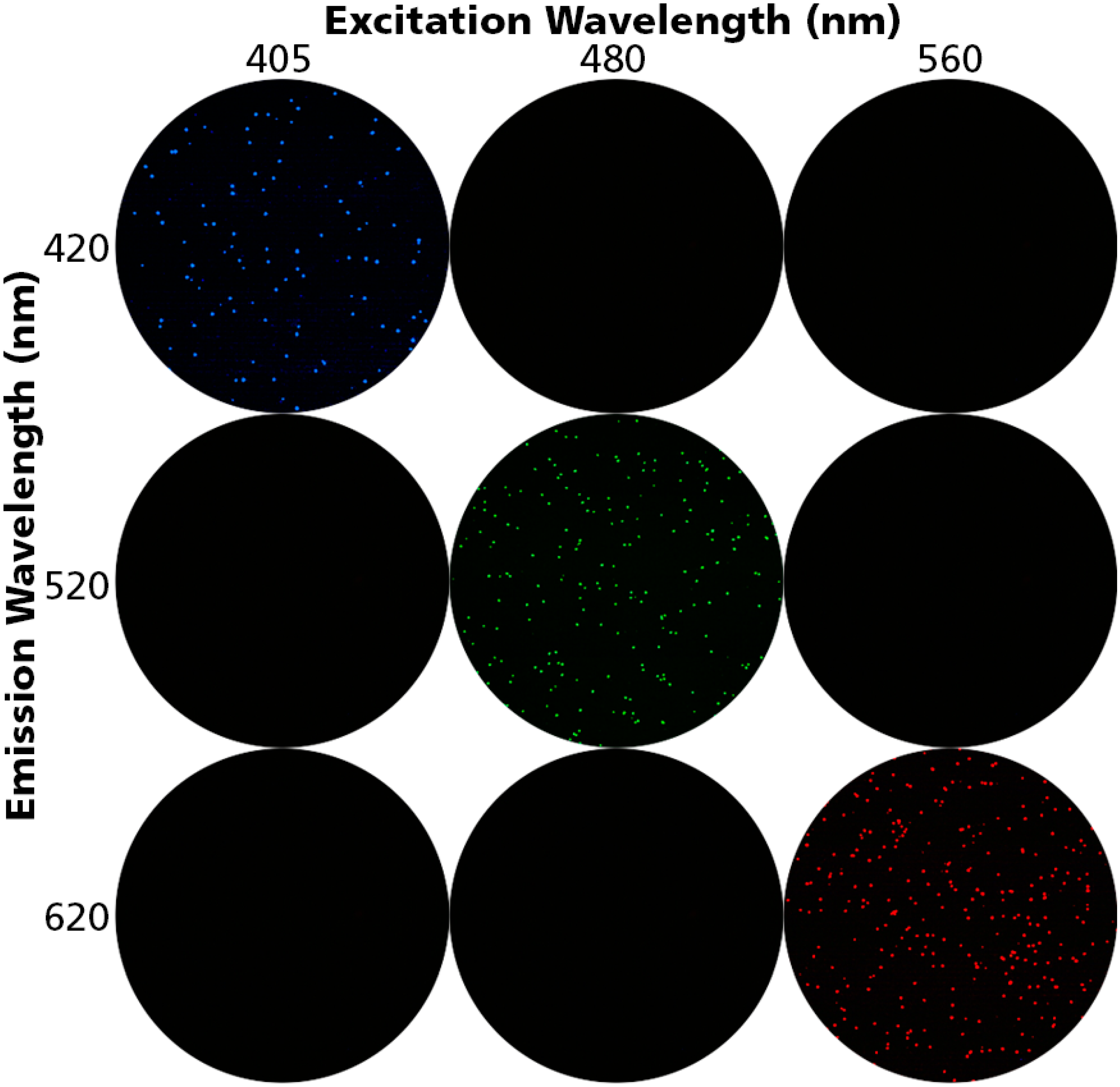
3.2. Inducing Apoptosis in B Cells
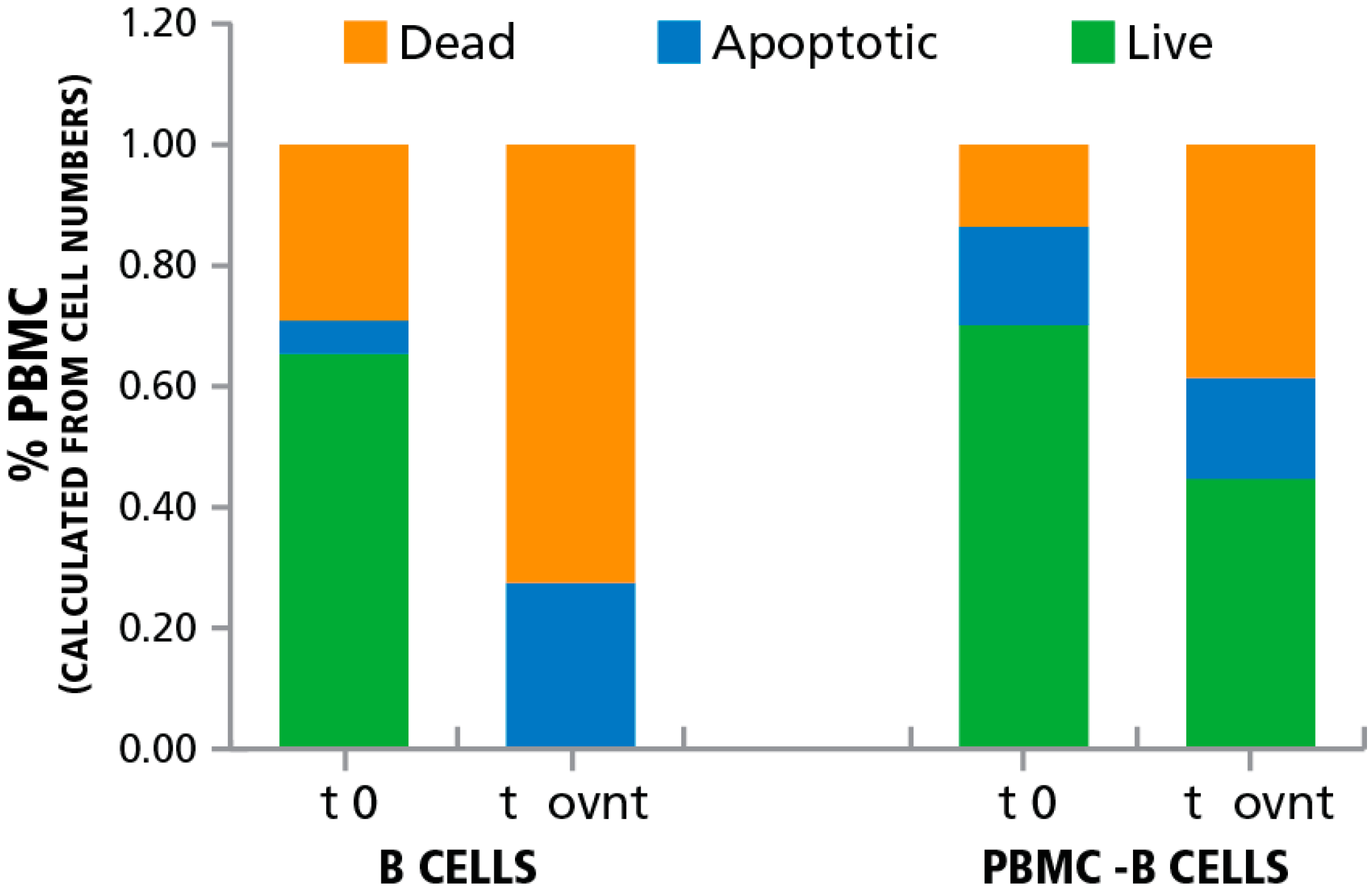
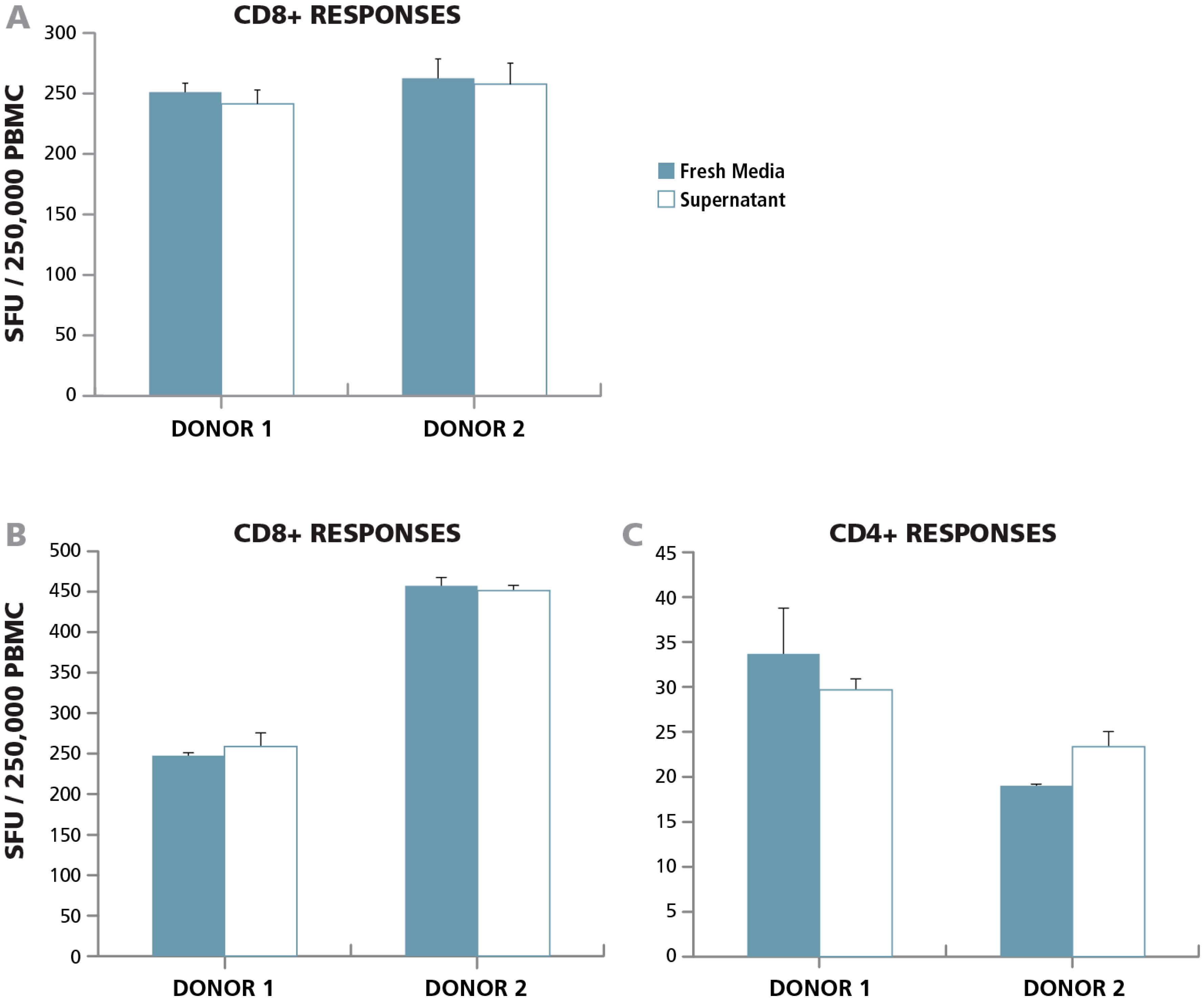
3.3. Apoptotic Bystander B Cells Do Not Affect T Cell Function
3.3.1. Lack of Paracrine Effects
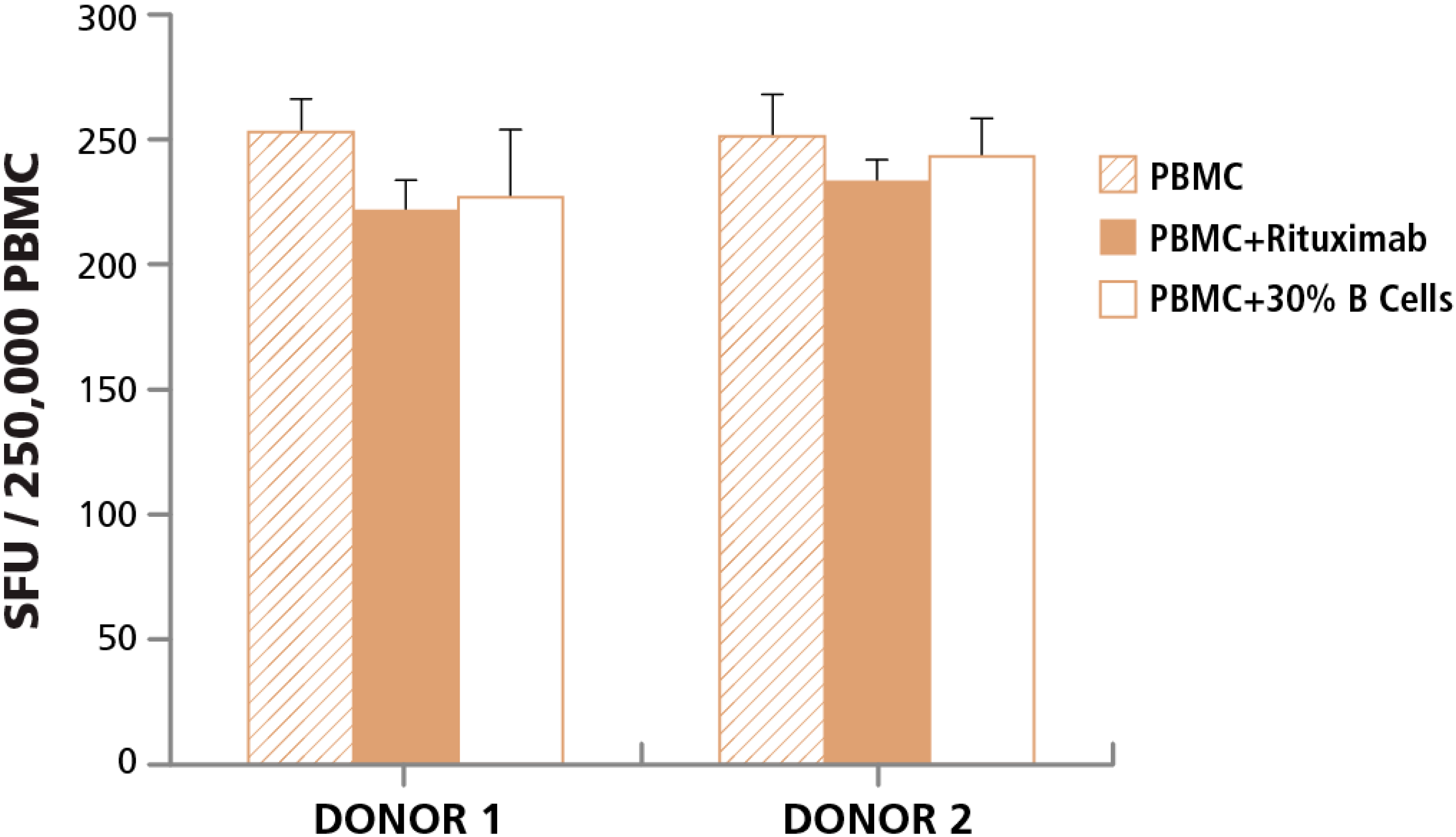
3.3.2. Lack of Cell Contact-Mediated Effect
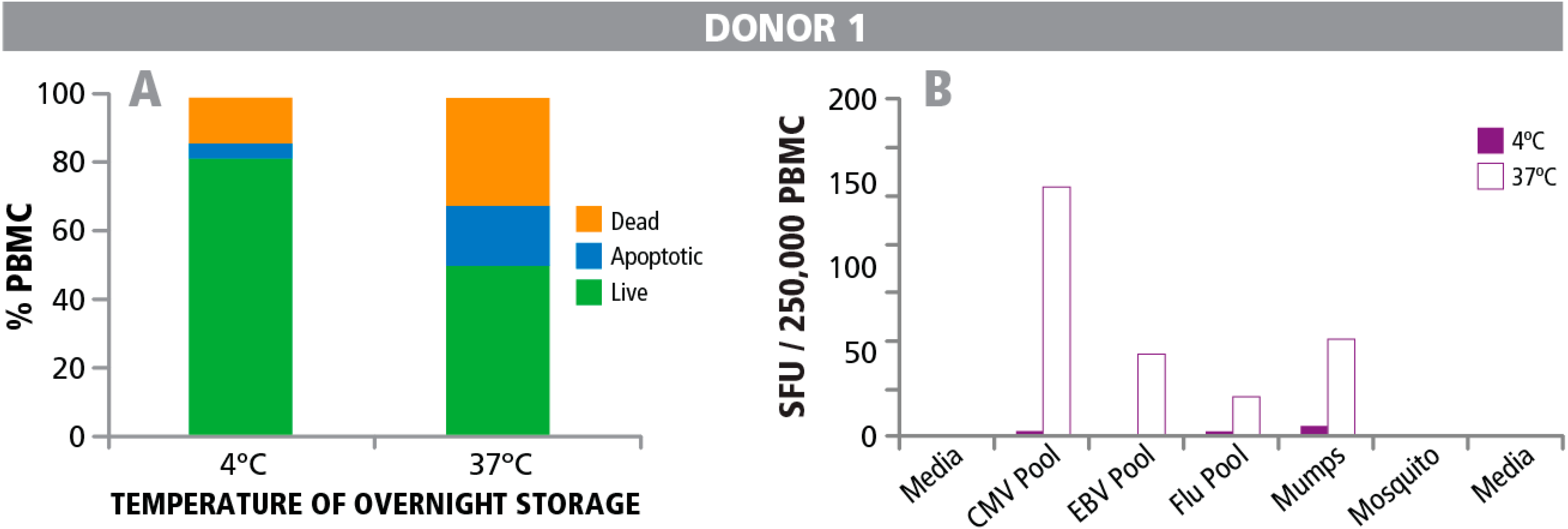
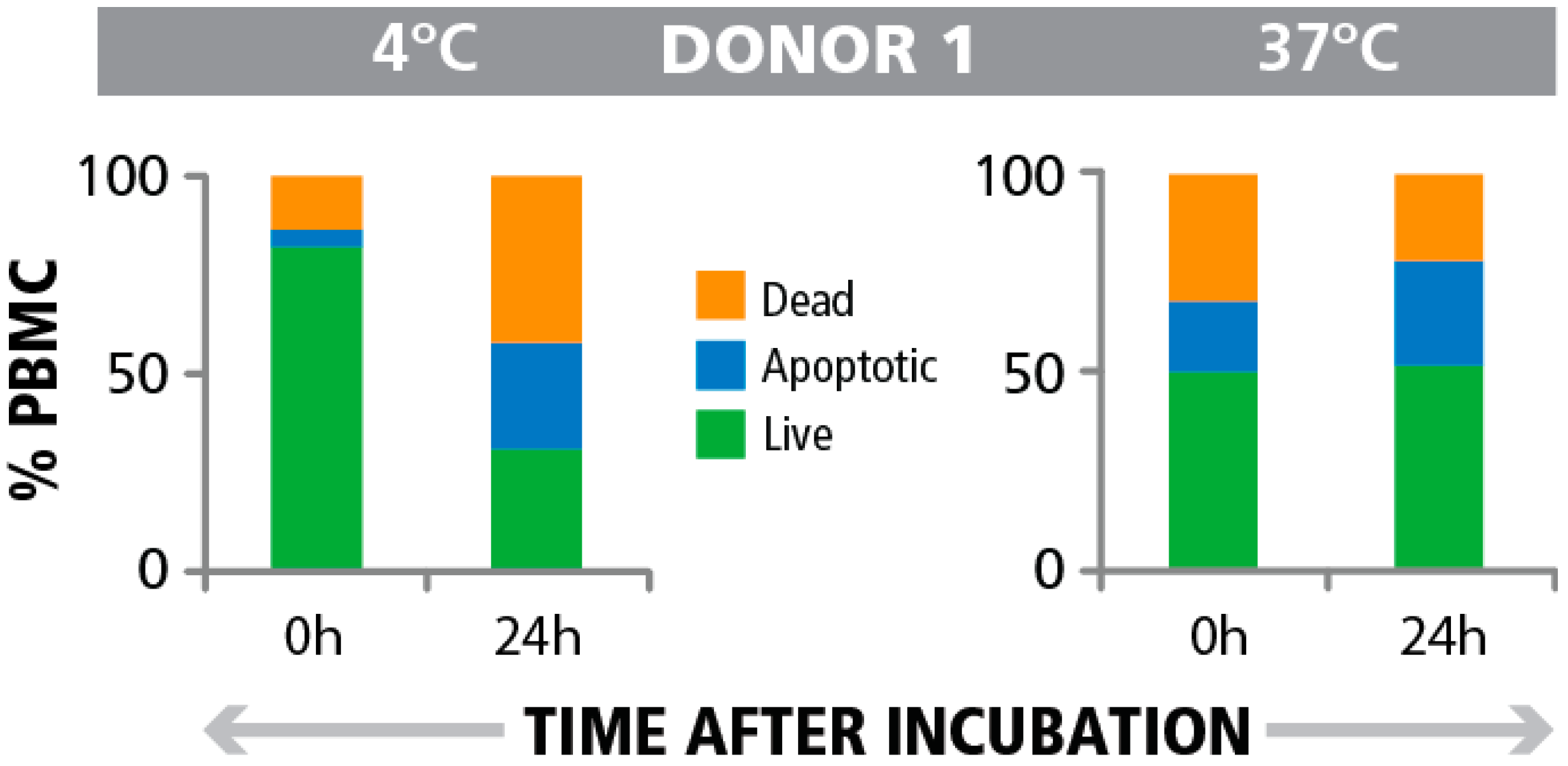
3.4. Post- Rather than Pre-T cell Assay Detection of Apoptotic Cells in PBMC Indicates Impaired T Cell Function
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kreher, C.R.; Dittrich, M.T.; Guerkov, R.; Boehm, B.O.; Tary-Lehmann, M. CD4+ and CD8+ cells in cryopreserved human pbmc maintain full functionality in cytokine ELISPOT assays. J. Immunol. Methods 2003, 278, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, S.; Faresjo, M.; Hedman, M.; Ludvigsson, J.; Casas, R. Cryopreserved peripheral blood mononuclear cells are suitable for the assessment of immunological markers in type 1 diabetic children. Cryobiology 2008, 57, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Maecker, H.T.; Moon, J.; Bhatia, S.; Ghanekar, S.A.; Maino, V.C.; Payne, J.K.; Kuus-Reichel, K.; Chang, J.C.; Summers, A.; Clay, T.M.; et al. Impact of cryopreservation on tetramer, cytokine flow cytometry, and ELISPOT. BMC Immunol. 2005, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, A.; Song, L.Y.; Wilkening, C.L.; Fenton, T.; Hural, J.; Louzao, R.; Ferrari, G.; Etter, P.E.; Berrong, M.; Canniff, J.D.; et al. Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays. J. Immunol. Methods 2010, 363, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.S.; Jaoko, W.; Vardas, E.; Panayotakopoulos, G.; Fast, P.; Schmidt, C.; Gilmour, J.; Bogoshi, M.; Omosa-Manyonyi, G.; Dally, L.; et al. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus ankara (MVA) with and without DNA priming: Effects of dosage and route on safety and immunogenicity. Vaccine 2007, 25, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Lagos, R.; Munoz, A.; Picon, T.; Rosa, R.; Alfonso, A.; Abriata, G.; Gentile, A.; Romanin, V.; Regueira, M.; et al. Impact of vaccination against haemophilus influenzae type B with and without a booster dose on meningitis in four south american countries. Vaccine 2012, 30, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.; Hazenfeld, S.; Brady, R.C.; Subbramanian, R.A. Comparison of antibody and T-cell responses elicited by licensed inactivated- and live-attenuated influenza vaccines against H3N2 hemagglutinin. Hum. Immunol. 2011, 72, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.F.; Huang, W.; Li, Y.F.; Gao, Z.G. Current status of non-viral vectors for sirna delivery. Yao Xue Xue Bao 2011, 46, 1436–1443. [Google Scholar] [PubMed]
- Ramachandran, H.; Laux, J.; Moldovan, I.; Caspell, R.; Lehmann, P.V.; Subbramanian, R.A. Optimal thawing of cryopreserved peripheral blood mononuclear cells for use in high-throughput human immune monitoring studies. Cells 2012, 1, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Areman, E.M.; Simonis, T.B.; Carter, C.S.; Read, E.J.; Klein, H.G. Bulk cryopreservation of lymphocytes in glycerol. Transfusion 1988, 28, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.H.; Ferrari, G.; Janetzki, S. Measurement of cytokine release at the single cell level using the ELISPOT assay. Methods 2006, 38, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.K.; Huang, Y.; Levine, G.L.; Sambor, A.; Carter, D.K.; Sato, A.; Kopycinski, J.; Hayes, P.; Hahn, B.; Birungi, J.; et al. Equivalence of ELISPOT assays demonstrated between major HIV network laboratories. PloS One 2010, 5, e14330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Caspell, R.; Karulin, A.Y.; Ahmad, M.; Haicheur, N.; Abdelsalam, A.; Johannesen, K.; Vignard, V.; Dudzik, P.; Georgakopoulou, K.; et al. ELISPOT assays provide reproducible results among different laboratories for T-cell immune monitoring—even in hands of ELISPOT-inexperienced investigators. J. Immunotoxicol. 2009, 6, 227–234. [Google Scholar]
- Smith, J.G.; Joseph, H.R.; Green, T.; Field, J.A.; Wooters, M.; Kaufhold, R.M.; Antonello, J.; Caulfield, M.J. Establishing acceptance criteria for cell-mediated-immunity assays using frozen peripheral blood mononuclear cells stored under optimal and suboptimal conditions. Clin. Vaccine Immunol. 2007, 14, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Mascotti, K.; McCullough, J.; Burger, S.R. Hpc viability measurement: Trypan blue versus acridine orange and propidium iodide. Transfusion 2000, 40, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.B.; Young, M.T.; Adinolfi, E.; Surprenant, A. Pseudoapoptosis induced by brief activation of atp-gated P2X7 receptors. J. Biol. Chem. 2005, 280, 33968–33976. [Google Scholar] [CrossRef] [PubMed]
- Cankurtaran-Sayar, S.; Sayar, K.; Ugur, M. P2x7 receptor activates multiple selective dye-permeation pathways in raw 264.7 and human embryonic kidney 293 cells. Mol. Pharmacol. 2009, 76, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.; Dransfield, I.; Gregory, C.; Haslett, C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002, 2, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar] [PubMed]
- Torr, E.E.; Gardner, D.H.; Thomas, L.; Goodall, D.M.; Bielemeier, A.; Willetts, R.; Griffiths, H.R.; Marshall, L.J.; Devitt, A. Apoptotic cell-derived ICAM-3 promotes both macrophage chemoattraction to and tethering of apoptotic cells. Cell Death Differ. 2012, 19, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.S.; Henson, P.M.; Haslett, C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel “harge-sensitive” ecognition mechanism. J. Clin. Invest. 1989, 84, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Lauber, K.; Bohn, E.; Krober, S.M.; Xiao, Y.J.; Blumenthal, S.G.; Lindemann, R.K.; Marini, P.; Wiedig, C.; Zobywalski, A.; Baksh, S.; et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 2003, 113, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Scannell, M.; Flanagan, M.B.; deStefani, A.; Wynne, K.J.; Cagney, G.; Godson, C.; Maderna, P. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J. Immunol. 2007, 178, 4595–4605. [Google Scholar] [CrossRef] [PubMed]
- Truman, L.A.; Ford, C.A.; Pasikowska, M.; Pound, J.D.; Wilkinson, S.J.; Dumitriu, I.E.; Melville, L.; Melrose, L.A.; Ogden, C.A.; Nibbs, R.; et al. Cx3cl1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood 2008, 112, 5026–5036. [Google Scholar] [CrossRef] [PubMed]
- Bournazou, I.; Pound, J.D.; Duffin, R.; Bournazos, S.; Melville, L.A.; Brown, S.B.; Rossi, A.G.; Gregory, C.D. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J. Clin. Invest. 2009, 119, 20–32. [Google Scholar]
- Voll, R.E.; Herrmann, M.; Roth, E.A.; Stach, C.; Kalden, J.R.; Girkontaite, I. Immunosuppressive effects of apoptotic cells. Nature 1997, 390, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, P.V. Image analysis and data management of ELISPOT assay results. Methods Mol. Biol. 2005, 302, 117–132. [Google Scholar] [PubMed]
- Shan, D.; Ledbetter, J.A.; Press, O.W. Apoptosis of malignant human B cells by ligation of Cd20 with monoclonal antibodies. Blood 1998, 91, 1644–1652. [Google Scholar] [PubMed]
- Pedersen, I.M.; Buhl, A.M.; Klausen, P.; Geisler, C.H.; Jurlander, J. The chimeric anti-CD20 antibody rituximab induces apoptosis in B-cell chronic lymphocytic leukemia cells through a p38 mitogen activated protein-kinase-dependent mechanism. Blood 2002, 99, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.M.; O’Donovan, N.; McGowan, P.M.; O’Sullivan, F.; Duffy, M.J.; Crown, J. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in her-2-non-amplified breast cancer cell lines. Ann. Oncol. ESMO 2012, 23, 1788–1795. [Google Scholar] [CrossRef]
- Kimura, H.; Sakai, K.; Arao, T.; Shimoyama, T.; Tamura, T.; Nishio, K. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci. 2007, 98, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter-Hufford, A.; Ravichandran, K.S. Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harbor Perspect. Biol. 2013, 5, a008748. [Google Scholar] [CrossRef]
- Kuerten, S.; Batoulis, H.; Recks, M.S.; Karacsony, E.; Zhang, W.; Subbramanian, R.A.; Lehmann, P.V. Resting of cryopreserved PBMC does not generally benefit the performance of antigen-specific T cell ELISPOT assays. Cells 2012, 1, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Lenders, K.; Ogunjimi, B.; Beutels, P.; Hens, N.; Van Damme, P.; Berneman, Z.N.; Van Tendeloo, V.F.; Smits, E.L. The effect of apoptotic cells on virus-specific immune responses detected using IFN-gamma ELISPOT. J. Immunol. Methods 2010, 357, 51–54. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wunsch, M.; Caspell, R.; Kuerten, S.; Lehmann, P.V.; Sundararaman, S. Serial Measurements of Apoptotic Cell Numbers Provide Better Acceptance Criterion for PBMC Quality than a Single Measurement Prior to the T Cell Assay. Cells 2015, 4, 40-55. https://doi.org/10.3390/cells4010040
Wunsch M, Caspell R, Kuerten S, Lehmann PV, Sundararaman S. Serial Measurements of Apoptotic Cell Numbers Provide Better Acceptance Criterion for PBMC Quality than a Single Measurement Prior to the T Cell Assay. Cells. 2015; 4(1):40-55. https://doi.org/10.3390/cells4010040
Chicago/Turabian StyleWunsch, Marie, Richard Caspell, Stefanie Kuerten, Paul V. Lehmann, and Srividya Sundararaman. 2015. "Serial Measurements of Apoptotic Cell Numbers Provide Better Acceptance Criterion for PBMC Quality than a Single Measurement Prior to the T Cell Assay" Cells 4, no. 1: 40-55. https://doi.org/10.3390/cells4010040




