Journal Description
Cells
Cells
is an international, peer-reviewed, open access journal on cell biology, molecular biology, and biophysics, published semimonthly online by MDPI. The Nordic Autophagy Society (NAS) and the Spanish Society of Hematology and Hemotherapy (SEHH) are affiliated with Cells and their members receive discounts on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, MEDLINE, PMC, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q2 (Cell Biology) / CiteScore - Q1 (General Biochemistry, Genetics and Molecular Biology)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 16 days after submission; acceptance to publication is undertaken in 2.7 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
- Sections: published in 21 topical sections.
- Companion journal: Organoids.
Impact Factor:
5.2 (2024);
5-Year Impact Factor:
6.1 (2024)
Latest Articles
Culture Strategy Determines the Differentiation Status of Sweat Gland Cells
Cells 2025, 14(20), 1643; https://doi.org/10.3390/cells14201643 - 21 Oct 2025
Abstract
►
Show Figures
Reliable methods for the isolation and culture of human eccrine sweat gland cells (SGCs) are essential for studying glandular biology and developing tissue-engineered skin substitutes (TESs) that restore full skin function. However, maintaining the glandular phenotype of SGCs in vitro remains a major
[...] Read more.
Reliable methods for the isolation and culture of human eccrine sweat gland cells (SGCs) are essential for studying glandular biology and developing tissue-engineered skin substitutes (TESs) that restore full skin function. However, maintaining the glandular phenotype of SGCs in vitro remains a major challenge. In this study, we present an optimized isolation protocol combining enzymatic digestion with mechanical separation to improve SGC yield and purity, while also enabling keratinocyte isolation from a single human skin biopsy. We then evaluated two culture strategies, 2D monolayers and 3D spheroids, to determine their impact on SGC identity and proliferation. While 2D culture supported cell expansion, SGCs and keratinocytes exhibited highly similar marker expression profiles, with the absence of functional SGC markers (AQP5, α-SMA) reflecting a shift toward less differentiated phenotypes. In contrast, SGCs cultured in 3D spheroids preserved the expression of SGC-specific markers (AQP5, K18, α-SMA), distinguishing them from keratinocytes; however, their growth and structural organization were suboptimal under these 3D conditions. Moreover, SGCs expanded in 2D did not regain their glandular features when reintroduced into 3D culture, suggesting potential limitations in phenotype recovery. These results highlight the need for improved culture systems that maintain SGC identity while supporting expansion. Advancing such methods is a critical step toward integrating functional sweat glands into TESs and achieving complete skin regeneration for clinical applications.
Full article
Open AccessArticle
Vascular Endothelial Growth Factor B Modulates Cardiac Functions via Ferroptosis Pathways in Post-Myocardial Infarction
by
Sai Manasa Varanasi, Ankit Sabharwal, Shreyartha Mukherjee, Huzaifa Muhammad, Riya Kar, Carter Magnano, Anya Dorairaj, Enfeng Wang, Shamit Dutta, Pritam Das, Stephen C. Ekker, Ying Wang, Debabrata Mukhopadhyay and Ramcharan Singh Angom
Cells 2025, 14(20), 1642; https://doi.org/10.3390/cells14201642 - 21 Oct 2025
Abstract
Myocardial infarction (MI) remains a leading cause of mortality worldwide, yet effective cardioprotective strategies remain limited in clinical settings. Vascular endothelial growth factor B (VEGFB) has emerged as a promising therapeutic candidate in MI, but the role of its co-receptor, Neuropilin-1 (NRP1
[...] Read more.
Myocardial infarction (MI) remains a leading cause of mortality worldwide, yet effective cardioprotective strategies remain limited in clinical settings. Vascular endothelial growth factor B (VEGFB) has emerged as a promising therapeutic candidate in MI, but the role of its co-receptor, Neuropilin-1 (NRP1), in cardiomyocyte (CM) survival under ischemic stress remains poorly understood. Here, we investigated VEGFB-NRP1 signaling using an in vivo zebrafish model of cardiac injury as well as in vitro hypoxia models in CMs. We demonstrated that VEGFB overexpression conferred protection against ischemic injury and enhanced cardiac regeneration in the zebrafish heart. Mechanistically, we showed that VEGFB treatment enhances CM viability through reducing reactive oxygen species (ROS), ferroptosis activation, and preserving mitochondrial integrity. We also demonstrated that NRP1 knockdown in the CMs abolished the VEGFB-mediated protective effects, indicating the significant role of NRP1 signaling in VEGFB-induced cardioprotective effects in MI. Lastly, using transcriptome analysis, we confirmed that VEGFB induces anti-apoptotic and anti-ferroptosis gene programs in CMs in response to hypoxic stress. Collectively, our findings provide mechanistic insight into cell death activation pathways, including ferroptosis, in response to ischemic stress and further validate the therapeutic potential of VEGFB in promoting CM survival in ischemic heart disease.
Full article
(This article belongs to the Section Cellular Pathology)
►▼
Show Figures
Figure 1
Open AccessArticle
C-Kit Is Essential for Vascular Smooth Muscle Cell Phenotypic Switch In Vitro and In Vivo after Injury
by
Chiara Siracusa, Giovanni Canino, Mariangela Scalise, Fabiola Marino, Loredana Pagano, Gianluca Santamaria, Annalaura Torella, Salvatore De Rosa, Daniele Torella and Eleonora Cianflone
Cells 2025, 14(20), 1641; https://doi.org/10.3390/cells14201641 (registering DOI) - 21 Oct 2025
Abstract
Pathological vascular remodeling—central to restenosis, atherosclerosis, and vasculo-proliferative diseases—depends on the phenotypic switching of vascular smooth muscle cells (VSMCs) from a quiescent, contractile state to a synthetic, proliferative program. Although the receptor tyrosine kinase c-Kit is implicated in proliferation, migration, and tissue repair,
[...] Read more.
Pathological vascular remodeling—central to restenosis, atherosclerosis, and vasculo-proliferative diseases—depends on the phenotypic switching of vascular smooth muscle cells (VSMCs) from a quiescent, contractile state to a synthetic, proliferative program. Although the receptor tyrosine kinase c-Kit is implicated in proliferation, migration, and tissue repair, its role in VSMC plasticity has yet to be fully understood. Using c-Kit haploinsufficient mice subjected to right carotid artery ligation (CAL) and primary aortic VSMC cultures, we show that c-Kit is required for the contractile-to-synthetic transition. In vitro, c-Kit haploinsufficiency halved c-Kit expression, reduced 5-bromo-2′-deoxyuridine (BrdU) incorporation, and blunted platelet-derived growth factor BB (PDGF-BB)-induced repression of contractile genes. c-Kit–deficient VSMCs exhibited a senescence program with increased p16INK4a/p21 expression and upregulated senescence-associated secretory phenotype (SASP) mediators. RNA-Seq of carotid arteries 7 days post-ligation revealed that wild-type arteries activated cell-cycle pathways and suppressed contractile signatures, whereas c-Kit-deficient carotid arteries failed to fully engage proliferative programs and instead maintained contractile gene expression. At 28 days post CAL in vivo, c-Kit haploinsufficiency produced markedly reduced neointima, fewer Ki67+ VSMCs, more p16INK4a+ cells, and impaired re-endothelialization. Because progenitor-to-VSMC differentiation contributes to remodeling, we tested adult cardiac stem/progenitor cells (CSCs) as a model system of adult progenitor differentiation. Wild-type CSCs efficiently generated induced VSMCs (iVSMCs) with appropriate smooth-muscle gene upregulation; c-Kit–deficient rarely did so. Restoring c-Kit with a BAC transgene rescued both the smooth-muscle differentiation and proliferative competence of c-Kit-deficient iVSMCs. Collectively, our data identified c-Kit as a gatekeeper of reparative VSMC plasticity. Adequate c-Kit enables progenitor-to-VSMC commitment and the expansion of newly formed VSMCs while permitting injury-induced proliferation and matrix synthesis; reduced c-Kit locks cells in a hypercontractile, senescence-prone state and limits neointima formation. Modulating the c-Kit axis may therefore offer a strategy to fine-tune vascular repair while mitigating pathological remodeling.
Full article
(This article belongs to the Special Issue Emerging Topics in Smooth Muscle Cell Fate and Plasticity in Atherosclerosis)
Open AccessSystematic Review
From Adhesion to Invasion: Integrins, Focal Adhesion Signaling, and Actin Binding Proteins in Cervical Cancer Progression—A Scoping Review
by
Marta Hałas-Wiśniewska, Patryk Zawadka, Wioletta Arendt and Magdalena Izdebska
Cells 2025, 14(20), 1640; https://doi.org/10.3390/cells14201640 (registering DOI) - 21 Oct 2025
Abstract
►▼
Show Figures
Background: Cervical cancer (CC) is one of the most common malignancies in women worldwide. Its progression involves a cascade of processes, including proliferation, migration, invasion, and metastasis. Each stage is regulated by specific signaling pathways. Objective: This scoping review aimed to map current
[...] Read more.
Background: Cervical cancer (CC) is one of the most common malignancies in women worldwide. Its progression involves a cascade of processes, including proliferation, migration, invasion, and metastasis. Each stage is regulated by specific signaling pathways. Objective: This scoping review aimed to map current evidence on the role of cell adhesion-related molecules, including integrins, focal adhesion (FA) proteins, and actin-binding proteins (ABPs), in CC progression. These protein groups act in a coordinated manner—integrins perceive and transmit extracellular matrix (ECM) signals, FA proteins mediate intracellular signaling, and ABPs reorganize the cytoskeleton, ensuring the continuity of adhesion and motility processes. Methods: A structured literature search was conducted for studies published between 2015 and 2025. Eligible articles described the role of adhesion-related proteins in migration, invasion, or EMT in CC. Data were synthesized thematically according to protein families. Results: The evidence highlights integrins, FA/FAK, and ABPs as interconnected regulators coordinating ECM signaling and cytoskeletal remodeling during CC progression. Their dysregulation is associated with enhanced migration, EMT induction, angiogenesis, and therapy resistance. Conclusions: This review provides a unique, integrated perspective linking adhesion molecules with invasion mechanisms in CC progression, providing new insights into their interplay. Understanding the interaction between these proteins is therefore a crucial step in the treatment of CC and may facilitate the discovery of biomarkers and support the development of targeted therapies.
Full article
Figure 1
Open AccessReview
Alcohol Consumption and Cervical Carcinogenesis: Time to Draw Conclusions
by
Vivek K. Kashyap, Divya B. Kenchappa, Ajay K. Singh, Bhupesh Singh, Murali M. Yallapu, Everardo Cobos and Subhash C. Chauhan
Cells 2025, 14(20), 1639; https://doi.org/10.3390/cells14201639 (registering DOI) - 21 Oct 2025
Abstract
Cervical cancer is the fourth most common cancer among women worldwide and remains a significant cause of cancer-related mortality. Alcohol consumption is linked to an increased risk of several cancers and is a controversial risk factor for developing cervical cancer. This review updates
[...] Read more.
Cervical cancer is the fourth most common cancer among women worldwide and remains a significant cause of cancer-related mortality. Alcohol consumption is linked to an increased risk of several cancers and is a controversial risk factor for developing cervical cancer. This review updates existing information on the correlation between alcohol consumption and the risk of developing cervical cancer. Several comprehensive studies from different geographical regions have shown that moderate and heavy drinking is positively correlated with the development of cervical cancer. There is a synergistic relationship between human papillomavirus (HPV) viral load and alcohol use among drinkers with a high HPV viral load. Excessive alcohol consumption and exposure to second-hand smoke may elevate the risk of persistent HPV infection. Furthermore, high-risk behaviors associated with Human immunodeficiency virus (HIV)/HPV co-infection are more common among binge drinkers. However, several observations failed to establish a relationship between these factors. Despite some inconsistency in the literature, evidence suggests a modest association between alcohol consumption and increased risk of persistent HPV infection, causing cervical cancer.
Full article
(This article belongs to the Special Issue The Cellular and Molecular Basis of Lifestyle Factors in Cancer Progression and Prevention)
►▼
Show Figures
Figure 1
Open AccessArticle
Phospho-Tau Signature During Mitosis: AT8, p-T217 and p-S422 as Key Phospho-Epitopes
by
Marion Goussard, Kelly Zarka, Morgane Denus, Thomas Curel, Sylvie Claeysen, Bruno Lefebvre, Malika Hamdane, Philippe Marin, Julien Villeneuve and Marie-Laure Parmentier
Cells 2025, 14(20), 1638; https://doi.org/10.3390/cells14201638 (registering DOI) - 21 Oct 2025
Abstract
Tau was initially identified as a microtubule-binding protein critical for microtubule stabilization. It is also a pathological hallmark of tauopathies, a group of neurodegenerative diseases that include Alzheimer’s disease. Under pathological conditions, Tau becomes hyperphosphorylated at numerous sites and aggregates into filamentous deposits,
[...] Read more.
Tau was initially identified as a microtubule-binding protein critical for microtubule stabilization. It is also a pathological hallmark of tauopathies, a group of neurodegenerative diseases that include Alzheimer’s disease. Under pathological conditions, Tau becomes hyperphosphorylated at numerous sites and aggregates into filamentous deposits, contributing to neuronal cell death and disease progression. While significant research has focused on Tau phosphorylation dynamics and their consequences in pathological contexts, comparatively few studies have investigated Tau phosphorylation during physiological processes, despite the potential relevance to the early onset of pathology. Previous findings have suggested similarities between mitotic Tau phosphorylation and hyperphosphorylation observed in tauopathies, particularly at sites such as AT8, PHF1, S214, and S422. In this study, we quantified the relative levels of phosphorylation at 12 Tau phospho-epitopes during interphase and mitosis in vitro to establish a preliminary mitotic phospho-Tau signature, which was subsequently validated in vivo. Our results demonstrated pronounced phosphorylation of Tau at AT8, p-T217, and p-S422 epitopes during mitosis, both in vitro and in vivo. These findings provide new insights into the physiological phosphorylation of Tau and its potential links to pathological processes.
Full article
(This article belongs to the Collection Molecular Insights into Neurodegenerative Diseases)
►▼
Show Figures
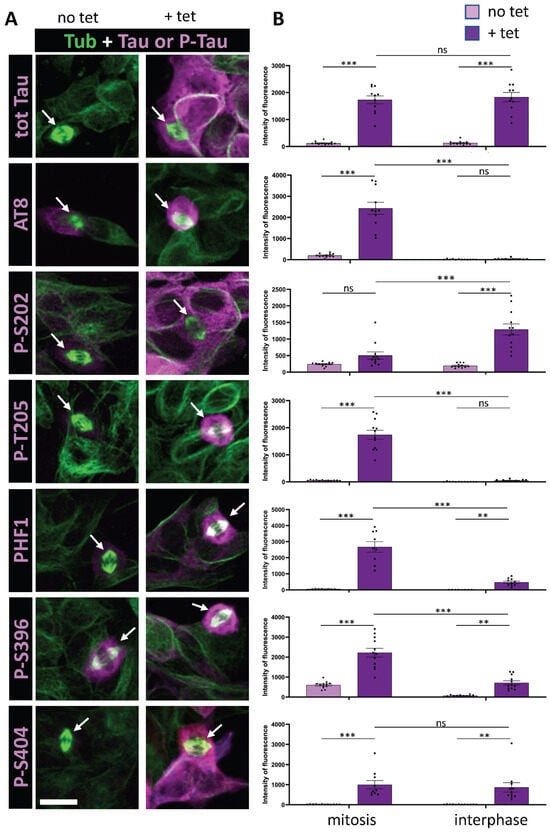
Figure 1
Open AccessReview
Biological Nanoparticles for Enhancing Chronic Wound Regeneration
by
Daniil Zotikov, Natalia Ponomareva, Sergey Brezgin, Anastasiia Kostyusheva, Anastasiya Frolova, Vladimir Chulanov, Alexander Lukashev, Peter Timashev and Dmitry Kostyushev
Cells 2025, 14(20), 1637; https://doi.org/10.3390/cells14201637 (registering DOI) - 21 Oct 2025
Abstract
►▼
Show Figures
Chronic wounds (CWs) represent a growing global health concern with profound clinical and socioeconomic implications. Studies indicate that approximately 15% of CWs remain unhealed one year after the initial treatment. At the same time, it is assumed that from 1% to 2% of
[...] Read more.
Chronic wounds (CWs) represent a growing global health concern with profound clinical and socioeconomic implications. Studies indicate that approximately 15% of CWs remain unhealed one year after the initial treatment. At the same time, it is assumed that from 1% to 2% of the population of developed countries will suffer from chronic wounds during their lifetime. CWs severely impair patients’ quality of life. Current therapies (compression bandages, antibiotics, hyperbaric oxygen, and skin grafts) face limitations, including toxicity, contraindications, inefficacy in patients with comorbidities like diabetes, and high cost. Biological nanoparticles (BNPs), particularly extracellular vesicles (EVs), emerge as transformative solutions due to their innate biocompatibility, targeted biodistribution, and multifunctional regenerative properties. This review examines the mechanisms by which BNPs promote CW healing and drug delivery. Innovative BNP delivery platforms (chitosan hydrogels, alginate films) are evaluated, enabling sustained release and responsiveness to the wound microenvironment. Clinical advances, including exosome-laden hydrogels that accelerate healing in diabetic ulcers, underscore BNPs’ potential to overcome conventional therapy limitations. By addressing the challenges of both pathophysiological complexity and healthcare system burden, BNPs demonstrate the potential to improve patient outcomes in the management of chronic wounds.
Full article
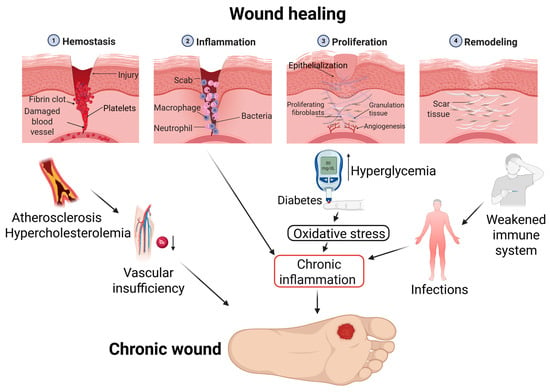
Figure 1
Open AccessArticle
Circulating CD16-Positive Monocyte-like Myeloid-Derived Suppressor Cells and Intermediate Monocytes Associated with Clinical and Immunological Complications in Pars Planitis Patients
by
Agata Kosmaczewska, Joanna Przeździecka-Dołyk, Lidia Ciszak, Zofia Rojek-Gajda, Irena Frydecka, Anna Turno-Kręcicka, Marta Misiuk-Hojło and Edyta Pawlak
Cells 2025, 14(20), 1636; https://doi.org/10.3390/cells14201636 (registering DOI) - 21 Oct 2025
Abstract
►▼
Show Figures
Recently, we observed that pars planitis (PP) patients present alterations in peripheral blood (PB) Th17/Treg associated with dysregulation in the Th1 response. Yet, little is known about the systemic distribution of myeloid cells, which drive the recruitment and differentiation of the adaptive effectors
[...] Read more.
Recently, we observed that pars planitis (PP) patients present alterations in peripheral blood (PB) Th17/Treg associated with dysregulation in the Th1 response. Yet, little is known about the systemic distribution of myeloid cells, which drive the recruitment and differentiation of the adaptive effectors toward pathogenic inflammatory Th1 and Th17 as well as regulatory lymphocytes in PP. Although myeloid populations in patients with uveitis have previously been addressed, the data did not provide an exact description of PP patients. Using flow cytometry, we evaluated monocyte and IDO-expressing monocyte-like myeloid-derived suppressor cell (MDSC) subpopulations in PB samples from 15 patients with different courses of PP (cystoid macular edema and non-macular edema subgroups; CME and nCME, respectively) and 17 healthy controls (HCs) in relation to the Th1, Th17, and immunoregulatory subsets. We observed that only PP patients from the CME subgroup presented a significantly higher fraction of CD16+ IDO-expressing MDSCs and intermediate CD14highCD16+ monocytes compared to the HCs; this corresponded with relative up-regulation of Th1 and Th17, and down-regulation of Treg. In addition, alongside the increased percentage of IDO-expressing CD16+ MDSCs, the MDSC compartment displayed an inappropriate level of IDO (more pronounced in the CD16− subset) only in CME patients. At the same time, the fraction of CD16− myeloid cells did not differ significantly among the patient cohorts and healthy participants. Our study is the first to evaluate subpopulations of circulating myeloid cells in PP patients and indicates that an increased fraction of CD16+ myeloid cells might reflect the immunological and clinical severity of PP.
Full article
Figure 1
Open AccessCommunication
RAGE Cytosolic Partner Diaph1 Does Not Play an Essential Role in Diabetic Peripheral Neuropathy Progression
by
Kamila Zglejc-Waszak, Bernard Kordas, Agnieszka Korytko, Andrzej Pomianowski, Bogdan Lewczuk, Joanna Wojtkiewicz, Krzysztof Wąsowicz, Izabella Babińska, Konark Mukherjee and Judyta Juranek
Cells 2025, 14(20), 1635; https://doi.org/10.3390/cells14201635 (registering DOI) - 21 Oct 2025
Abstract
►▼
Show Figures
Receptor for advanced glycation end-products (RAGE) activation by hyperglycemia-induced AGE (advanced glycation end-products) accumulation is likely to play a crucial role in the development of complications such as diabetic peripheral neuropathy (DPN). RAGE signaling is mediated via its cytosolic tail. Through its cytosolic
[...] Read more.
Receptor for advanced glycation end-products (RAGE) activation by hyperglycemia-induced AGE (advanced glycation end-products) accumulation is likely to play a crucial role in the development of complications such as diabetic peripheral neuropathy (DPN). RAGE signaling is mediated via its cytosolic tail. Through its cytosolic tail, RAGE recruits diaphanous-related formin 1 (Diaph1), a protein involved in actin filament organization. Disruption of RAGE–Diaph1 interactions using small molecules alleviates diabetic complications in mice; however, the role of Diaph1 in DPN progression has not been rigorously tested. In this study, we employed a Diaph1 knockout mouse (DKO) to investigate the role of Diaph1 in DPN progression. Herein, we demonstrate that, at the systemic level, CRISPR deletion of Diaph1 fails to ameliorate diabetes-induced weight loss in mice. Within the sciatic nerve (SCN), the lack of Diaph1 failed to prevent hyperglycemia-induced loss of β-actin in the nerve fibers. At a morphological level, the lack of Diaph1 leads to a partial rescue in DPN. While we observed improvements in axonal and fiber diameters in diabetic DKO mice, the g-ratio (an indicator of myelination) and myelin invaginations displayed incomplete rescue. Furthermore, the lack of Diaph1 failed to rescue motor or sensory nerve conduction defects resulting from hyperglycemia over 6 months. Overall, our data thus indicate that the complete loss of Diaph1 is insufficient to halt the progression of DPN. However, across a range of parameters including blood glucose levels, body weight measurements, axon and fiber diameters, and nerve conduction velocity, DKO diabetic mice show improvement when compared to wild-type diabetic mice.
Full article
Figure 1
Open AccessReview
RNA Degradation in Pluripotent Stem Cells: Mechanisms, Crosstalk, and Fate Regulation
by
Seunghwa Jeong, Myunggeun Oh, Jaeil Han and Seung-Kyoon Kim
Cells 2025, 14(20), 1634; https://doi.org/10.3390/cells14201634 - 20 Oct 2025
Abstract
Pluripotent stem cells (PSCs) exhibit remarkable self-renewal capacity and differentiation potential, necessitating tight regulation of gene expression at both transcriptional and post-transcriptional levels. Among post-transcriptional mechanisms, RNA turnover and degradation together play pivotal roles in maintaining transcriptome homeostasis and controlling RNA stability. RNA
[...] Read more.
Pluripotent stem cells (PSCs) exhibit remarkable self-renewal capacity and differentiation potential, necessitating tight regulation of gene expression at both transcriptional and post-transcriptional levels. Among post-transcriptional mechanisms, RNA turnover and degradation together play pivotal roles in maintaining transcriptome homeostasis and controlling RNA stability. RNA degradation plays a pivotal role in determining transcript stability for both messenger RNAs (mRNAs) and non-coding RNAs (ncRNAs), thereby influencing cell identity and fate transitions. The core RNA decay machinery, which includes exonucleases, decapping complexes, RNA helicases, and the RNA exosome, ensures timely and selective decay of transcripts. In addition, RNA modifications such as 5′ capping and N6-methyladenosine (m6A) further modulate RNA stability, contributing to the fine-tuning of gene regulatory networks essential for maintaining PSC states. Recent single-cell and multi-omics studies have revealed that RNA degradation exhibits heterogeneous and dynamic kinetics during cell fate transitions, highlighting its role in preserving transcriptome homeostasis. Conversely, disruption of RNA decay pathways has been implicated in developmental defects and disease, underscoring their potential as therapeutic targets. Collectively, RNA degradation emerges as a central regulator of PSC biology, integrating the decay of both mRNAs and ncRNAs to orchestrate pluripotency maintenance, lineage commitment, and disease susceptibility.
Full article
(This article belongs to the Special Issue Advances and Breakthroughs in Stem Cell Research)
►▼
Show Figures

Figure 1
Open AccessArticle
SIRT3 Mediates Coordination Between Energy Metabolism and SOD Activity in Melatonin-Enhanced Boar Sperm Motility
by
Naisheng Lu, Hulong Lei, Xueyuan Jiang, Peng Jia, Bushe Li and Dong Xia
Cells 2025, 14(20), 1633; https://doi.org/10.3390/cells14201633 - 20 Oct 2025
Abstract
Previous studies have demonstrated that melatonin (MLT) enhances boar sperm motility by modulating energy metabolism status, yet the underlying mechanisms remain incompletely understood. This study aims to investigate whether sirtuin 3 (SIRT3), a key mitochondrial deacetylase, mediates MLT’s effects. Herein, the semen of
[...] Read more.
Previous studies have demonstrated that melatonin (MLT) enhances boar sperm motility by modulating energy metabolism status, yet the underlying mechanisms remain incompletely understood. This study aims to investigate whether sirtuin 3 (SIRT3), a key mitochondrial deacetylase, mediates MLT’s effects. Herein, the semen of six Landrace boars (16–18 months of age) was treated with 1.0 μM MLT with/without the SIRT3 inhibitor 3-TYP, preserved at 17 °C for 3 days, and subsequently maintained at 37 °C for a duration of 10 min. We demonstrated that MLT upregulated SIRT3 protein expression and reduced the acetylation level in mitochondrial proteins. MLT significantly increased glucose uptake and suppressed lactate release in the sperm, while elevating levels of pyruvate and acetyl-CoA, the substrates of pyruvate dehydrogenase (PDH) and the tricarboxylic acid (TCA) cycle, respectively, and the protein expression of PDH, indicating enhanced metabolic flux. Notably, inhibition of SIRT3 reversed MLT’s effects: it blocked the increases in SIRT3 expression, glucose consumption, PDH expression, complex I activity, ATP content, and superoxide dismutase (SOD) activity, and prevented the decreases in the levels of acetylation and lactate, as well as pyruvate kinase (PK) activity, confirming the essential role of SIRT3. Functionally, the MLT-induced improvements in sperm motility parameters (total, progressive, fast motility, immotile) were also reversed by 3-TYP. Collectively, these findings demonstrate that the SIRT3-mediated pathway is essential for MLT to enhance boar sperm energy metabolism and antioxidant defense, thereby increasing ATP production and enhancing sperm motility. Targeting SIRT3 represents a promising therapeutic strategy for improving boar fertility and may also provide insights for research into human male infertility.
Full article
(This article belongs to the Collection Research Advances in Cellular Metabolism)
►▼
Show Figures

Figure 1
Open AccessArticle
Identification of Regulatory RNA-Binding Genes in Spermatogonial Stem Cell Reprogramming to ES-like Cells Using Machine Learning–Integrated Transcriptomic and Network Analysis
by
Ali Shakeri Abroudi, Hossein Azizi, Hewa Khalid Abdullah, Marwa Fadhil Alsaffar and Thomas Skutella
Cells 2025, 14(20), 1632; https://doi.org/10.3390/cells14201632 - 20 Oct 2025
Abstract
►▼
Show Figures
Spermatogonial stem cells (SSCs) are unipotent germline cells with emerging pluripotent potential under specific in vitro conditions. Understanding their capacity for reprogramming and the molecular mechanisms involved offers valuable insights into regenerative medicine and fertility preservation. SSCs were isolated from Oct4-GFP C57BL/6 transgenic
[...] Read more.
Spermatogonial stem cells (SSCs) are unipotent germline cells with emerging pluripotent potential under specific in vitro conditions. Understanding their capacity for reprogramming and the molecular mechanisms involved offers valuable insights into regenerative medicine and fertility preservation. SSCs were isolated from Oct4-GFP C57BL/6 transgenic mice using enzymatic digestion and cultured in defined media. Under these conditions, ES-like colonies emerged expressing pluripotency markers. These cells were characterized by immunocytochemistry, teratoma assays, and transcriptomic analyses using bulk and single-cell RNA sequencing datasets. Gene expression profiles were compared with ESCs and SSCs using datasets from GEO (GSE43850, GSE38776, GSE149512). Protein–protein interaction (PPI) networks and co-expression modules were explored through STRING, Cytoscape, and WGCNA. ES-like cells derived from SSCs exhibited strong expression of OCT4, DAZL, and VASA. Transcriptomic analysis revealed key differentially expressed genes and shared regulatory networks with ESCs. WGCNA identified key co-expression modules and hub regulatory RNA binding genes (Ctdsp1, Rest, and Stra8) potentially responsible for the reprogramming process. Teratoma assays confirmed pluripotency, and single-cell RNA-seq validated expression of critical markers in cultured SSCs. This study demonstrates that SSCs can acquire pluripotency features and be reprogrammed into ES-like cells. The integration of transcriptomic and network-based analyses reveals novel insights into the molecular drivers of SSC reprogramming, highlighting their potential utility in stem cell-based therapies and male fertility preservation.
Full article

Figure 1
Open AccessArticle
BelloStage™-3000 Bioreactor Versus Conventional Cultivation of Recombinant Capripoxvirus Expressing Brucella Antigens in Vero Cells: A Step Towards the Development of a New Human Brucellosis Vaccine
by
Zhanat Amanova, Zhanna Sametova, Olga Chervyakova, Sholpan Turyskeldi, Alina Kurmasheva, Ruslan Abitayev, Abdurakhman Ussembay, Zhanat Kondibayeva, Dariya Toktyrova, Dana Mazbayeva and Yerbol Bulatov
Cells 2025, 14(20), 1631; https://doi.org/10.3390/cells14201631 - 20 Oct 2025
Abstract
Brucellosis remains one of the most significant zoonotic diseases, posing a serious threat to both human health and livestock. This issue is particularly relevant for Kazakhstan, which is among the countries endemic for brucellosis with a high incidence rate. Such circumstances highlight the
[...] Read more.
Brucellosis remains one of the most significant zoonotic diseases, posing a serious threat to both human health and livestock. This issue is particularly relevant for Kazakhstan, which is among the countries endemic for brucellosis with a high incidence rate. Such circumstances highlight the urgent need for the development and implementation of effective preventive measures, including modern vaccine platforms capable of providing reliable protection for the population and reducing the economic impact on the agricultural sector. Recombinant capripoxviruses are considered promising vector platforms for vaccine development, as they ensure high expression of target antigens, elicit strong immune responses, and are safe for humans. In this study, the replication of recombinant capripoxviruses expressing Brucella antigens (SPPV (TK-) OMP19/SODC and SPPV (TK-) OMP25) was evaluated in Vero cells using the BelloStage™-3000 bioreactor system in combination with BioNOC II® macrocarriers. Application of the bioreactor resulted in nearly a 100-fold increase in Vero cell density compared with static cultures and provided optimal conditions for cell adhesion, growth, and metabolic activity. Consequently, a significant increase in viral titers was observed: for SPPV (TK-) OMP19/SODC, mean titers reached 7.50 log10 TCID50/mL versus 4.50 in static culture (p < 0.0001), while SPPV (TK-) OMP25 achieved 7.08 log10 TCID50/mL versus 4.33 (p < 0.001). These findings confirm the reliability, reproducibility, and scalability of this bioreactor-based approach, demonstrating clear advantages over conventional cultivation methods. Overall, the study highlights the high potential of the BelloStage™-3000 system with BioNOC II® macrocarriers for the industrial production of recombinant capripoxvirus-based vaccines against brucellosis and for the broader development of other recombinant viral vaccines.
Full article
(This article belongs to the Special Issue 3D Cultures and Organ-on-a-Chip in Cell and Tissue Cultures)
►▼
Show Figures

Figure 1
Open AccessArticle
MK3 Gene Upregulates Granulosa Cell Apoptosis Through the TNF/P38 MAPK Pathway in Chicken
by
Li Chen, Jia Liu, Ying Zhang, Jinsong Pi and Yan Wu
Cells 2025, 14(20), 1630; https://doi.org/10.3390/cells14201630 (registering DOI) - 20 Oct 2025
Abstract
In poultry production, the laying rate is a critical economic trait, as high egg production significantly enhances profitability. Mitogen-activated protein kinase-activated protein kinase 3 (MK3) is a member of the mitogen-activated protein kinase (MAPK) family, which plays an important role in
[...] Read more.
In poultry production, the laying rate is a critical economic trait, as high egg production significantly enhances profitability. Mitogen-activated protein kinase-activated protein kinase 3 (MK3) is a member of the mitogen-activated protein kinase (MAPK) family, which plays an important role in follicular development. Our previous RNA-seq analysis revealed that MK3 expression was significantly altered in the ovaries of laying hens exposed to normal versus light-deprivation conditions. Based on previous RNA-seq analysis of chicken ovaries, this study focused on the MK3 gene to explore its role in regulating apoptosis of follicular granulosa cells in laying hens. The results demonstrated that MK3 overexpression induced granulosa cell apoptosis by modulating the expression of key proliferation- and apoptosis-related genes, including FAS, Caspase3, BCL2, and C-myc. These findings were further validated using specific siRNA-mediated knockdown of MK3. Flow cytometry, CCK-8, and EdU assays consistently showed that MK3 facilitated apoptosis and inhibited granulosa cell proliferation. Additionally, dual-luciferase reporter assays revealed that the transcription factor WT1 bound to the MK3 promoter and enhanced its transcriptional activity. Mechanistically, MK3 regulated granulosa cell apoptosis through the TNF/P38 MAPK pathway. This conclusion was corroborated by treatment with the P38 inhibitor GS-444217 and specific siRNA targeting components of the pathway. In summary, MK3 promotes granulosa cell apoptosis in the follicles of laying hens, is transcriptionally regulated by WT1, and exerts its pro-apoptotic effects via the TNF/P38 MAPK pathway.
Full article
(This article belongs to the Special Issue Focus on Machinery of Cell Death)
►▼
Show Figures
Figure 1
Open AccessReview
HPV Oncoproteins and Mitochondrial Reprogramming: The Central Role of ROMO1 in Oxidative Stress and Metabolic Shifts
by
Eva Tsoneva and Angel Yordanov
Cells 2025, 14(20), 1629; https://doi.org/10.3390/cells14201629 - 19 Oct 2025
Abstract
High-risk human papillomaviruses (HPVs), particularly types 16 and 18, drive carcinogenesis by rewiring host metabolism and mitochondrial function. The oncoproteins E5, E6, and E7 collectively induce mitochondrial fragmentation, increase reactive oxygen species (ROS), and promote a metabolic shift from oxidative phosphorylation (OXPHOS) to
[...] Read more.
High-risk human papillomaviruses (HPVs), particularly types 16 and 18, drive carcinogenesis by rewiring host metabolism and mitochondrial function. The oncoproteins E5, E6, and E7 collectively induce mitochondrial fragmentation, increase reactive oxygen species (ROS), and promote a metabolic shift from oxidative phosphorylation (OXPHOS) to glycolysis (the Warburg effect). A redox-sensitive mitochondrial protein, Reactive Oxygen Species Modulator 1 (ROMO1), has emerged as a key mediator of these processes. ROMO1 contributes to mitochondrial morphology, regulates ROS homeostasis, and interacts with key stress-response pathways. While ROMO1 is overexpressed in many cancers and correlates with poor prognosis, recent data suggest that HPV-associated cervical lesions exhibit a unique biphasic expression pattern, with high ROMO1 levels in early stages and reduced expression in advanced tumors. The underlying molecular mechanisms remain unclear, but may involve HPV genome integration, NF-κB suppression, or epigenetic silencing. Key mechanisms such as how HPV modulates ROMO1 expression and how this contributes to stage-dependent metabolic vulnerability remain incompletely understood. This review highlights the current understanding of how HPV oncoproteins impact mitochondrial structure and function, emphasizes the role of ROMO1 in this context, and compares findings with other cancer types. Although no ROMO1-targeted therapies currently exist, the protein may serve as a redox-sensitive biomarker and potential vulnerability in HPV-driven tumors. We propose that targeting mitochondrial fragmentation, ROS signaling, or metabolic reprogramming may offer new avenues for therapeutic intervention. Further research is needed to clarify ROMO1’s dual role in early vs. late-stage disease and to validate its relevance as a clinical target. Our review fills a gap in the current literature by being the first to systematically explore ROMO1’s contribution to HPV-induced mitochondrial dysfunction and metabolic rewiring, and we outline research priorities for future studies.
Full article
(This article belongs to the Special Issue From Energy Metabolism to Disease: The Multifaceted Roles of Mitochondria)
►▼
Show Figures

Figure 1
Open AccessArticle
Non-Competitive AMPA Receptor Antagonist Perampanel Inhibits Ischemia-Induced Neurodegeneration and Behavioral Deficits in Focal Cortical Pial Vessel Disruption Stroke Model
by
Michael G. Zaki, Mohamed Taha Moutaoufik, Mahboubeh Pordeli, Mohan Babu, Changiz Taghibiglou and Francisco S. Cayabyab
Cells 2025, 14(20), 1628; https://doi.org/10.3390/cells14201628 - 19 Oct 2025
Abstract
Glutamate receptors represent a potential target for neuroprotection in neurodegenerative neurological conditions. Perampanel, a non-competitive α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor (AMPAR) antagonist, is clinically approved for the management of epilepsy. Perampanel’s neuroprotective effects have been reported in global and focal cerebral ischemia models, but the
[...] Read more.
Glutamate receptors represent a potential target for neuroprotection in neurodegenerative neurological conditions. Perampanel, a non-competitive α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor (AMPAR) antagonist, is clinically approved for the management of epilepsy. Perampanel’s neuroprotective effects have been reported in global and focal cerebral ischemia models, but the cellular mechanisms remain incompletely understood. Therefore, we studied the potential neuroprotective effects of perampanel in rats using the pial vessel disruption (PVD) stroke model, an established focal cortical non-reperfusion ischemic stroke model. Perampanel was given once intraperitoneally (3 mg/kg body weight) 1 h after PVD surgery and repeated on days 2–3 post-surgery. On the fourth day post PVD, animal behavioral assays and imaging, biochemical, and electrophysiological analyses were performed. Compared to vehicle control, perampanel in PVD-treated rats significantly inhibited hippocampal neurodegeneration and long-term potentiation deficits. Perampanel also attenuated PVD-induced motor deficits, depressive/anxiety-like behaviors, and hippocampal-dependent cognitive impairment. In addition, perampanel prevented the PVD-induced downregulation of surface-expressed GluA1 and GluA2 AMPARs and increased phosphorylation of GluA1 at S831 and S845. Molecular docking analysis revealed perampanel binding to transmembrane regions M1, M3 and M4 of GluA1 and GluA2 subunits. Together, our results show that perampanel attenuated PVD-induced neurodegeneration and behavioral deficits by blocking AMPARs and decreasing GluA1 and GluA2 internalization. In addition, this study shows the neuroprotective potential of perampanel through the inhibition of neuroinflammation mediated by activated microglia and astrocytes following cerebral ischemia. This study is the first to evaluate perampanel in the pial vessel disruption model of ischemia without reperfusion, a clinically relevant stroke paradigm that differs fundamentally from middle cerebral carotid artery occlusion and photothrombosis stroke models.
Full article
(This article belongs to the Topic Application of Animal Models: From Physiology to Pathology)
►▼
Show Figures

Figure 1
Open AccessReview
Heat Shock Proteins in Pancreatic Cancer: Pathogenic Mechanisms and Clinical Implications
by
Jacek Kabut, Jakub Sokołowski, Wiktoria Żelazna, Mateusz Stępień, Marta Strauchman, Natalia Jaworska, Jakub Wnuk, Anita Gorzelak-Magiera, Łukasz Michalecki and Iwona Gisterek-Grocholska
Cells 2025, 14(20), 1627; https://doi.org/10.3390/cells14201627 - 18 Oct 2025
Abstract
Heat shock proteins (HSPs) are highly conserved molecular chaperones that play a key role in maintaining protein homeostasis, or proteostasis, especially under stressful environmental conditions such as hyperthermia, hypoxia, or the presence of reactive oxygen species. In pancreatic cancer, the expression of many
[...] Read more.
Heat shock proteins (HSPs) are highly conserved molecular chaperones that play a key role in maintaining protein homeostasis, or proteostasis, especially under stressful environmental conditions such as hyperthermia, hypoxia, or the presence of reactive oxygen species. In pancreatic cancer, the expression of many HSP isoforms is dysregulated, contributing to the activation of mechanisms that promote tumor development, including proliferation, invasion, angiogenesis, treatment resistance, and cancer cachexia syndrome. HSPs are significant diagnostic and prognostic biomarkers. Some of them, such as HSP27, HSP70, and HSP90, have been shown to correlate with treatment response and patient survival. Others, including HSPA2 and HSPB6, may indicate an increased risk of disease recurrence. These proteins also represent promising therapeutic targets. Preclinical and clinical studies suggest that inhibiting HSP activity and associated signaling pathways may inhibit tumor growth and increase treatment efficacy. These therapeutic effects include inducing apoptosis, autophagy, and ferroptosis, as well as sensitizing cancer cells to chemotherapy and immunotherapy. This article summarizes the current knowledge about the role of HSPs in pancreatic cancer biology, their significance as biomarkers, and their potential therapeutic applications in treating pancreatic ductal adenocarcinoma (PDAC). Most studies conducted so far have been preclinical, and due to the promising results, further clinical investigation is warranted.
Full article
(This article belongs to the Special Issue Heat Shock Proteins and Human Cancers)
►▼
Show Figures
Figure 1
Open AccessArticle
Generating a Preclinical Model for PITPNM3 and Evaluating Genotype–Phenotype Concordance: Insights from a Mouse Model
by
Aykut Demirkol, Joanne Li and Stephen H. Tsang
Cells 2025, 14(20), 1626; https://doi.org/10.3390/cells14201626 - 18 Oct 2025
Abstract
►▼
Show Figures
PITPNM3 has been identified as a crucial gene associated with various phenotypes of retinal disease in humans; however, detailed mechanisms through which PITPNM3 mutations result in these conditions are not fully understood. In this study, we aimed to generate such a preclinical mouse
[...] Read more.
PITPNM3 has been identified as a crucial gene associated with various phenotypes of retinal disease in humans; however, detailed mechanisms through which PITPNM3 mutations result in these conditions are not fully understood. In this study, we aimed to generate such a preclinical mouse model and evaluate its relevance to human PITPNM3-related conditions. Heterozygous mice were bred to obtain a homozygous genotype, aiming to mimic the human genetic condition. Subsequent phenotyping and genetic segregation analyses were conducted along with electrophysiological studies and histological examinations. Full-field electroretinogram analysis revealed a reduced cone response although the severity was not as pronounced as observed in humans with PITPNM3-related conditions. Histologically, the retinal structure appeared largely unchanged, indicating a discordance between functional impairment and morphological changes. In our preclinical mouse model, the observed phenotypic changes were not as severe as those found in humans with PITPNM3-related conditions and this discrepancy points to a potentially different disease progression trajectory in the mouse model. These findings highlight the importance of longer follow-up periods in such studies and the need for further research to elucidate the genotype–phenotype relationship in PITPNM3.
Full article

Figure 1
Open AccessArticle
Ginsenoside Derivative AD-1 Suppresses Pathogenic Phenotypes of Rheumatoid Arthritis Fibroblast-like Synoviocytes by Modulating the PI3K/Akt Signaling Pathway
by
Yuan Fu, Fangfang Li, Biao Cui, Zhongyu Zhou, Xizhu Fang, Shengnan Huang, Xingguo Quan, Yuqing Zhao and Dan Jin
Cells 2025, 14(20), 1625; https://doi.org/10.3390/cells14201625 - 18 Oct 2025
Abstract
Rheumatoid arthritis (RA) is a systemic autoimmune disorder marked by chronic inflammation of small synovial joints, with frequent extra-articular involvement of the skin and eyes. Prolonged methotrexate therapy for RA is often accompanied by serious side effects. Therefore, new drugs with less toxicity
[...] Read more.
Rheumatoid arthritis (RA) is a systemic autoimmune disorder marked by chronic inflammation of small synovial joints, with frequent extra-articular involvement of the skin and eyes. Prolonged methotrexate therapy for RA is often accompanied by serious side effects. Therefore, new drugs with less toxicity and greater effectiveness need to be developed. The ginsenoside 20(R)-25-methoxyl-dammarane-3β,12β,20-triol (AD-1), purified from Panax ginseng berry, exhibits potent anti-inflammatory and anti-cancer activities. However, the pharmacological mechanism of AD-1 in RA remains unclear. This study explored the potential anti-RA effects of AD-1 using an integrative strategy that combined network pharmacology, molecular docking, molecular dynamics simulation, and in vitro pharmacological validation. Enrichment analyses of KEGG and GO terms based on network pharmacology pointed to the PI3K/Akt signaling axis as a key regulatory pathway modulated by AD-1. Molecular docking and dynamics simulations revealed that AD-1 may have a close interaction with PIK3R1 and AKT1, demonstrating a stabilizing effect. Then, after experimental verification using human rheumatoid arthritis fibroblasts (MH7A), it was found that AD-1 suppressed cell proliferation, migration, and invasion and promoted apoptosis. Subsequent analysis of the RABC databases revealed that PIK3R1 and AKT1 were upregulated in RA, while AD-1 reduces phosphorylation of PI3K and Akt. In conclusion, these findings indicate that AD-1 exerts its anti-RA action, at least in part, through modulation of the PI3K/Akt signaling pathway and induction of apoptosis in synovial cells. This study provides a basis and new strategies for the role of ginsenosides in the treatment of RA.
Full article
(This article belongs to the Special Issue Study on Immune Activity of Natural Products)
►▼
Show Figures
Graphical abstract
Open AccessArticle
Propiconazole-Induced Testis Damage and MAPK-Mediated Apoptosis and Autophagy in Germ Cells
by
Won-Young Lee, Ran Lee, Hyeon Woo Sim and Hyun-Jung Park
Cells 2025, 14(20), 1624; https://doi.org/10.3390/cells14201624 - 17 Oct 2025
Abstract
►▼
Show Figures
Propiconazole (PRO), a triazole fungicide, controls fungal diseases by disrupting ergosterol production in fungal cells. It is used in crops such as cereals and fruits. However, there are concerns regarding its potential to disrupt the endocrine system and cause reproductive toxicity. This study
[...] Read more.
Propiconazole (PRO), a triazole fungicide, controls fungal diseases by disrupting ergosterol production in fungal cells. It is used in crops such as cereals and fruits. However, there are concerns regarding its potential to disrupt the endocrine system and cause reproductive toxicity. This study examined the effects of PRO on mouse testes, germ cells, and GC-1 spermatogonia. After eight weeks, PRO reduced testicular diameter and downregulated key germ cell genes (Sall4, Piwil, Nanos2, and Dazl). A histological examination revealed smaller seminiferous tubules and fewer SALL4+ cells. PRO also impaired steroidogenesis by downregulating genes (StAR, Cyp11a1, 3β-HSD1) and reducing sperm motility, with a decline in Velocity Straight Line (VSL), Linearity (LIN), Straightness (STR), and motile sperm. PRO caused dose-dependent cytotoxicity in GC-1 spermatogonia, decreased proliferation, and increased apoptosis, marked by cleaved caspase-3 and BAX. PRO also induced autophagy, as presented by elevated levels of autophagy-related genes (LC3 and ATG12) and proteins (ATG5 and LC3A/B). 3-Methyladenine (3-MA), an autophagy inhibitor, downregulates levels of autophagy- and apoptosis-related proteins when 3-MA and PRO are simultaneously treated in vitro. This suggests that both apoptosis and autophagy contribute to PRO-induced testicular cytotoxicity. This study is the first to detail that PRO affects sperm motility in mice and induces autophagy-mediated apoptosis in GC-1 spg.
Full article
Graphical abstract

Journal Menu
► ▼ Journal Menu-
- Cells Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Analytica, Metabolites, Toxins, Molecules, Cells, Chemosensors
Application of Analytical Technology in Metabolomics
Topic Editors: Preeti Chandra, Raúl G. EnríquezDeadline: 31 October 2025
Topic in
Cells, Chemistry, IJMS, Molecules, Metabolites
Bioactive Compounds and Therapeutics: Molecular Aspects, Metabolic Profiles, and Omics Studies 2nd Edition
Topic Editors: Michele Costanzo, Giovanni N. Roviello, Armando CeveniniDeadline: 20 November 2025
Topic in
Animals, Cells, Life, Veterinary Sciences
Application of Animal Models: From Physiology to Pathology
Topic Editors: Juan Carlos Illera del Portal, Sara Cáceres Ramos, Felisbina Luisa QueirogaDeadline: 20 December 2025
Topic in
Biology, Biomolecules, Cancers, Cells, IJMS
RNAs and Phase Separation Phenomena
Topic Editors: Ana Lúcia Leitão, Afshin Beheshti, Francisco J. EnguitaDeadline: 31 December 2025

Conferences
Special Issues
Special Issue in
Cells
Adult Stem Cells in Human Disease
Guest Editors: Esther Wolfs, Tim Vangansewinkel, Annelies BronckaersDeadline: 25 October 2025
Special Issue in
Cells
Recent Advances in Regenerative Dentistry—Second Edition
Guest Editor: Li XiaoDeadline: 25 October 2025
Special Issue in
Cells
Molecular Anatomy and Function of Sensory Organs and Sensory Tissues
Guest Editor: Frank SchmitzDeadline: 25 October 2025
Special Issue in
Cells
3D Cultures and Organ-on-a-Chip in Cell and Tissue Cultures
Guest Editors: Annunziata Mauro, Barbara Barboni, Giovanna Della PortaDeadline: 25 October 2025
Topical Collections
Topical Collection in
Cells
Insulin-Like Growth Factors in Development, Cancers and Aging
Collection Editor: Haim Werner
Topical Collection in
Cells
Advances in Epithelial-Mesenchymal Transition (EMT)
Collection Editors: Oriana Trubiani, Francesca Diomede, Jacopo Pizzicannella, Guya Diletta Marconi
Topical Collection in
Cells
How Perinatal Stress Affects Brain Plasticity in Ontogenesis
Collection Editors: Alexander V. Arutjunyan, Natalia V. Gulyaeva











