Induced Pluripotent Stem Cell-Derived Red Blood Cells and Platelet Concentrates: From Bench to Bedside
Abstract
:1. Why We Need Alternatives to Donated RBCs and Platelet Concentrates
2. iPS Cells Technology
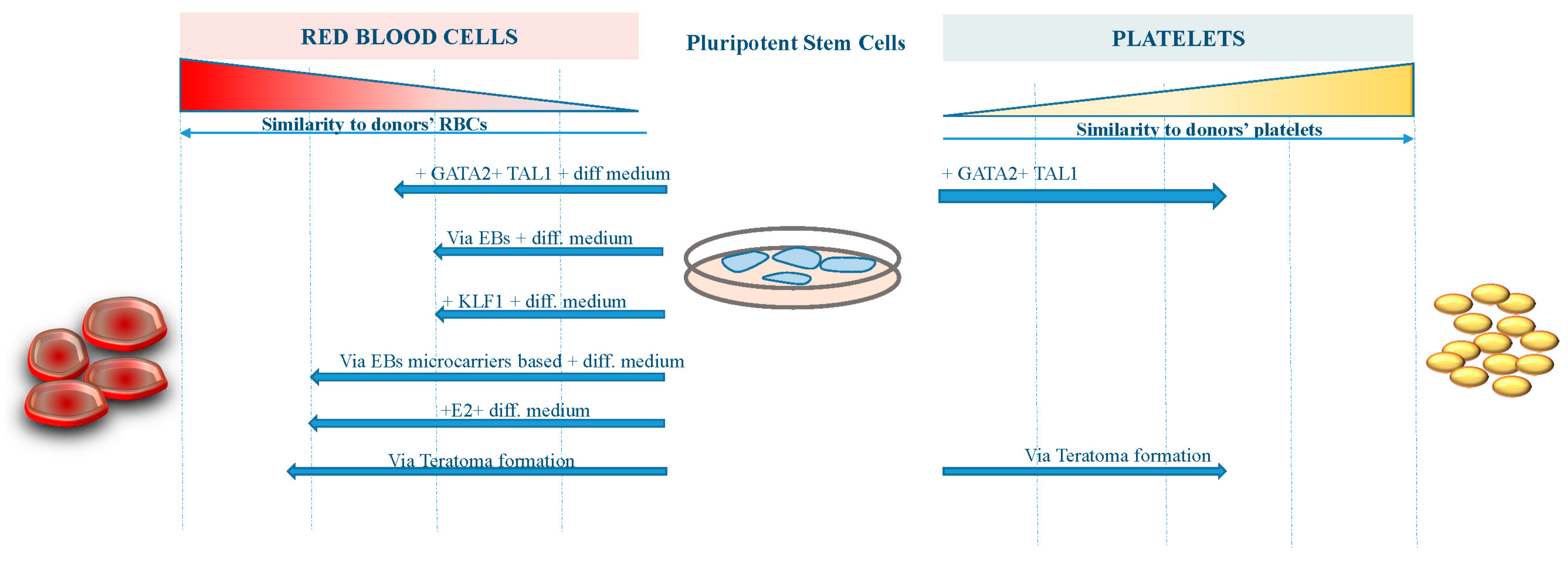
3. Advances in RBCs Derivation from iPS Cells
4. Advances in Platelet Derivation from iPS Cells
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Conflicts of Interest
Abbreviations
| iPS cells | Induced pluripotent stem cells |
| RBCs | Red blood cells |
| PLT | Platelets |
References
- WHO Model List of Essential Medicines for Adults, 19th List (April 2015) Rev. August 2015. Available online: http://www.who.int/selection_medicines/committees/expert/20/EML_2015_FINAL_amended_AUG2015.pdf?ua=1 (accessed on 24 December 2017).
- Ali, A.; Auvinen, M.K.; Rautonen, J. The aging population poses a global challenge for blood services. Transfusion 2010, 50, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Baek, E.J.; Kim, H.S.; Kim, S.; Jin, H.; Choi, T.Y.; Kim, H.O. In vitro clinical-grade generation of red blood cells from human umbilical cord blood CD34+ cells. Transfusion 2008, 48, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Vodyanik, M.A.; Thomson, J.A.; Slukvin, I.I. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood 2006, 108, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Klimchenko, O.; Mori, M.; Distefano, A.; Langlois, T.; Larbret, F.; Lecluse, Y.; Feraud, O.; Vainchenker, W.; Norol, F.; Debili, N. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood 2009, 114, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.J.; Feng, Q.; Park, J.S.; Vida, L.; Lee, B.S.; Strausbauch, M.; Wettstein, P.J.; Honig, G.R.; Lanza, R. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood 2008, 112, 4475–4484. [Google Scholar] [CrossRef] [PubMed]
- Elcheva, I.; Brok-Volchanskaya, V.; Kumar, A.; Liu, P.; Lee, J.H.; Tong, L.; Vodyanik, M.; Swanson, S.; Stewart, R.; Kyba, M.; et al. Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nat. Commun. 2014, 5, 4372. [Google Scholar] [CrossRef] [PubMed]
- Acosta, N.D.; Golub, S.H. The New Federalism: State Policies Regarding Embryonic Stem Cell Research. J. Law Med. Ethics 2016, 44, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Vodyanik, M.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.; Tian, S.; Nie, J.; Jonsdottir, G.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Jincho, Y.; Araki, R.; Hoki, Y.; Tamura, C.; Nakamura, M.; Ando, S.; Kasama, Y.; Abe, M. Generation of genome integration-free induced pluripotent stem cells from fibroblasts of C57BL/6 mice without c-Myc transduction. J. Biol. Chem. 2010, 285, 26384–26389. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Chien, Y.; Chen, Y.J.; Chen, S.F.; Chang, Y.L.; Chiang, C.H.; Jeng, S.Y.; Chang, C.M.; Wang, M.L.; Chen, L.K.; et al. Reprogramming induced pluripotent stem cells in the absence of c-Myc for differentiation into hepatocyte-like cells. Biomaterials 2011, 32, 5994–6005. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.-H.; Jiang, B.-H.; Yu, Y.-L.; Chou, S.-J.; Tsai, P.-H.; Chang, W.-C.; Chen, L.-K.; Chen, L.-H.; Chien, Y.; Chiou, G.-Y. Poly(ADP-ribose) polymerase 1 regulates nuclear reprogramming and promotes iPSC generation without c-Myc. J. Exp. Med. 2013, 210, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Doege, C.A.; Inoue, K.; Yamashita, T.; Rhee, D.B.; Travis, S.; Fujita, R.; Guarnieri, P.; Bhagat, G.; Vanti, W.B.; Shih, A.; et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature 2012, 488, 652–655. [Google Scholar] [CrossRef] [PubMed]
- De Kelver, R.C.; Choi, V.M.; Moehle, E.A.; Paschon, D.E.; Hockemeyer, D.; Meijsing, S.H.; Sancak, Y.; Cui, X.; Steine, E.J.; Miller, J.C.; et al. Functional genomics, proteomics, and regulatory DNA analysis in isogenic settings using zinc finger nuclease-driven transgenesis into a safe harbor locus in the human genome. Genome Res. 2010, 20, 1133–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayart, E.; Cohen-Haguenauer, O. Technological overview of iPS induction from human adult somatic cells. Curr. Gene Ther. 2013, 13, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Amabile, G.; Welner, R.S.; Nombela-Arrieta, C.; D’Alise, A.M.; Di Ruscio, A.; Ebralidze, A.K.; Kraytsberg, Y.; Ye, M.; Kocher, O.; Neuberg, D.S.; et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood 2013, 121, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Amabile, G.; Meissner, A. Induced pluripotent stem cells: Current progress and potential for regenerative medicine. Trends Mol. Med. 2009, 15, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Amabile, G.; Di Ruscio, A.; Quaranta, P.; Tenen, D.G.; Pistello, M. Induced pluripotent stem cells in hematology: Current and future applications. Blood Cancer J. 2014, 4, e211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amabile, G.; Di Ruscio, A.; Muller, F.; Welner, R.S.; Yang, H.; Ebralidze, A.K.; Zhang, H.; Levantini, E.; Qi, L.; Martinelli, G.; et al. Dissecting the role of aberrant DNA methylation in human leukaemia. Nat. Commun. 2015, 6, 7091. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cascino, P.; Ummarino, S.; Di Ruscio, A. Application of Induced Pluripotent Stem Cell Technology to the Study of Hematological Diseases. Cells 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Jackman, R.P.; Deng, X.; Bolgiano, D.; Utter, G.H.; Schechterly, C.; Lebedeva, M.; Operskalski, E.; Luban, N.L.; Alter, H.; Busch, M.P.; et al. Leukoreduction and ultraviolet treatment reduce both the magnitude and the duration of the HLA antibody response. Transfusion 2014, 54, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Fast, L.D.; Nevola, M.; Tavares, J.; Reddy, H.L.; Goodrich, R.P.; Marschner, S. Treatment of whole blood with riboflavin plus ultraviolet light, an alternative to gamma irradiation in the prevention of transfusion-associated graft-versus-host disease? Transfusion 2013, 53, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Pistello, M. Effect of Induced Pluripotent Stem Cell Technology in Blood Banking. Stem Cells Transl. Med. 2016, 5, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Nakamura, S.; Nakajima, M.; Endo, H.; Dohda, T.; Takayama, N.; Nakauchi, H.; Arai, F.; Fukuda, T.; Eto, K. Two differential flows in a bioreactor promoted platelet generation from human pluripotent stem cell-derived megakaryocytes. Exp. Hematol. 2013, 41, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.Y.; Tan, H.K.; Loh, Y.H. Derivation of Transgene-Free Induced Pluripotent Stem Cells from a Single Drop of Blood. Curr. Protoc. Stem Cell Biol. 2016, 38. [Google Scholar] [CrossRef]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Shabrani, N.; Thon, J.N.; Huo, H.; Thiel, A.; Machlus, K.R.; Kim, K.; Brooks, J.; Li, F.; Luo, C.; et al. Scalable Generation of Universal Platelets from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2014, 3, 817–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivier, E.N.; Marenah, L.; McCahill, A.; Condie, A.; Cowan, S.; Mountford, J.C. High-Efficiency Serum-Free Feeder-Free Erythroid Differentiation of Human Pluripotent Stem Cells Using Small Molecules. Stem Cells Transl. Med. 2016, 5, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Borst, S.; Sim, X.; Poncz, M.; French, D.L.; Gadue, P. Induced Pluripotent Stem Cell-Derived Megakaryocytes and Platelets for Disease Modeling and Clinical Use. Arterioscler. Thromb. Vasc. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Asano, H.; Lee, C.Y.; Fox-Talbot, K.; Koh, C.M.; Erdinc, M.M.; Marschner, S.; Keil, S.; Goodrich, R.P.; Baldwin, W.M., 3rd. Treatment with riboflavin and ultraviolet light prevents alloimmunization to platelet transfusions and cardiac transplants. Transplantation 2007, 84, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Mitra, K.; Koya, M.; Velho, M.; Desprat, R.; Lenz, J.; Bouhassira, E.E. Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells. PLoS ONE 2011, 6, e25761. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Gumenyuk, M.; Kang, H.; Vodyanik, M.; Yu, J.; Thomson, J.A.; Slukvin, I.I. Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev. 2011, 20, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, H.; Kobari, L.; Mazurier, C.; Tropel, P.; Giarratana, M.C.; Zanella-Cleon, I.; Kiger, L.; Wattenhofer-Donze, M.; Puccio, H.; Hebert, N.; et al. Red blood cell generation from human induced pluripotent stem cells: Perspectives for transfusion medicine. Haematologica 2010, 95, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Kobari, L.; Yates, F.; Oudrhiri, N.; Francina, A.; Kiger, L.; Mazurier, C.; Rouzbeh, S.; El-Nemer, W.; Hebert, N.; Giarratana, M.C.; et al. Human induced pluripotent stem cells can reach complete terminal maturation: In vivo and in vitro evidence in the erythropoietic differentiation model. Haematologica 2012, 97, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Kessel, K.U.; Bluemke, A.; Scholer, H.R.; Zaehres, H.; Schlenke, P.; Dorn, I. Emergence of CD43-Expressing Hematopoietic Progenitors from Human Induced Pluripotent Stem Cells. Transfus. Med. Hemother. 2017, 44, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Merryweather-Clarke, A.T.; Tipping, A.J.; Lamikanra, A.A.; Fa, R.; Abu-Jamous, B.; Tsang, H.P.; Carpenter, L.; Robson, K.J.; Nandi, A.K.; Roberts, D.J. Distinct gene expression program dynamics during erythropoiesis from human induced pluripotent stem cells compared with adult and cord blood progenitors. BMC Genom. 2016, 17, 817. [Google Scholar] [CrossRef] [PubMed]
- Trakarnsanga, K.; Wilson, M.C.; Griffiths, R.E.; Toye, A.M.; Carpenter, L.; Heesom, K.J.; Parsons, S.F.; Anstee, D.J.; Frayne, J. Qualitative and quantitative comparison of the proteome of erythroid cells differentiated from human iPSCs and adult erythroid cells by multiplex TMT labelling and nanoLC-MS/MS. PLoS ONE 2014, 9, e100874. [Google Scholar] [CrossRef] [PubMed]
- Razaq, M.A.; Taylor, S.; Roberts, D.J.; Carpenter, L. A molecular roadmap of definitive erythropoiesis from human induced pluripotent stem cells. Br. J. Haematol. 2017, 176, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sankaran, V.G.; Ni, M.; Menne, T.F.; Puram, R.V.; Kim, W.; Orkin, S.H. Transcriptional silencing of γ-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev. 2010, 24, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Siatecka, M.; Bieker, J.J. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood 2011, 118, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Haro-Mora, J.J.; Fujita, A.; Lee, D.Y.; Winkler, T.; Hsieh, M.M.; Tisdale, J.F. Efficient Generation of beta-Globin-Expressing Erythroid Cells Using Stromal Cell-Derived Induced Pluripotent Stem Cells from Patients with Sickle Cell Disease. Stem Cells 2017, 35, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; Ma, R.; Axton, R.A.; Jackson, M.; Taylor, A.H.; Fidanza, A.; Marenah, L.; Frayne, J.; Mountford, J.C.; Forrester, L.M. Activation of KLF1 Enhances the Differentiation and Maturation of Red Blood Cells from Human Pluripotent Stem Cells. Stem Cells 2017, 35, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, J.; Lam, A.T.; Chen, H.Y.; Yang, B.X.; Chen, A.K.; Reuveny, S.; Loh, Y.H.; Oh, S.K. Superior Red Blood Cell Generation from Human Pluripotent Stem Cells Through a Novel Microcarrier-Based Embryoid Body Platform. Tissue Eng. Part C Methods 2016, 22, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, M.; Chonabayashi, K.; Nomura, M.; Tanaka, A.; Nakamura, M.; Inagaki, A.; Nishikawa, M.; Takei, I.; Oishi, A.; Tanabe, K.; et al. Epigenetic Variation between Human Induced Pluripotent Stem Cell Lines Is an Indicator of Differentiation Capacity. Cell Stem Cell 2016, 19, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Lee, J.H.; Heo, H.R.; Yang, S.R.; Ha, K.S.; Park, W.S.; Han, E.T.; Song, H.; Hong, S.H. Improved hematopoietic differentiation of human pluripotent stem cells via estrogen receptor signaling pathway. Cell Biosci. 2016, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Giani, F.C.; Fiorini, C.; Wakabayashi, A.; Ludwig, L.S.; Salem, R.M.; Jobaliya, C.D.; Regan, S.N.; Ulirsch, J.C.; Liang, G.; Steinberg-Shemer, O.; et al. Targeted Application of Human Genetic Variation Can Improve Red Blood Cell Production from Stem Cells. Cell Stem Cell 2016, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Doulatov, S.; Vo, L.T.; Macari, E.R.; Wahlster, L.; Kinney, M.A.; Taylor, A.M.; Barragan, J.; Gupta, M.; McGrath, K.; Lee, H.Y.; et al. Drug discovery for Diamond-Blackfan anemia using reprogrammed hematopoietic progenitors. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Phanthong, P.; Borwornpinyo, S.; Kitiyanant, N.; Jearawiriyapaisarn, N.; Nuntakarn, L.; Saetan, J.; Nualkaew, T.; Sa-Ngiamsuntorn, K.; Anurathapan, U.; Dinnyes, A.; et al. Enhancement of beta-Globin Gene Expression in Thalassemic IVS2-654 Induced Pluripotent Stem Cell-Derived Erythroid Cells by Modified U7 snRNA. Stem Cells Transl. Med. 2017, 6, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Gianotti-Sommer, A.; Molina-Estevez, F.J.; Vanuytsel, K.; Skvir, N.; Leung, A.; Rozelle, S.S.; Shaikho, E.M.; Weir, I.; Jiang, Z.; et al. A Comprehensive, Ethnically Diverse Library of Sickle Cell Disease-Specific Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 8, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Wang, Y.; Suzuki, H.; Okamoto, S.; Ikeda, Y.; Murata, M.; Poncz, M.; Matsubara, Y. Induction of functional platelets from mouse and human fibroblasts by p45NF-E2/Maf. Blood 2012, 120, 3812–3821. [Google Scholar] [CrossRef] [PubMed]
- Pulecio, J.; Alejo-Valle, O.; Capellera-Garcia, S.; Vitaloni, M.; Rio, P.; Mejia-Ramirez, E.; Caserta, I.; Bueren, J.A.; Flygare, J.; Raya, A. Direct Conversion of Fibroblasts to Megakaryocyte Progenitors. Cell Rep. 2016, 17, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, R.; Priora, R.; Alessandrello Liotta, A.; Abbate, R. Platelet function tests: A comparative review. Vasc. Health Risk Manag. 2015, 11, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Takayama, N.; Nishimura, S.; Nakamura, S.; Shimizu, T.; Ohnishi, R.; Endo, H.; Yamaguchi, T.; Otsu, M.; Nishimura, K.; Nakanishi, M.; et al. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J. Exp. Med. 2010, 207, 2817–2830. [Google Scholar] [CrossRef] [PubMed]
- Gekas, C.; Graf, T. Induced pluripotent stem cell-derived human platelets: One step closer to the clinic. J. Exp. Med. 2010, 207, 2781–2784. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hatoya, S.; Kanegi, R.; Sugiura, K.; Wijewardana, V.; Kuwamura, M.; Tanaka, M.; Yamate, J.; Izawa, T.; Takahashi, M.; et al. Generation of functional platelets from canine induced pluripotent stem cells. Stem Cells Dev. 2013, 22, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Thon, J.N.; Mazutis, L.; Wu, S.; Sylman, J.L.; Ehrlicher, A.; Machlus, K.R.; Feng, Q.; Lu, S.; Lanza, R.; Neeves, K.B.; et al. Platelet bioreactor-on-a-chip. Blood 2014, 124, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Takayama, N.; Hirata, S.; Seo, H.; Endo, H.; Ochi, K.; Fujita, K.; Koike, T.; Harimoto, K.; Dohda, T.; et al. Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell 2014, 14, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Eto, K. Platelet production from induced pluripotent stem cells. J. Thromb. Haemost. 2017, 15, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Murata, T.; Suzuki, D.; Nakamura, S.; Jono-Ohnishi, R.; Hirose, H.; Sawaguchi, A.; Nishimura, S.; Sugimoto, N.; Eto, K. Selective Inhibition of ADAM17 Efficiently Mediates Glycoprotein Ibalpha Retention During Ex Vivo Generation of Human Induced Pluripotent Stem Cell-Derived Platelets. Stem Cells Transl. Med. 2017, 6, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Borger, A.K.; Eicke, D.; Wolf, C.; Gras, C.; Aufderbeck, S.; Schulze, K.; Engels, L.; Eiz-Vesper, B.; Schambach, A.; Guzman, C.A.; et al. Generation of HLA-universal iPSCs-derived megakaryocytes and platelets for survival under refractoriness conditions. Mol. Med. 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- Gras, C.; Schulze, K.; Goudeva, L.; Guzman, C.A.; Blasczyk, R.; Figueiredo, C. HLA-universal platelet transfusions prevent platelet refractoriness in a mouse model. Hum. Gene Ther. 2013, 24, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Kammers, K.; Taub, M.A.; Ruczinski, I.; Martin, J.; Yanek, L.R.; Frazee, A.; Gao, Y.; Hoyle, D.; Faraday, N.; Becker, D.M.; et al. Integrity of Induced Pluripotent Stem Cell (iPSC) Derived Megakaryocytes as Assessed by Genetic and Transcriptomic Analysis. PLoS ONE 2017, 12, e0167794. [Google Scholar] [CrossRef] [PubMed]
- Moreau, T.; Evans, A.L.; Vasquez, L.; Tijssen, M.R.; Yan, Y.; Trotter, M.W.; Howard, D.; Colzani, M.; Arumugam, M.; Wu, W.H.; et al. Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat. Commun. 2016, 7, 11208. [Google Scholar] [CrossRef] [PubMed]
- Fast, L.D.; DiLeone, G.; Marschner, S. Inactivation of human white blood cells in platelet products after pathogen reduction technology treatment in comparison to gamma irradiation. Transfusion 2011, 51, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Focosi, D.; Amabile, G. Induced Pluripotent Stem Cell-Derived Red Blood Cells and Platelet Concentrates: From Bench to Bedside. Cells 2018, 7, 2. https://doi.org/10.3390/cells7010002
Focosi D, Amabile G. Induced Pluripotent Stem Cell-Derived Red Blood Cells and Platelet Concentrates: From Bench to Bedside. Cells. 2018; 7(1):2. https://doi.org/10.3390/cells7010002
Chicago/Turabian StyleFocosi, Daniele, and Giovanni Amabile. 2018. "Induced Pluripotent Stem Cell-Derived Red Blood Cells and Platelet Concentrates: From Bench to Bedside" Cells 7, no. 1: 2. https://doi.org/10.3390/cells7010002




