Understanding the Synergy of NKp46 and Co-Activating Signals in Various NK Cell Subpopulations: Paving the Way for More Successful NK-Cell-Based Immunotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monoclonal Antibodies
2.2. Cell isolation and Culture
2.3. Microbead Stimulation of NK Cells
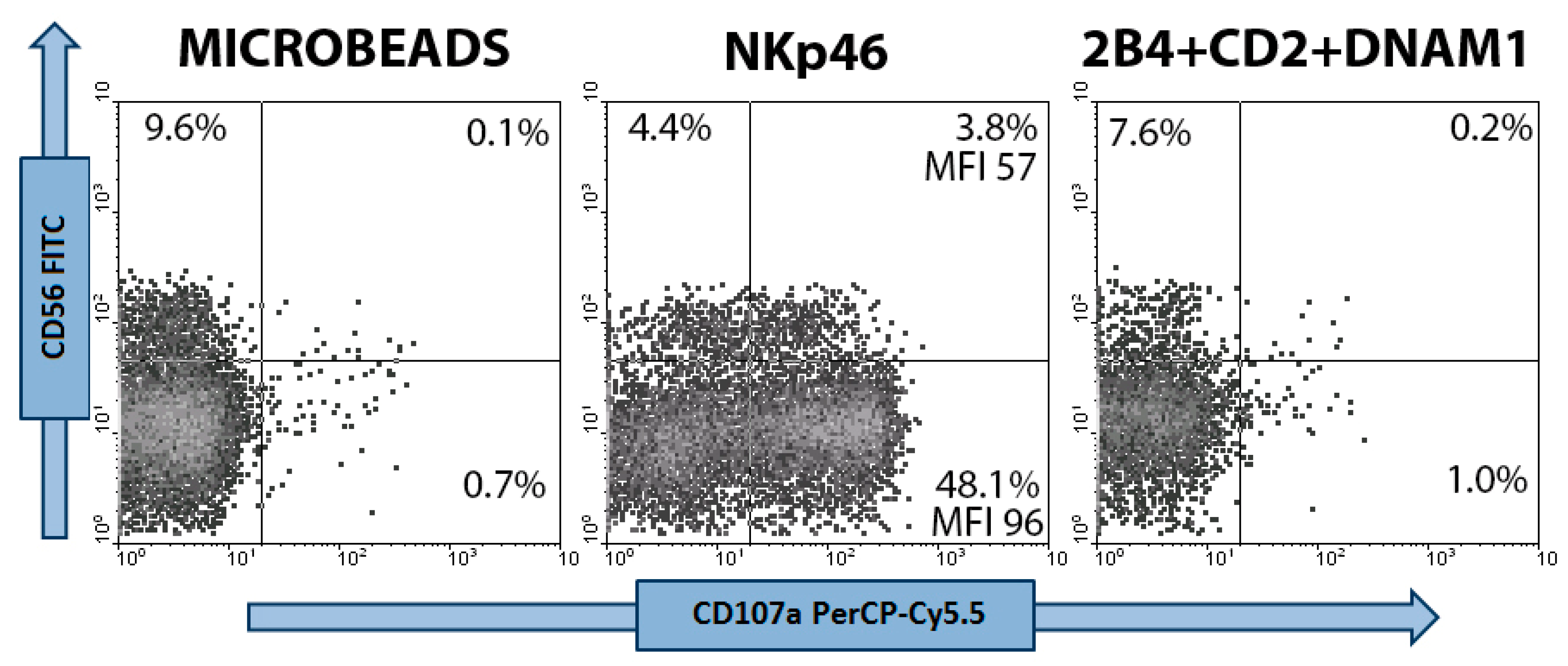
2.4. CD107a Degranulation Assay
2.5. Flow Cytometry
2.6. Confocal Microscopy and ImageStream
2.7. Statistical Analysis
3. Results
3.1. NKp-46 Induced Degranulation in Total Resting PB NK Cells
3.2. Degranulation of Resting PB NK Cell Subsets: CD56bright and CD56dim NK Cells and Licensed and Unlicensed CD56dim NK Cells
3.3. Degranulation of in Vitro Cultured NK Cell Cubsets: CD56bright and CD56dim NK Cells and Licensed and Unlicensed CD56dim NK Cells
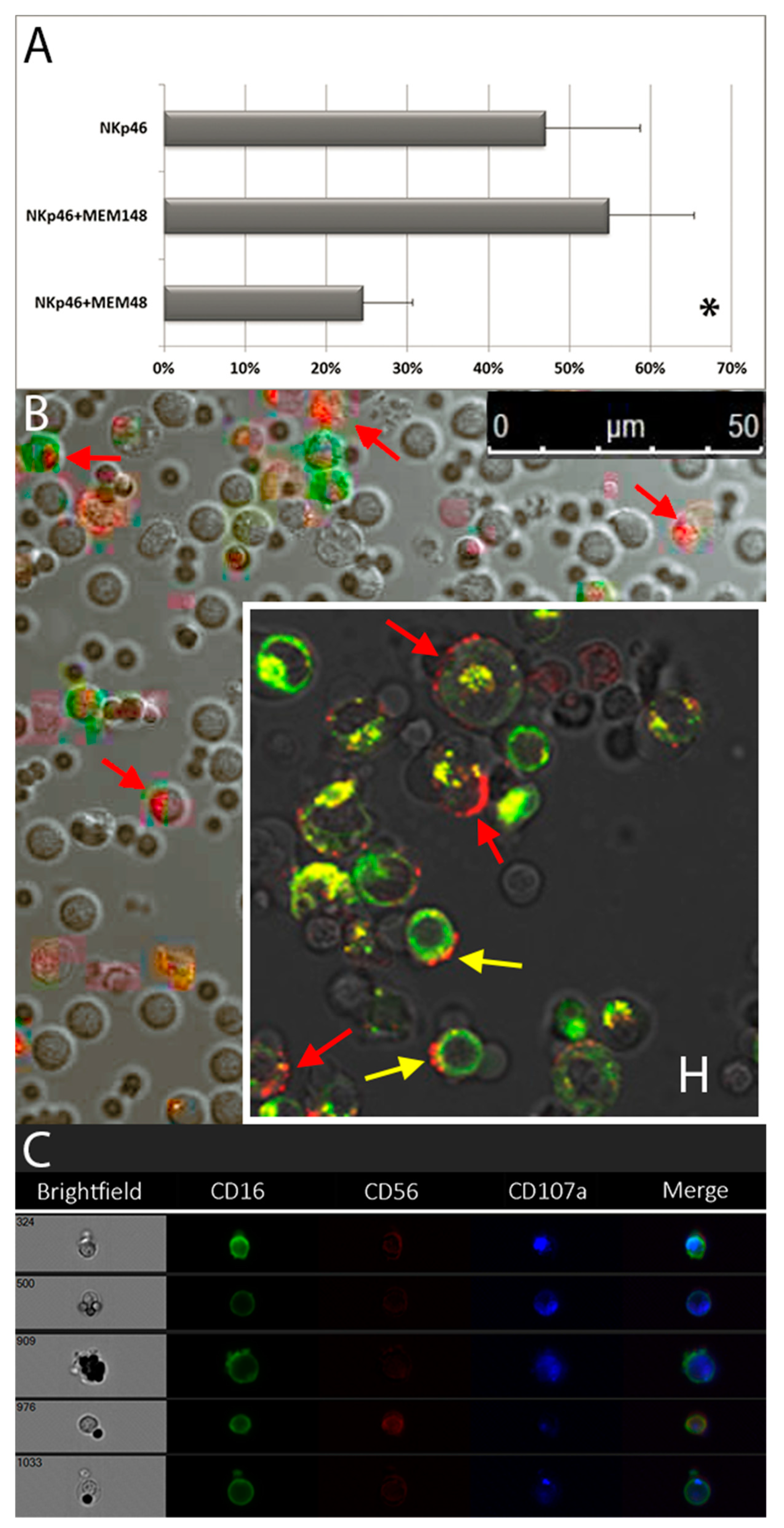
3.4. Granule Polarization of in Vitro Vultured NK Cells and LFA-1-Mediated Inhibition of Degranulation
4. Discussion
4.1. Assay Sensitivity
4.2. 2B4 Activating Co-Operation on CD56dim NK Cells
4.3. 2B4 Preferentially Drives NK Cell Function against Hematopoietic Cells (Exemplified in Haploidentical Transplantation)
4.4. 2B4 Signalling on Unlicensed CD56dim NK Cells
4.5. CD56dim and CD56bright Converging Phenotypes and Functions Possibly from Different Precursors
4.6. NK Spontaneous Activation After Blood withdrawal and Cytokine Effect
4.7. LFA-1 Mediated Inhibition of NK Cell Degranulation: Multi-Functional Receptors
4.8. Cis Receptor/Ligand Interactions as Regulatory Mechanism for Receptor Multifunctionality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moretta, A.; Pende, D.; Locatelli, F.; Moretta, L. Activating and inhibitory killer immunoglobulin-like receptors (KIR) in haploidentical haemopoietic stem cell transplantation to cure high-risk leukaemias. Clin. Exp. Immunol. 2009, 157, 325–331. [Google Scholar] [CrossRef]
- Zamai, L.; Ponti, C.; Mirandola, P.; Gobbi, G.; Papa, S.; Galeotti, L.; Cocco, L.; Vitale, M. NK cells and cancer. J. Immunol. 2007, 178, 4011–4016. [Google Scholar] [CrossRef] [Green Version]
- Gabrielli, S.; Ortolani, C.; Del Zotto, G.; Luchetti, F.; Canonico, B.; Buccella, F.; Artico, M.; Papa, S.; Zamai, L. The Memories of NK Cells: Innate-Adaptive Immune Intrinsic Crosstalk. J. Immunol. Res. 2016, 2016, 1376595. [Google Scholar] [CrossRef] [PubMed]
- Velardi, A.; Ruggeri, L.; Mancusi, A.; Aversa, F.; Christiansen, F.T. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: A tool for immunotherapy of leukemia. Curr. Opin. Immunol. 2009, 21, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Marcenaro, S.; Falco, M.; Martini, S.; Bernardo, M.E.; Montagna, D.; Romeo, E.; Cognet, C.; Martinetti, M.; Maccario, R.; et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: Evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood 2009, 113, 3119–3129. [Google Scholar] [CrossRef] [Green Version]
- Minetto, P.; Guolo, F.; Pesce, S.; Greppi, M.; Obino, V.; Ferretti, E.; Sivori, S.; Genova, C.; Lemoli, R.M.; Marcenaro, E. Harnessing NK Cells for Cancer Treatment. Front. Immunol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Cantoni, C.; Mingari, M.C.; Biassoni, R.; Moretta, L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001, 19, 197–223. [Google Scholar] [CrossRef]
- Moretta, L.; Moretta, A. Unravelling natural killer cell function: Triggering and inhibitory human NK receptors. EMBO J. 2004, 23, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Braud, V.M.; Allan, D.S.J.; O’Callaghan, C.A.; Soderstrom, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, N.; André, P.; Guia, S.; Falk, C.S.; Roetynck, S.; Stewart, C.A.; Breso, V.; Frassati, C.; Reviron, D.; Middleton, D.; et al. Human NK Cell Education by Inhibitory Receptors for MHC Class I. Immunity 2006, 25, 331–342. [Google Scholar] [CrossRef]
- Nagler, A.; Lanier, L.L.; Cwirla, S.; Phillips, J.H. Comparative studies of human FcRIII-positive and negative natural killer cells. J. Immunol. 1989, 143, 3183–3191. [Google Scholar] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, W.E.; Caligiuri, M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56bright subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef]
- Fauriat, C.; Long, E.O.; Ljunggren, H.G.; Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Maria, A.; Bozzano, F.; Cantoni, C.; Moretta, L. Revisiting human natural killer cell subset function revealed cytolytic CD56dimCD16+ NK cells as rapid producers of abundant IFN-γ on activation. Proc. Natl. Acad. Sci. USA 2011, 108, 728–732. [Google Scholar] [CrossRef] [Green Version]
- Freud, A.G.; Caligiuri, M.A. Human natural killer cell development. Immunol. Rev. 2006, 214, 56–72. [Google Scholar] [CrossRef]
- Zamai, L.; Del Zotto, G.; Buccella, F.; Galeotti, L.; Canonico, B.; Luchetti, F.; Papa, S. Cytotoxic functions and susceptibility to apoptosis of human CD56 bright NK cells differentiated in vitro from CD34 + hematopoietic progenitors. Cytom. Part. A 2012, 81 A, 294–302. [Google Scholar] [CrossRef]
- Parrish-Novak, J.; Dillon, S.R.; Nelson, A.; Hammond, A.; Sprecher, C.; Gross, J.A.; Johnston, J.; Madden, K.; Xu, W.; West, J.; et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000, 408, 57–63. [Google Scholar] [CrossRef]
- Zamai, L.; Galeotti, L.; Del Zotto, G.; Canonico, B.; Mirandola, P.; Papa, S. Identification of a NCR+/NKG2D+/LFA-1 low/CD94- immature human NK cell subset. Cytom. Part. A 2009, 75, 893–901. [Google Scholar] [CrossRef]
- Carrega, P.; Bonaccorsi, I.; Di Carlo, E.; Morandi, B.; Paul, P.; Rizzello, V.; Cipollone, G.; Navarra, G.; Mingari, M.C.; Moretta, L.; et al. CD56 bright Perforin low Noncytotoxic Human NK Cells Are Abundant in Both Healthy and Neoplastic Solid Tissues and Recirculate to Secondary Lymphoid Organs via Afferent Lymph. J. Immunol. 2014, 192, 3805–3815. [Google Scholar] [CrossRef] [Green Version]
- Bozzano, F.; Marras, F.; Ascierto, M.L.; Cantoni, C.; Cenderello, G.; Dentone, C.; Di Biagio, A.; Orofino, G.; Mantia, E.; Boni, S.; et al. “Emergency exit” of bone-marrow-resident CD34+ DNAM-1 bright CXCR4+-committed lymphoid precursors during chronic infection and inflammation. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, D.S.J.; Rybalov, B.; Awong, G.; Zúñiga-Pflücker, J.C.; Kopcow, H.D.; Carlyle, J.R.; Strominger, J.L. TGF-β affects development and differentiation of human natural killer cell subsets. Eur. J. Immunol. 2010, 40, 2289–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, W.E.; Giri, J.G.; Lindemann, M.J.; Linett, M.L.; Ahdieh, M.; Paxton, R.; Anderson, D.; Eisenmann, J.; Grabstein, K.; Caligiuri, M.A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 1994, 180, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Wendt, K.; Wilk, E.; Buyny, S.; Schmidt, R.E.; Jacobs, R. Interleukin-21 differentially affects human natural killer cell subsets. Immunology 2007, 122, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Cooley, S.; Xiao, F.; Pitt, M.; Gleason, M.; McCullar, V.; Bergemann, T.L.; McQueen, K.L.; Guethlein, L.A.; Parham, P.; Miller, J.S. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood 2007, 110, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006, 107, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Bryceson, Y.T.; Ljunggren, H.G.; Long, E.O. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood 2009, 114, 2657–2666. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, A.; Campbell, D.; Hannum, C.; Yssel, H.; Franz-Bacon, K.; McClanashan, T.; Kitamura, T.; Nicholl, J.; Sutherland, G.R.; Lanier, L.L.; et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 1996, 4, 573–581. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, K.; Lanier, L.L.; Phillips, J.H.; Ochs, H.D.; Shimizu, K.; Nakayama, E.; Nakauchi, H.; Shibuya, A. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity 1999, 11, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Pessino, A.; Sivori, S.; Bottino, C.; Malaspina, A.; Morelli, L.; Moretta, L.; Biassoni, R.; Moretta, A. Molecular cloning of NKp46: A novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J. Exp. Med. 1998, 188, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Long, E.O. Complement factor P: Promoting the antibacterial activity of natural killer cells. Cell. Mol. Immunol. 2017, 14, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Vitale, M.; Morelli, L.; Sanseverino, L.; Augugliaro, R.; Bottino, C.; Moretta, L.; Moretta, A. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J. Exp. Med. 1997, 186, 1129–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gahmberg, C.G.; Tolvanen, M.; Kotovuori, P. Leukocyte adhesion--structure and function of human leukocyte beta2-integrins and their cellular ligands. Eur. J. Biochem. 1997, 245, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.F.; Faure, M.; Long, E.O. LFA-1 Contributes an Early Signal for NK Cell Cytotoxicity. J. Immunol. 2004, 173, 3653–3659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, C.C.; Brzostowski, J.A.; Liu, D.; Long, E.O. Tethering of intercellular adhesion molecule on target cells is required for LFA-1-dependent NK cell adhesion and granule polarization. J. Immunol. 2010, 185, 2918–2926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryceson, Y.T.; March, M.E.; Barber, D.F.; Ljunggren, H.G.; Long, E.O. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 2005, 202, 1001–1012. [Google Scholar] [CrossRef]
- Claus, M.; Meinke, S.; Bhat, R.; Watzl, C. Regulation of NK cell activity by 2B4, NTB-A and CRACC. Front. Biosci. 2008, 13, 956–965. [Google Scholar] [CrossRef] [Green Version]
- Sivori, S.; Parolini, S.; Falco, M.; Marcenaro, E.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. 2B4 functions as a co-receptor in human NK cell activation. Eur. J. Immunol. 2000, 30, 787–793. [Google Scholar] [CrossRef]
- Reymond, N.; Imbert, A.M.; Devilard, E.; Fabre, S.; Chabannon, C.; Xerri, L.; Farnarier, C.; Cantoni, C.; Bottino, C.; Moretta, A.; et al. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J. Exp. Med. 2004, 199, 1331–1341. [Google Scholar] [CrossRef] [Green Version]
- Pende, D.; Bottino, C.; Castriconi, R.; Cantoni, C.; Marcenaro, S.; Rivera, P.; Spaggiari, G.M.; Dondero, A.; Carnemolla, B.; Reymond, N.; et al. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: Involvement in tumor cell lysis. Mol. Immunol. 2005, 42, 463–469. [Google Scholar] [CrossRef]
- Pende, D.; Spaggiari, G.M.; Marcenaro, S.; Martini, S.; Rivera, P.; Capobianco, A.; Falco, M.; Lanino, E.; Pierri, I.; Zambello, R.; et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: Evidence for the involvement of the Polio virus receptor (CD 155) and Nectin-2 (CD 112). Blood 2005, 105, 2066–2073. [Google Scholar] [CrossRef]
- Pende, D.; Castriconi, R.; Romagnani, P.; Spaggiari, G.M.; Marcenaro, S.; Dondero, A.; Lazzeri, E.; Lasagni, L.; Martini, S.; Rivera, P.; et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: Relevance for natural killer-dendritic cell interaction. Blood 2006, 107, 2030–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvaraj, P.; Plunkett, M.L.; Dustin, M.; Sanders, M.E.; Shaw, S.; Springer, T.A. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. Nature 1987, 326, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.; Zamai, L.; Rezzani, R.; Artico, M.; Peri, G.; Falconi, M.; Facchini, A.; Pelusi, G.; Vitale, M. Interleukin 2 and interleukin 15 differentially predispose natural killer cells to apoptosis mediated by endothelial and tumour cells. Br. J. Haematol. 2001, 115, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Perussia, B.; Campbell, K.S. Prostaglandin D 2 Suppresses Human NK Cell Function via Signaling through D Prostanoid Receptor. J. Immunol. 2007, 179, 2766–2773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangye, S.G.; Phillips, J.H.; Lanier, L.L.; Nichols, K.E. Cutting Edge: Functional Requirement for SAP in 2B4-Mediated Activation of Human Natural Killer Cells as Revealed by the X-Linked Lymphoproliferative Syndrome. J. Immunol. 2000, 165, 2932–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reim, F.; Dombrowski, Y.; Ritter, C.; Buttmann, M.; Häusler, S.; Ossadnik, M.; Krockenberger, M.; Beier, D.; Beier, C.P.; Dietl, J.; et al. Immunoselection of breast and ovarian cancer cells with trastuzumab and natural killer cells: Selective escape of CD44high/CD24 low/HER2low breast cancer stem cells. Cancer Res. 2009, 69, 8058–8066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Ferzly, M.; Takagi, J.; Springer, T.A. Epitope mapping of antibodies to the C-terminal region of the integrin beta 2 subunit reveals regions that become exposed upon receptor activation. J. Immunol. 2001, 166, 5629–5637. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.-H.; Tng, E.; Law, S.K.A.; Tan, S.-M. Epitope mapping of monoclonal antibody to integrin alphaL beta2 hybrid domain suggests different requirements of affinity states for intercellular adhesion molecules (ICAM)-1 and ICAM-3 binding. J. Biol. Chem. 2005, 280, 29208–29216. [Google Scholar] [CrossRef] [Green Version]
- Drbal, K.; Angelisová, P.; Cerný, J.; Hilgert, I.; Horejsí, V. A novel anti-CD18 mAb recognizes an activation-related epitope and induces a high-affinity conformation in leukocyte integrins. Immunobiology 2001, 203, 687–698. [Google Scholar] [CrossRef]
- Drbal, K.; Angelisová, P.; Hilgert, I.; Černý, J.; Novák, P.; Hořejší, V. A proteolytically truncated form of free CD18, the common chain of leukocyte integrins, as a novel marker of activated myeloid cells. Blood 2001, 98, 1561–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, M.R.; Brenchley, J.M.; Price, D.A.; De Rosa, S.C.; Douek, D.C.; Roederer, M.; Koup, R.A. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 2003, 281, 65–78. [Google Scholar] [CrossRef]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef]
- Zamai, L.; Ahmad, M.; Bennett, I.M.; Azzoni, L.; Alnemri, E.S.; Perussia, B. Natural killer (NK) cell-mediated cytotoxicity: Differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J. Exp. Med. 1998, 188, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 2006, 214, 73–91. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [Green Version]
- Dolstra, H.; Roeven, M.W.H.; Spanholtz, J.; Hangalapura, B.N.; Tordoir, M.; Maas, F.; Leenders, M.; Bohme, F.; Kok, N.; Trilsbeek, C.; et al. Successful transfer of umbilical cord blood CD34+ hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin. Cancer Res. 2017, 23, 4107–4118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hubeshy, Z.B.; Coleman, A.; Nelson, M.; Goodier, M.R. A rapid method for assessment of natural killer cell function after multiple receptor crosslinking. J. Immunol. Methods 2011, 366, 52–59. [Google Scholar] [CrossRef]
- Jacobs, R.; Hintzen, G.; Kemper, A.; Beul, K.; Kempf, S.; Behrens, G.; Sykora, K.W.; Schmidt, R.E. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur. J. Immunol. 2001, 31, 3121–3126. [Google Scholar] [CrossRef]
- Tassi, I.; Klesney-Tait, J.; Colonna, M. Dissecting natural killer cell activation pathways through analysis of genetic mutations in human and mouse. Immunol. Rev. 2006, 214, 92–105. [Google Scholar] [CrossRef]
- Schlums, H.; Cichocki, F.; Tesi, B.; Theorell, J.; Beziat, V.; Holmes, T.D.; Han, H.; Chiang, S.C.C.; Foley, B.; Mattsson, K.; et al. Cytomegalovirus Infection Drives Adaptive Epigenetic Diversification of NK Cells with Altered Signaling and Effector Function. Immunity 2015, 42, 443–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, S.; Reardon, J.; Peterson, E.; Poole, D.; Bosch, R.; Alter, G.; Altfeld, M. Expansion of 2B4+ natural killer (NK) cells and decrease in NKp46+ NK cells in response to influenza. Immunology 2011, 132, 516–526. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, Y.M.; Meade, J.L.; Holmes, T.D.; McGonagle, D.; Mackie, S.L.; Morgan, A.W.; Cook, G.; Feyler, S.; Richards, S.J.; Davies, F.E.; et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007, 67, 8444–8449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McArdel, S.L.; Terhorst, C.; Sharpe, A.H. Roles of CD48 in regulating immunity and tolerance. Clin. Immunol. 2016, 164, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollard, C.M.; Heslop, H.E. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood 2016, 127, 3331–3340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottaviano, G.; Chiesa, R.; Feuchtinger, T.; Vickers, M.; Dickinson, A.; Gennery, A.; Veys, P.; Todryk, S. Adoptive T Cell Therapy Strategies for Viral Infections in Patients Receiving Haematopoietic Stem Cell Transplantation. Cells 2019, 8, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, C.; Wang, Y.; Reppel, L.; D’Aveni, M.; Campidelli, A.; Decot, V.; Bensoussan, D. Viral-specific T-cell transfer from HSCT donor for the treatment of viral infections or diseases after HSCT. Bone Marrow Transplant. 2018, 53, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Hatton, O.L.; Harris-Arnold, A.; Schaffert, S.; Krams, S.M.; Martinez, O.M. The interplay between Epstein-Barr virus and B lymphocytes: Implications for infection, immunity, and disease. Immunol. Res. 2014, 58, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Hahn, G.; Jores, R.; Mocarski, E.S. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA 1998, 95, 3937–3942. [Google Scholar] [CrossRef] [Green Version]
- Zambello, R.; Teramo, A.; Barilà, G.; Gattazzo, C.; Semenzato, G. Activating KIRs in chronic lymphoproliferative disorder of NK cells: Protection from viruses and disease induction? Front. Immunol. 2014, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Zambello, R.; Loughran, T.P.; Trentin, L.; Pontisso, P.; Battistella, L.; Raimondi, R.; Facco, M.; Sancetta, R.; Agostini, C.; Pizzolo, G. Serologic and molecular evidence for a possible pathogenetic role of viral infection in CD3-negative natural killer-type lymphoproliferative disease of granular lymphocytes. Leukemia 1995, 9, 1207–1211. [Google Scholar] [PubMed]
- Vosshenrich, C.A.J.; García-Ojeda, M.E.; Samson-Villéger, S.I.; Pasqualetto, V.; Enault, L.; Goff, O.R.L.; Corcuff, E.; Guy-Grand, D.; Rocha, B.; Cumano, A.; et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 2006, 7, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- De Rham, C.; Ferrari-Lacraz, S.; Jendly, S.; Schneiter, G.; Dayer, J.M.; Villard, J. The proinflammatory cytokines IL-2, IL-15 and IL-21 modulate the repertoire of mature human natural killer cell receptors. Arthritis Res. Ther. 2007, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, M.E.; Schnier, G.S.; Beecher, M.S.; Ashman, L.K.; Williams, D.E.; Caligiuri, M.A. Expression of a functional c-kit receptor on a subset of natural killer cells. J. Exp. Med. 1993, 178, 1079–1084. [Google Scholar] [CrossRef] [Green Version]
- Freud, A.G.; Becknell, B.; Roychowdhury, S.; Mao, H.C.; Ferketich, A.K.; Nuovo, G.J.; Hughes, T.L.; Marburger, T.B.; Sung, J.; Baiocchi, R.A.; et al. A human CD34 (+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 2005, 22, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.J.; Qin, S.; Unutmaz, D.; Soler, D.; Murphy, K.E.; Hodge, M.R.; Wu, L.; Butcher, E.C. Unique Subpopulations of CD56 + NK and NK-T Peripheral Blood Lymphocytes Identified by Chemokine Receptor Expression Repertoire. J. Immunol. 2001, 166, 6477–6482. [Google Scholar] [CrossRef] [Green Version]
- Frey, M.; Packianathan, N.B.; Fehniger, T.A.; Ross, M.E.; Wang, W.C.; Stewart, C.C.; Caligiuri, M.A.; Evans, S.S. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J. Immunol. 1998, 161, 400–408. [Google Scholar]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Chalifour, A.; Scarpellino, L.; Back, J.; Brodin, P.; Devèvre, E.; Gros, F.; Lévy, F.; Leclercq, G.; Höglund, P.; Beermann, F.; et al. A Role for cis Interaction between the Inhibitory Ly49A Receptor and MHC Class I for Natural Killer Cell Education. Immunity 2009, 30, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Perez, O.D.; Mitchell, D.; Jager, G.C.; Nolan, G.P. LFA-1 signaling through p44/42 is coupled to perforin degranulation in CD56+CD8+ natural killer cells. Blood 2004, 104, 1083–1093. [Google Scholar] [CrossRef] [Green Version]
- DiFranco, K.M.; Gupta, A.; Galusha, L.E.; Perez, J.; Nguyen, T.V.K.; Fineza, C.D.; Kachlany, S.C. Leukotoxin (Leukothera®) targets active Leukocyte Function Antigen-1 (LFA-1) protein and triggers a lysosomal mediated cell death pathway. J. Biol. Chem. 2012, 287, 17618–17627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, A.; Taga, K.; Mostowski, H.S.; Bloom, E.T. Target cell-induced apoptosis of interleukin-2-activated human natural killer cells: Roles of cell surface molecules and intracellular events. Blood 1996, 87, 5127–5135. [Google Scholar] [CrossRef] [Green Version]
- Reina, M.; Espel, E. Role of LFA-1 and ICAM-1 in cancer. Cancers (Basel) 2017, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Parolini, S.; Bottino, C.; Falco, M.; Augugliaro, R.; Giliani, S.; Franceschini, R.; Ochs, H.D.; Wolf, H.; Bonnefoy, J.Y.; Biassoni, R.; et al. X-linked lymphoproliferative disease: 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J. Exp. Med. 2000, 192, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Falco, M.; Marcenaro, E.; Parolini, S.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Early expression of triggering receptors and regulatory role of 2B4 in human natural killer cell precursors undergoing in vitro differentiation. Proc. Natl. Acad. Sci. USA 2002, 99, 4526–4531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chlewicki, L.K.; Velikovsky, C.A.; Balakrishnan, V.; Mariuzza, R.A.; Kumar, V. Molecular Basis of the Dual Functions of 2B4 (CD244). J. Immunol. 2008, 180, 8159–8167. [Google Scholar] [CrossRef] [Green Version]
- Claus, M.; Wingert, S.; Watzl, C. Modulation of natural killer cell functions by interactions between 2B4 and CD48 in cis and in trans. Open Biol. 2016, 6, 160010. [Google Scholar] [CrossRef] [Green Version]
- Fenwick, C.; Loredo-Varela, J.L.; Joo, V.; Pellaton, C.; Farina, A.; Rajah, N.; Esteves-Leuenberger, L.; Decaillon, T.; Suffiotti, M.; Noto, A.; et al. Tumor suppression of novel anti-PD-1 antibodies mediated through CD28 costimulatory pathway. J. Exp. Med. 2019, 216, 1525–1541. [Google Scholar] [CrossRef]
- Held, W.; Mariuzza, R.A. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat. Rev. Immunol. 2008, 8, 269–278. [Google Scholar] [CrossRef]
- Miner, C.A.; Giri, T.K.; Meyer, C.E.; Shabsovich, M.; Tripathy, S.K. Acquisition of Activation Receptor Ligand by Trogocytosis Renders NK Cells Hyporesponsive. J. Immunol. 2015, 194, 1945–1953. [Google Scholar] [CrossRef] [Green Version]
- Perussia, B.; Ramoni, C.; Anegon, I.; Cuturi, M.C.; Faust, J.; Trinchieri, G. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat. Immun. Cell Growth Regul. 1987, 6, 171–188. [Google Scholar] [PubMed]
- Perussia, B.; Loza, M.J. Purification of peripheral blood natural killer cells. Methods Mol. Med. 2005, 107, 147–161. [Google Scholar] [PubMed]
- Little, R.D.; Schildkraut, C.L. Initiation of latent DNA replication in the Epstein-Barr virus genome can occur at sites other than the genetically defined origin. Mol. Cell. Biol. 1995, 15, 2893–2903. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, S.; Staunton, D.; Fisher, R.; Amiot, M.; Fortin, J.J.; Thorley-Lawson, D.A. Expression of the Blast-1 activation/adhesion molecule and its identification as CD48. J. Immunol. 1991, 146, 2192–2200. [Google Scholar] [PubMed]
- Klaman, L.D.; Thorley-Lawson, D.A. Characterization of the CD48 gene demonstrates a positive element that is specific to Epstein-Barr virus-immortalized B-cell lines and contains an essential NF-kappa B site. J. Virol. 1995, 69, 871–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzi, T.; Lünemann, A.; Murer, A.; Ueda, S.; Béziat, V.; Malmberg, K.J.; Staubli, G.; Gysin, C.; Berger, C.; Münz, C.; et al. Role for early-differentiated natural killer cells in infectious mononucleosis. Blood 2014, 124, 2533–2543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendricks, D.W.; Balfour, H.H.; Dunmire, S.K.; Schmeling, D.O.; Hogquist, K.A.; Lanier, L.L. Cutting Edge: NKG2C hi CD57 + NK Cells Respond Specifically to Acute Infection with Cytomegalovirus and Not Epstein–Barr Virus. J. Immunol. 2014, 192, 4492–4496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chijioke, O.; Müller, A.; Feederle, R.; Barros, M.H.M.; Emmel, V.; Marcenaro, E.; Leung, C.S.; Antsiferova, O.; Landtwing, V.; Bossart, W.; et al. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 2013, 5, 1489–1498. [Google Scholar] [CrossRef] [Green Version]
- Chijioke, O.; Landtwing, V.; Münz, C. NK cell influence on the outcome of primary Epstein-Barr virus infection. Front. Immunol. 2016, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mandelboim, O.; Lieberman, N.; Lev, M.; Paul, L.; Arnon, T.I.; Bushkin, Y.; Davis, D.M.; Strominger, J.L.; Yewdell, J.W.; Porgador, A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 2001, 409, 1055–1060. [Google Scholar] [CrossRef]
- McNerney, M.E.; Lee, K.M.; Kumar, V. 2B4 (CD244) is a non-MHC binding receptor with multiple functions on natural killer cells and CD8+ T cells. Mol. Immunol. 2005, 42, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Agresta, L.; Hoebe, K.H.N.; Janssen, E.M. The emerging role of CD244 signaling in immune cells of the tumor microenvironment. Front. Immunol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schlaphoff, V.; Lunemann, S.; Suneetha, P.V.; Jaroszewicz, J.; Grabowski, J.; Dietz, J.; Helfritz, F.; Bektas, H.; Sarrazin, C.; Manns, M.P.; et al. Dual function of the NK cell receptor 2B4 (CD244) in the regulation of HCV-specific CD8+ T cells. PLoS Pathog. 2011, 7, e1002045. [Google Scholar] [CrossRef]
- Poli, A.; Michel, T.; Patil, N.; Zimmer, J. Revisiting the Functional Impact of NK Cells. Trends Immunol. 2018, 39, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Marcenaro, E.; Pesce, S.; Sivori, S.; Carlomagno, S.; Moretta, L.; Moretta, A. KIR2DS1-dependent acquisition of CCR7 and migratory properties by human NK cells interacting with allogeneic HLA-C2+ DCs or T-cell blasts. Blood 2013, 121, 3396–3401. [Google Scholar] [CrossRef] [Green Version]
- Cho, F.N.; Chang, T.H.; Shu, C.W.; Ko, M.C.; Liao, S.K.; Wu, K.H.; Yu, M.S.; Lin, S.J.; Hong, Y.C.; Chen, C.H.; et al. Enhanced cytotoxicity of natural killer cells following the acquisition of chimeric antigen receptors through trogocytosis. PLoS ONE 2014, 9, e109352. [Google Scholar] [CrossRef] [PubMed]
- Caumartin, J.; Favier, B.; Daouya, M.; Guillard, C.; Moreau, P.; Carosella, E.D.; LeMaoult, J. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007, 26, 1423–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| MAb | Company | Clone | Isotype | Fluorochrome |
|---|---|---|---|---|
| CD2 (LFA-2) | Miltenyi Biotec | LT2 | IgG2b | Biotin |
| CD3(CD3 ε-chain) | Ancell | UCHT1 | IgG1 | FITC/APC |
| CD16(FcγRIII) | Ancell | 3G8 | IgG1 | FITC/PE |
| CD18(LFA-1 β2-chain) | Immunological Sciences | MEM-48 | IgG1 | Biotin |
| CD18(LFA-1 β2-chain) | Immunological Sciences | MEM-148 | IgG1 | Purified * |
| CD45(LCA) | Ancell | C11 | IgG2a, κ | FITC |
| CD56(NCAM) | Biolegend/Exbio | MEM-188 | IgG2a | FITC/PE/Biotin |
| CD107a(LAMP-1) | Biolegend | H4A3 | IgG1 | PerCP-Cy5.5 |
| CD158a/h(KIR2DL1/S1) | BD Biosciences | HP3E4 | IgM | PE |
| CD158b/j(KIR2DL2/3/S2) | BD Biosciences | CH-L | IgG2b | PE |
| CD158e(KIR3DL1) | Miltenyi Biotec | DX9 | IgG1 | PE |
| CD159a(NKG2A) | Beckman Coulter | Z199 | IgG2b | PE |
| CD226(DNAM-1) | AbCam | DX11 | IgG1 | Biotin |
| CD244(2B4) | eBiosciences | C1.7 | IgG1, κ | Biotin |
| CD335(NKp46) | Miltenyi | 9E2 | IgG1 | Biotin |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamai, L.; Del Zotto, G.; Buccella, F.; Gabrielli, S.; Canonico, B.; Artico, M.; Ortolani, C.; Papa, S. Understanding the Synergy of NKp46 and Co-Activating Signals in Various NK Cell Subpopulations: Paving the Way for More Successful NK-Cell-Based Immunotherapy. Cells 2020, 9, 753. https://doi.org/10.3390/cells9030753
Zamai L, Del Zotto G, Buccella F, Gabrielli S, Canonico B, Artico M, Ortolani C, Papa S. Understanding the Synergy of NKp46 and Co-Activating Signals in Various NK Cell Subpopulations: Paving the Way for More Successful NK-Cell-Based Immunotherapy. Cells. 2020; 9(3):753. https://doi.org/10.3390/cells9030753
Chicago/Turabian StyleZamai, Loris, Genny Del Zotto, Flavia Buccella, Sara Gabrielli, Barbara Canonico, Marco Artico, Claudio Ortolani, and Stefano Papa. 2020. "Understanding the Synergy of NKp46 and Co-Activating Signals in Various NK Cell Subpopulations: Paving the Way for More Successful NK-Cell-Based Immunotherapy" Cells 9, no. 3: 753. https://doi.org/10.3390/cells9030753





