Genetic Association between Amyotrophic Lateral Sclerosis and Cancer
Abstract
:1. Introduction
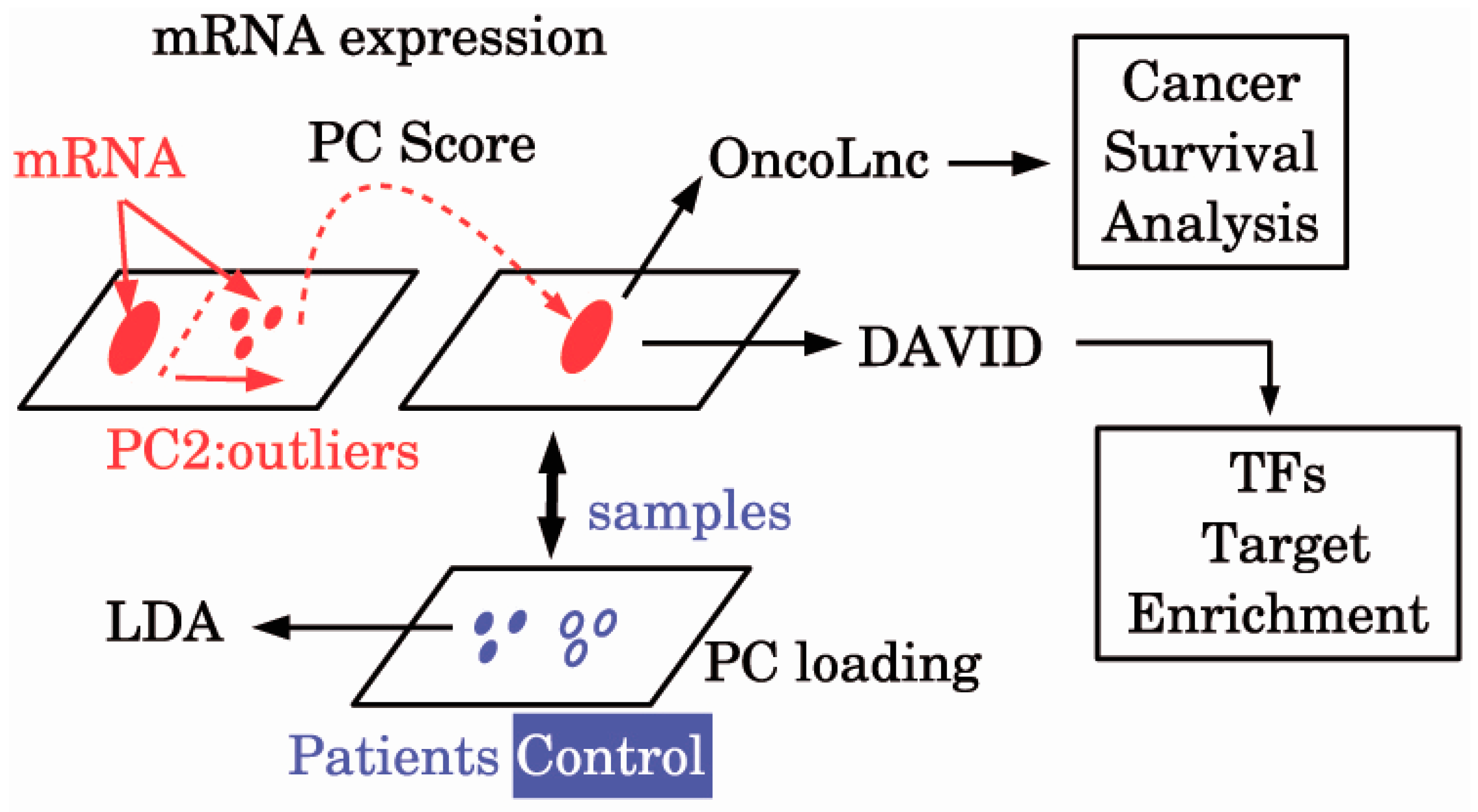
2. Materials and Methods
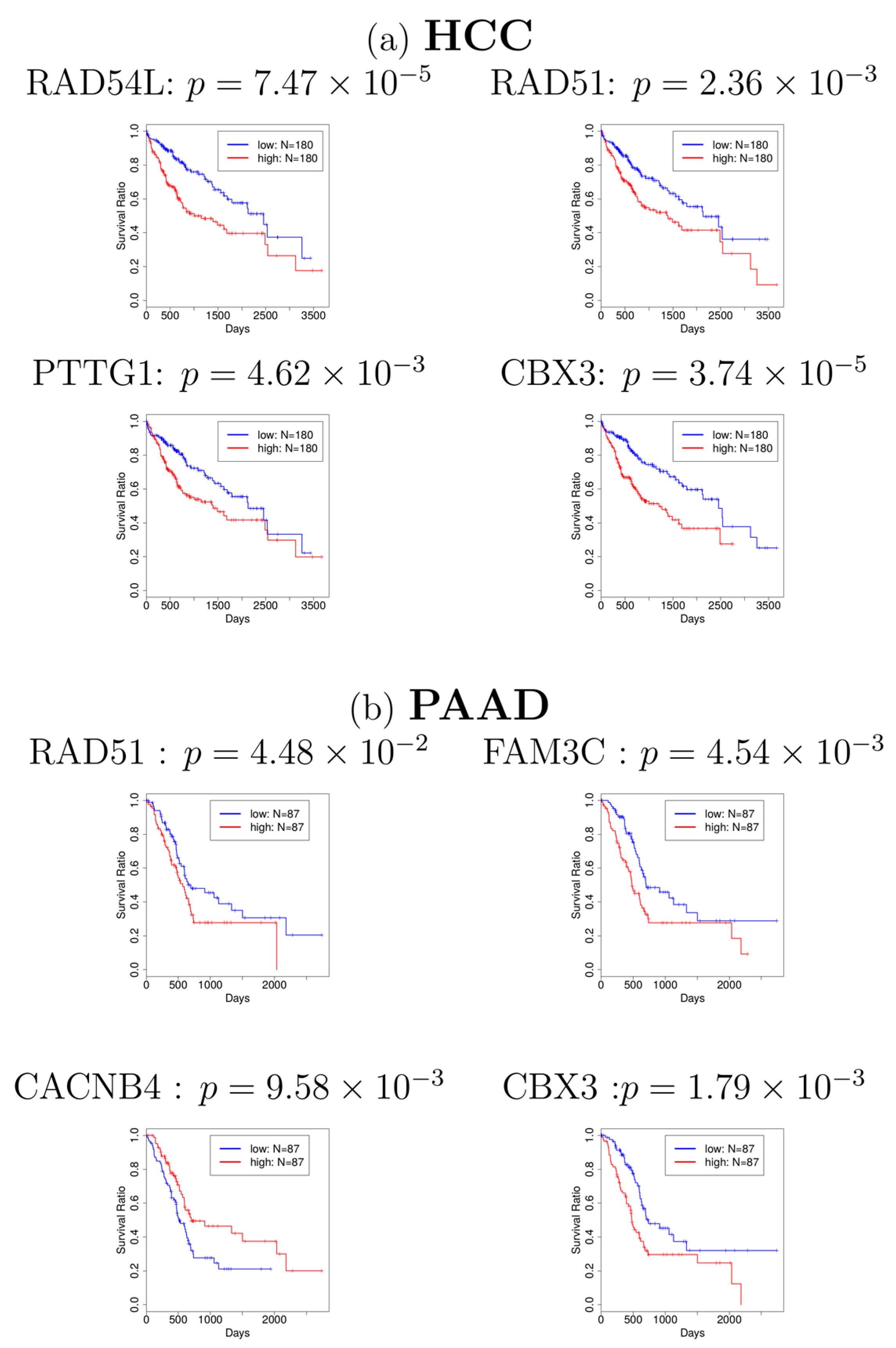
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef]
- Westeneng, H.J.; Walhout, R.; Straathof, M.; Schmidt, R.; Hendrikse, J.; Veldink, J.H.; van den Heuvel, M.P.; van den Berg, L.H. Widespread structural brain involvement in ALS is not limited to the C9orf72 repeat expansion. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Bergen, J.M.; Park, I.K.; Horner, P.J.; Pun, S.H. Nonviral approaches for neuronal delivery of nucleic acids. Pharm. Res. 2008, 25, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.M.; Curtis, R.E.; Daugherty, S.E.; Goedert, J.J.; Kuncl, R.W.; Tucker, M.A. The association between cancer and amyotrophic lateral sclerosis. Cancer Cause Control 2013, 24, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Al-Chalabi, A.; Ronnevi, L.O.; Turner, M.R.; Wirdefeldt, K.; Kamel, F.; Ye, W. Amyotrophic lateral sclerosis and cancer: A register-based study in Sweden. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Seol, H.S.; Lee, S.E.; Song, J.S.; Lee, H.Y.; Park, S.; Kim, I.; Singh, S.R.; Chang, S.; Jang, S.J. Glutamate release inhibitor, Riluzole, inhibited proliferation of human hepatocellular carcinoma cells by elevated ROS production. Cancer Lett. 2016, 382, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Geevasinga, N.; Burrell, J.R.; Hibbert, M.; Vucic, S.; Ng, K. C9ORF72 familial motor neuron disease—Frontotemporal dementia associated with lung adenocarcinoma and anti-Ma2/Ta antibodies: A chance association? Eur. J. Neurol. 2014, 21, e31–e33. [Google Scholar] [CrossRef] [PubMed]
- Macmillan-Crow, L.A.; Cruthirds, D.L. Invited review: Manganese superoxide dismutase in disease. Free Radic. Res. 2001, 34, 325–336. [Google Scholar] [CrossRef] [PubMed]
- McEachern, G.; Kassovska-Bratinova, S.; Raha, S.; Tarnopolsky, M.A.; Turnbull, J.; Bourgeois, J.; Robinson, B. Manganese superoxide dismutase levels are elevated in a proportion of amyotrophic lateral sclerosis patient cell lines. Biochem. Biophys. Res. Commun. 2000, 273, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Shaw, I.C.; Fitzmaurice, P.S.; Mitchell, J.D.; Lynch, P.G. Studies on cellular free radical protection mechanisms in the anterior horn from patients with amyotrophic lateral sclerosis. Neurodegeneration 1995, 4, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Oberley, L.W.; Buettner, G.R. Role of superoxide dismutase in cancer: A review. Cancer Res. 1979, 39, 1141–1149. [Google Scholar] [PubMed]
- Zhong, W.; Yan, T.; Lim, R.; Oberley, L.W. Expression of superoxide dismutases, catalase, and glutathione peroxidase in glioma cells. Free Radic. Biol. Med. 1999, 27, 1334–1345. [Google Scholar] [CrossRef]
- Pradat, P.F.; Dubourg, O.; de Tapia, M.; di Scala, F.; Dupuis, L.; Lenglet, T.; Bruneteau, G.; Salachas, F.; Lacomblez, L.; Corvol, J.C.; et al. Muscle gene expression is a marker of amyotrophic lateral sclerosis severity. Neurodegener. Dis. 2012, 9, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Kubo, S.; Tamori, A.; Itami, S.; Kawamura, E.; Iwaisako, K.; Ikeda, K.; Kawada, N.; Ochiya, T.; Taguchi, Y.H. Comprehensive analysis of transcriptome and metabolome analysis in intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Sci. Rep. 2015, 5, 16294. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.H. Identification of more feasible microRNA-mRNA interactions within multiple cancers using principal component analysis based unsupervised feature extraction. Int. J. Mol. Sci. 2016, 17, 696. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Dubowitz, V.; Sewry, C.A.; Oldfors, A.; Lane, R.J.M. Muscle Biopsy: A Practical Approach; Saunders: Oxford, UK, 2013; Available online: https://www.clinicalkey.com/dura/browse/bookChapter/3-s2.0-C2009063539X (accessed on 1 April 2013).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Anaya, J. OncoLnc: Linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput. Sci. 2016, 2, e674. [Google Scholar] [CrossRef]
- Han, H.Y.; Bearss, D.J.; Browne, L.W.; Calaluce, R.; Nagle, R.B.; Von Hoff, D.D. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res. 2002, 62, 2890–2896. [Google Scholar] [PubMed]
- Maacke, H.; Jost, K.; Opitz, S.; Miska, S.; Yuan, Y.; Hasselbach, L.; Lüttges, J.; Kalthoff, H.; Stürzbecher, H.W. DNA repair and recombination factor Rad51 is over-expressed in human pancreatic adenocarcinoma. Oncogene 2000, 19, 2791–2795. [Google Scholar] [CrossRef] [PubMed]
- Dighe, N.; Khoury, M.; Mattar, C.; Chong, M.; Choolani, M.; Chen, J.; Antoniou, M.N.; Chan, J.K. Long-term reproducible expression in human fetal liver hematopoietic stem cells with a UCOE-based lentiviral vector. PLoS ONE. 2014, 9, e104805. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, S.; Glesel, E.; Singh, G.; Chen, N.M.; Reutlinger, K.; Zhang, J.; Billadeau, D.D.; Fernandez–Zapico, M.E. Restricted heterochromatin formation Links NFATc2 repressor activity with growth promotion in pancreatic cancer. Gastroenterology 2012, 142, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Leucci, E.; Coe, E.A.; Marine, J.C.; Vance, K.W. The emerging role of long non-coding RNAs in cutaneous melanoma. Pigment Cell Melanoma Res. 2016, 29, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Tong, G.; Zhang, Y.; Liang, S.; Tang, K.; Yang, Q. PGK1 drives hepatocellular carcinoma metastasis by enhancing metabolic process. Int. J. Mol. Sci. 2017, 18, 1630. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zavadil, J.; Martin, L.; Parisi, F.; Friedman, E.; Levy, D.; Harding, H.; Ron, D.; Gardner, L.B. Inhibition of nonsense-mediated RNA decay by the tumor microenvironment promotes tumorigenesis. Mol. Cell. Biol. 2011, 31, 3670–3680. [Google Scholar] [CrossRef] [PubMed]
- Ionov, Y.; Nowak, N.; Perucho, M.; Markowitz, S.; Cowell, J.K. Manipulation of nonsense mediated decay identifies gene mutations in colon cancer cells with microsatellite instability. Oncogene 2004, 23, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.B. Nonsense-mediated RNA decay regulation by cellular stress: Implications for tumorigenesis. Mol. Cancer Res. 2010, 8, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Norrbom, M.; Chun, S.; Bennett, C.F.; Rigo, F. Nonsense-mediated decay as a terminating mechanism for antisense oligonucleotides. Nucleic Acids Res. 2014, 42, 5871–5879. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Vuong, J.K.; Zhang, M.; Stork, C.; Zheng, S. Inhibition of nonsense-mediated RNA decay by ER stress. RNA 2016, 23, 378–394. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.P.; Pletnikova, O.; Troncoso, J.C.; Wong, P.C. TDP-43 repression of nonconserved cryptic exons is compromised in ALS-FTD. Science 2015, 349, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Farrawell, N.E.; Lambert-Smith, I.A.; Warraich, S.T.; Blair, I.P.; Saunders, D.N.; Hatters, D.M.; Yerbury, J.J. Distinct partitioning of ALS associated TDP-43, FUS and SOD1 mutants into cellular inclusions. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Onodera, O.; Sugai, A.; Konno, T.; Tada, M.; Koyama, A.; Nishizawa, M. What is the key player in TDP-43 pathology in ALS: Disappearance from the nucleus or inclusion formation in the cytoplasm? Neurol. Clin. Neurosci. 2013, 1, 11–17. [Google Scholar] [CrossRef]
- Van Blitterswijk, M.; DeJesus-Hernandez, M.; Rademakers, R. How do C9ORF72 repeat expansions cause amyotrophic lateral sclerosis and frontotemporal dementia: Can we learn from other noncoding repeat expansion disorders? Curr. Opin. Neurol. 2012, 25, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Apiwattanakul, M.; Milone, M.; Pittock, S.J.; Kryzer, T.J.; Fryer, J.P.; O’Toole, O.; Mckeon, A.; Lennon, V.A. Signal recognition particle immunoglobulin g detected incidentally associates with autoimmune myopathy. Muscle Nerve 2016, 53, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.H.; Qin, S.; Wang, J.Y.; Roehrl, M.H. Proteomic expression analysis of surgical human colorectal cancer tissues: Up-regulation of PSB7, PRDX1, and SRP9 and hypoxic adaptation in cancer. J. Proteome Res. 2008, 7, 2959–2972. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, J.; Harty, L.; Mayer, N.; Critcher, V.; Ryan, J. Immune-mediated necrotizing myopathy, associated with antibodies to signal recognition particle, together with lupus nephritis: Case presentation and management. J. Clin. Med. Res. 2015, 7, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nishikawa, A.; Kuwana, M.; Nishimura, H.; Watanabe, Y.; Nakahara, J.; Hayashi, Y.K.; Suzuki, N.; Nishino, I. Inflammatory myopathy with anti-signal recognition particle antibodies: Case series of 100 patients. Orphanet J. Rare Dis. 2015, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, J.A.; Nath, A. Neuronal Cell Death and Inflammation. In Encyclopedia of Neuroscience; Springer: Berlin/Heidelberg, Germany, 2009; pp. 2772–2776. [Google Scholar]
- Simoes, J.; Amado, F.M.; Vitorino, R.; Helguero, L.A. A meta-analysis to evaluate the cellular processes regulated by the interactome of endogenous and over-expressed estrogen receptor alpha. Oncoscience 2015, 2, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, A.; Henderson, T.A.; Brubaker, D.; Bebek, G. Frequent subgraph mining of personalized signaling pathway networks groups patients with frequently dysregulated disease pathways and predicts prognosis. In Proceedings of the Pacific Symposium on Biocomputing Pacific Symposium on Biocomputing, Kohala Coast, HI, USA, 4–8 January 2016; Volume 22, pp. 402–413. [Google Scholar]
- Ikiz, B.; Alvarez, M.J.; Re, D.B.; Le Verche, V.; Politi, K.; Lotti, F.; Phani, S.; Pradhan, R.; Yu, C.; Croft, G.F.; et al. The regulatory machinery of neurodegeneration in in vitro models of amyotrophic lateral sclerosis. Cell Rep. 2015, 12, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Siddiqui, S.; Gabriely, G.; Lanser, A.J.; Dake, B.; Murugaiyan, G.; Doykan, C.E.; Wu, P.M.; Gali, R.R.; Iyer, L.K.; et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J. Clin. Investig. 2012, 122, 3063–3087. [Google Scholar] [CrossRef] [PubMed]
- Dini Modigliani, S.; Morlando, M.; Errichelli, L.; Sabatelli, M.; Bozzoni, I. An ALS-associated mutation in the FUS 3’-UTR disrupts a microRNA-FUS regulatory circuitry. Nat. Commun. 2014, 5, 4335. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Filippakopoulos, P.; Knapp, S. Bromodomains as therapeutic targets. Exp. Rev. Mol. Med. 2011, 13, e29. [Google Scholar] [CrossRef] [PubMed]
- Gayther, S.A.; Batley, S.J.; Linger, L.; Bannister, A.; Thorpe, K.; Chin, S.F.; Daigo, Y.; Russell, P.; Wilson, A.; Sowter, H.M.; et al. Mutations truncating the EP300 acetylase in human cancers. Nat. Genet. 2000, 24, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, W.; Mossmann, D.; Kleemann, J.; Mock, K.; Meisinger, C.; Brummer, T.; Herr, R.; Brabletz, S.; Stemmler, M.P.; Brabletz, T. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat. Commun. 2016, 7, 10498. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Slyper, M.; Shahar, A.; Bar-Ziv, A.; Granit, R.Z.; Hamburger, T.; Maly, B.; Peretz, T.; Ben-Porath, I. Control of breast cancer growth and initiation by the stem cell-associated transcription factor TCF3. Cancer Res. 2012, 72, 5613–5624. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Blobel, G.A. GATA Transcription Factors and Cancer. Genes Cancer 2010, 1, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
| True | |||
|---|---|---|---|
| Normal | ALS | ||
| Prediction | Normal | 10 | 1 |
| ALS | 0 | 8 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taguchi, Y.-h.; Wang, H. Genetic Association between Amyotrophic Lateral Sclerosis and Cancer. Genes 2017, 8, 243. https://doi.org/10.3390/genes8100243
Taguchi Y-h, Wang H. Genetic Association between Amyotrophic Lateral Sclerosis and Cancer. Genes. 2017; 8(10):243. https://doi.org/10.3390/genes8100243
Chicago/Turabian StyleTaguchi, Y-h., and Hsiuying Wang. 2017. "Genetic Association between Amyotrophic Lateral Sclerosis and Cancer" Genes 8, no. 10: 243. https://doi.org/10.3390/genes8100243






