Outcome of Full-Thickness Macular Hole Surgery in Choroideremia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
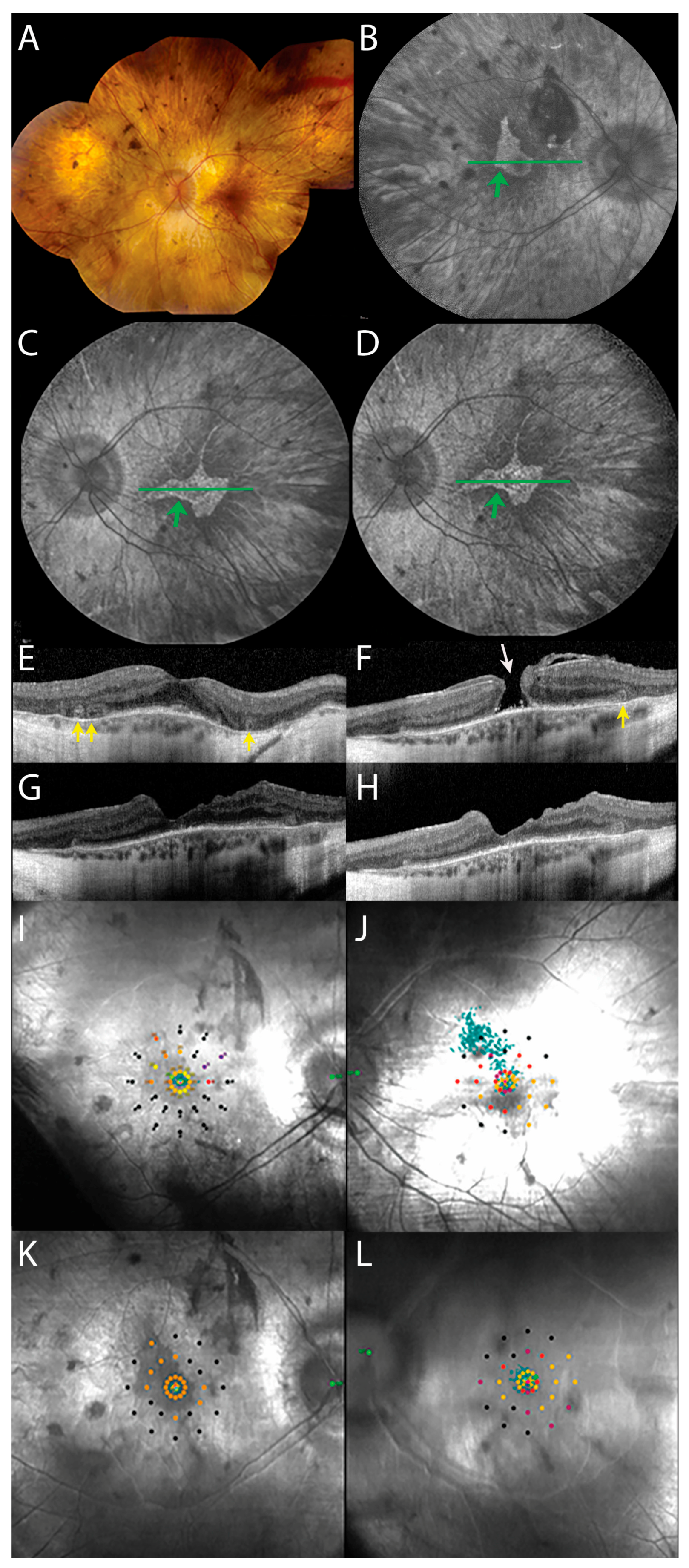
3.1. Preoperative Presentation
3.2. Postoperative Clinical Course
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coussa, R.G.; Kim, J.; Traboulsi, E.I. Choroideremia: Effect of age on visual acuity in patients and female carriers. Ophthalmic Genet. 2012, 33, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zinkernagel, M.S.; Groppe, M.; MacLaren, R.E. Macular hole surgery in patients with end-stage choroideremia. Ophthalmology 2013, 120, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, H.; Koto, T.; Fujiki, K.; Murakami, A.; Tsubota, K.; Ozawa, Y. Clinical findings in a choroideremia patient who underwent vitrectomy for retinal detachment associated with macular hole. Jpn J. Ophthalmol. 2011, 55, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Van Huet, R.A.; Oomen, C.J.; Plomp, A.S.; van Genderen, M.M.; Klevering, B.J.; Schlingemann, R.O.; Klaver, C.C.; van den Born, L.I.; Cremers, F.P.; RD5000 Study Group. The RD5000 database: Facilitating clinical, genetic, and therapeutic studies on inherited retinal diseases. Invest. Ophthalmol. Vis. Sci. 2014, 55, 7355–7360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagnelie, G. Conversion of planimetric visual field data into solid angles and retinal areas. Clin. Vis. Sci. 1990, 5, 95–100. [Google Scholar]
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.M.; Black, G.C.M.; et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014, 383, 1129–1137. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, S.W.; Lee, E.J.; Kim, J.; Kim, S.J.; Ahn, J. Temporal changes in foveal contour after macular hole surgery. Eye 2014, 28, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Aleman, T.S.; Han, G.; Serrano, L.W.; Fuerst, N.M.; Charlson, E.S.; Pearson, D.J.; Chung, D.C.; Traband, A.; Pan, W.; Ying, G.S.; et al. Natural History of the Central Structural Abnormalities in Choroideremia: A Prospective Cross-Sectional Study. Ophthalmology 2017, 124, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Smiddy, W.E.; Feuer, W.J.; Shi, W. Outcomes of sulfur hexafluoride (SF6) versus perfluoropropane (C3F8) gas tamponade for macular hole surgery. Retina 2008, 28, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Abbey, A.M.; Van Laere, L.; Shah, A.R.; Hassan, T.S. Recurrent Macular Holes in the Era of Small-Gauge Vitrectomy: A Review of Incidence, Risk Factors, and Outcomes. Retina 2017, 37, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Furukawa, M.; Ogino, N.; Larson, E. Incidence and factors related to macular hole reopening. Am. J. Ophthalmol. 2010, 149, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kishi, S. Pathogenesis of macular hole recurrence and its prevention by internal limiting membrane peeling. Retina 2007, 27, 169–173. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talib, M.; Koetsier, L.S.; MacLaren, R.E.; Boon, C.J.F. Outcome of Full-Thickness Macular Hole Surgery in Choroideremia. Genes 2017, 8, 187. https://doi.org/10.3390/genes8070187
Talib M, Koetsier LS, MacLaren RE, Boon CJF. Outcome of Full-Thickness Macular Hole Surgery in Choroideremia. Genes. 2017; 8(7):187. https://doi.org/10.3390/genes8070187
Chicago/Turabian StyleTalib, Mays, Leonoor S. Koetsier, Robert E. MacLaren, and Camiel J.F. Boon. 2017. "Outcome of Full-Thickness Macular Hole Surgery in Choroideremia" Genes 8, no. 7: 187. https://doi.org/10.3390/genes8070187



