Atmospheric Levels of Benzene and C1-C2 Carbonyls in San Nicolas de los Garza, Nuevo Leon, Mexico: Source Implications and Health Risk
Abstract
:1. Introduction
2. Materials and Methods
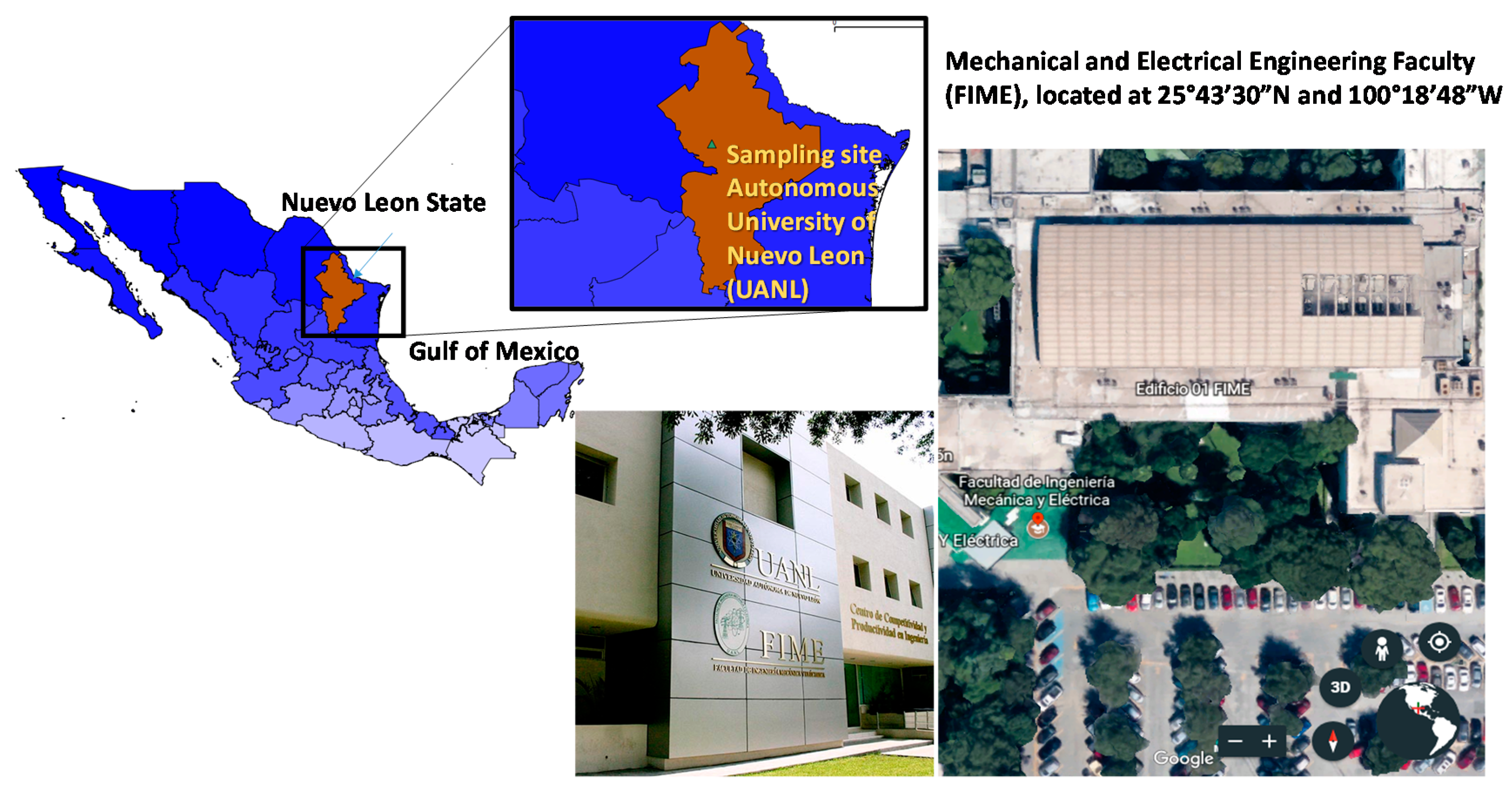
2.1. Sampling Site
2.2. Statistical Analysis
2.3. Measurement of VOCs (Carbonyls and Benzene), Meteorological Parameters, and Criteria Air Pollutants
2.3.1. Sampling and Analysis of Benzene
2.3.2. Sampling and Analysis of Carbonyls
2.4. Measurements of Criteria Air Pollutants and Meteorological Parameters
2.5. Principal Component Analysis
2.6. Health Risk Evaluation
3. Results and Discussion
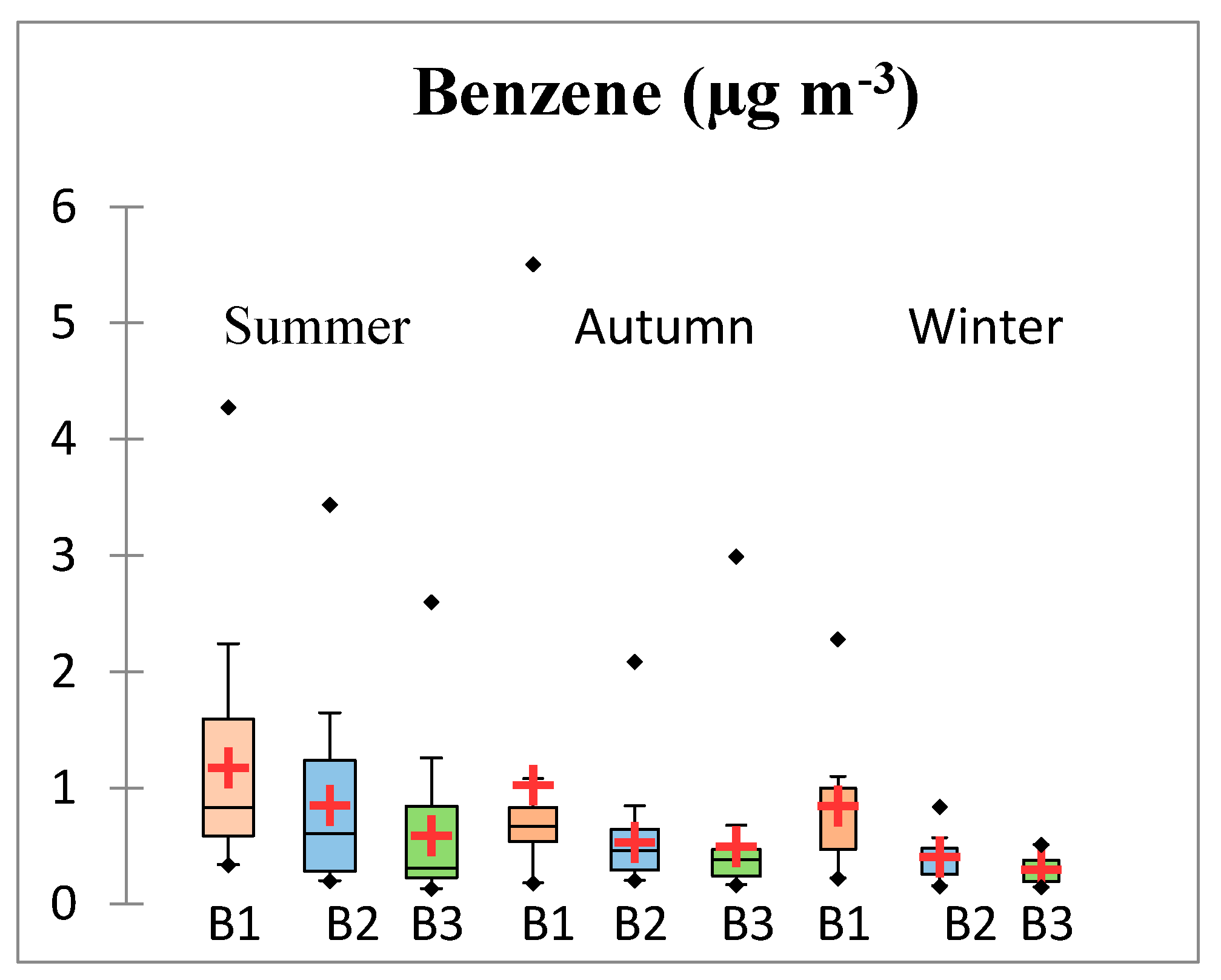
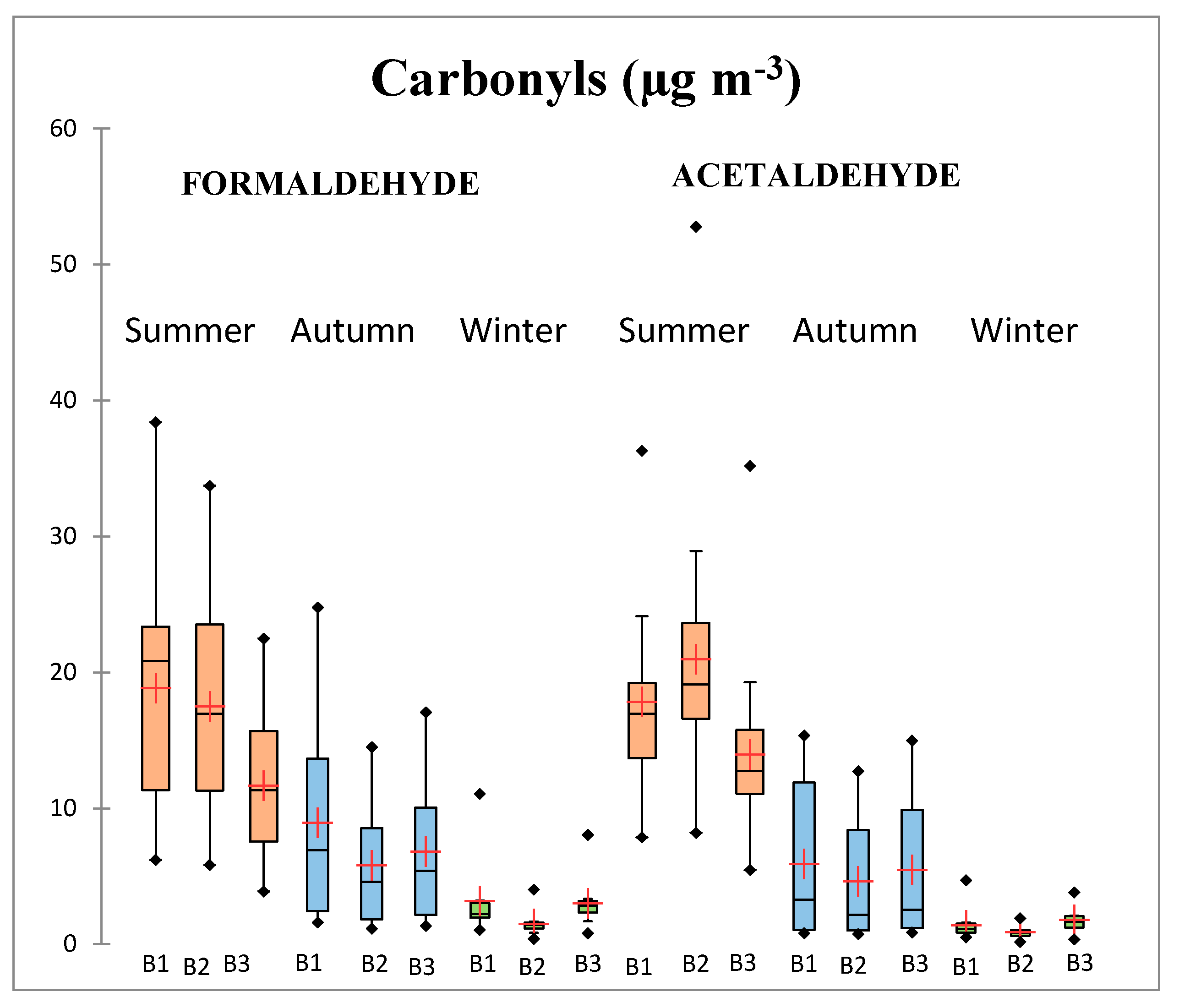
3.1. Diurnal and Seasonal Variations
3.1.1. Results for Benzene
3.1.2. Results for C1-C2 Carbonyls
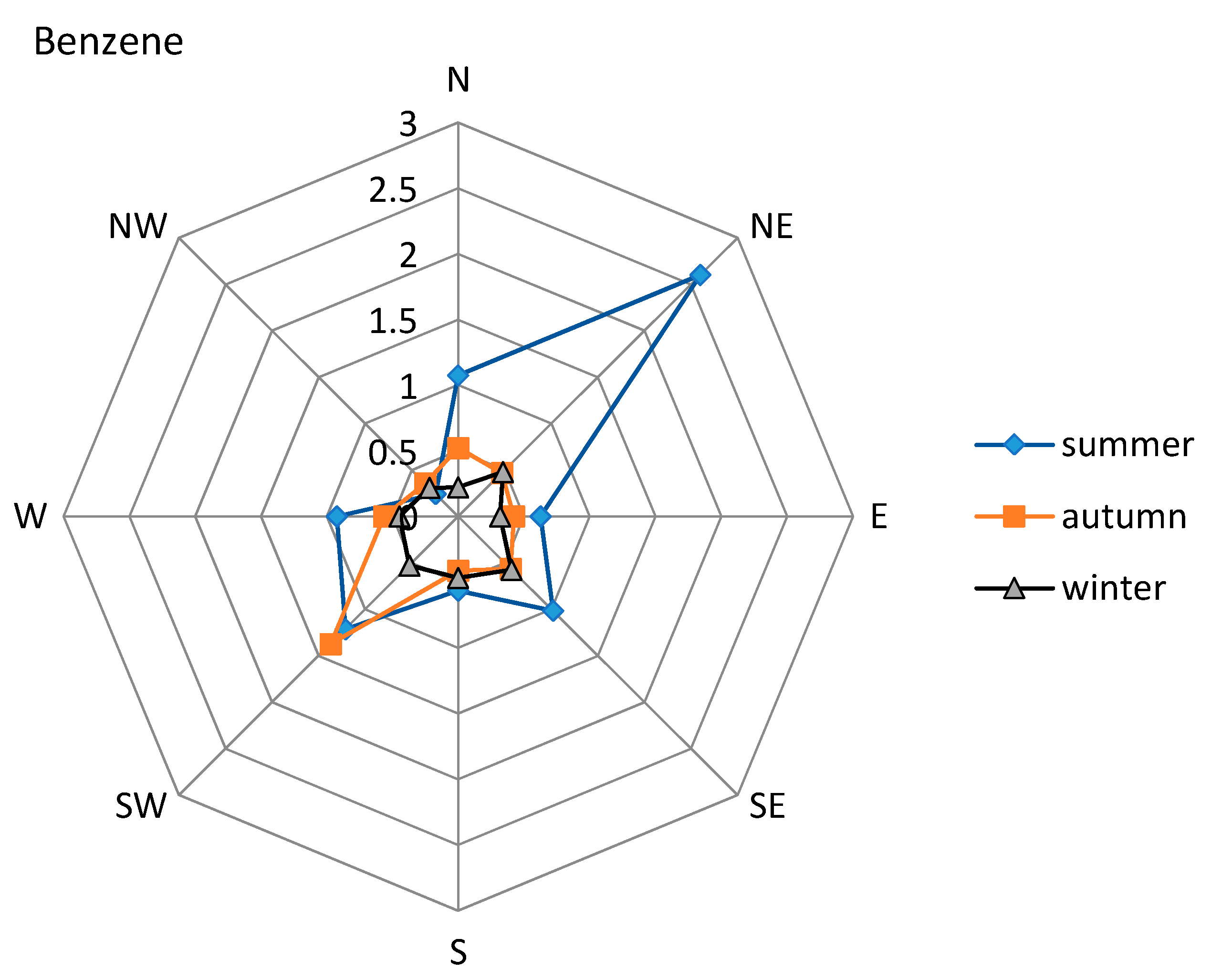
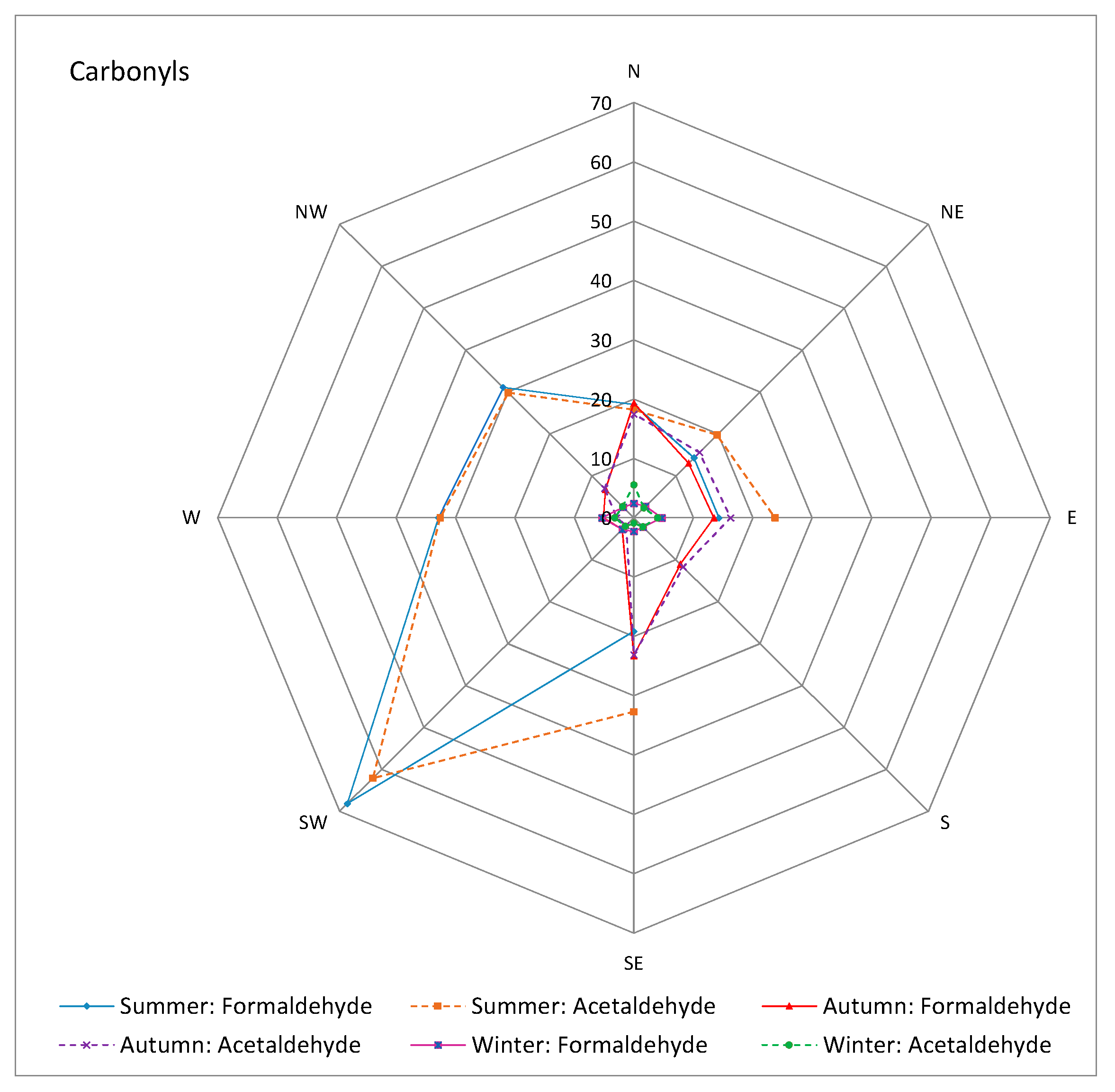
3.2. Meteorological Influence
3.3. Formaldehyde/Acetaldehyde Ratios
3.4. Pearson Correlation and Principal Component Analysis (PCA)
3.5. Health Risk
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Research Council (NRC). Human Exposure to Airborne Pollutants: Advances and Opportunities; National Academy Press: Washington, DC, USA, 1991. [Google Scholar]
- Atkinson, R. Atmospheric chemistry of VOCs and NOX. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Hoque, R.R.; Khillare, P.S.; Agarwal, T.; Shridhar, V.; Balachandran, S. Spatial and temporal variation of BTEX in the urban atmosphere of Delhi, India. Sci. Total Environ. 2008, 392, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Báez, A.; Padilla, H.; Garcia, R.; Torres, M.C.; Rosas, I.; Belmont, R. Carbonyl levels in indoor and outdoor air in Mexico City and Xalapa, Mexico. Sci. Total Environ. 2003, 302, 211–226. [Google Scholar] [CrossRef]
- WHO Regional Publications. Air Quality Guidelines for Europe. Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/74732/E71922.pdf (accessed on 16 August 2017).
- Civan, M.Y.; Elbir, T.; Seyfioglu, R.; Kuntasal, Ö.O.; Bayram, A.; Doğan, G.; Yurdakul, S.; Andiç, Ö.; Müezzinoğlu, A.; Sofuoglu, S.C.; et al. Spatial and temporal variations in atmospheric VOCs, NO2, SO2, and O3 concentrations at a heavily industrialized region in Western Turkey, and assessment of the carcinogenic risk levels of benzene. Atmos. Environ. 2015, 103, 102–113. [Google Scholar] [CrossRef]
- De Blas, M.; Navazo, M.; Alonso, L.; Durana, N.; Gomez, M.C.; Iza, J. Simultaneous indoor and outdoor on-line hourly monitoring of atmospheric volatile organic compounds in an urban building. The role of inside and outside sources. Sci. Total Environ. 2012, 426, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.G.; Lanning, J.A.; Barrell, R.; Miyagishima, J.; Jones, R.H.; Wolfe, P. Sources and sinks of formaldehyde and acetaldehyde: An analysis of Denver’s ambient concentration data. Atmos. Environ. 1996, 30, 2113–2123. [Google Scholar] [CrossRef]
- Wang, X.M.; Sheng, G.Y.; Fu, J.M.; Chan, C.Y.; Lee, S.C.; Chan, L.Y.; Wang, Z.S. Urban roadside aromatic hydrocarbons in three cities of the Pearl River Delta, People’s Republic of China. Atmos. Environ. 2002, 36, 5141–5148. [Google Scholar] [CrossRef]
- Vega, E.; Mugica, V.; Carmona, R.; Valencia, E. Hydrocarbon source apportionment in Mexico City using the chemical mass balance receptor model. Atmos. Environ. 2000, 34, 4121–4129. [Google Scholar] [CrossRef]
- Mugica, V.; Watson, J.; Vega, E.; Reyes, E.; Ruiz, M.E.; Chow, J. Receptor Model source apportionment of nonmethane hydrocarbons in Mexico City. Sci. World J. 2002, 2, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Arriaga-Colina, J.L.; West, J.J.; Sosa, G.; Escalona, S.S.; Ordúñez, R.M.; Cervantes, A.D.M. Measurements of VOCs in Mexico City (1992–2001) and evaluation of VOCs and CO in the emissions inventory. Atmos. Environ. 2004, 38, 2523–2533. [Google Scholar] [CrossRef]
- Cerón, R.M.; Cerón, J.G.; Muriel, M. Diurnal and seasonal trends in carbonyl levels in a semi-urban coastal site in the Gulf of Campeche, Mexico. Atmos. Environ. 2007, 41, 63–71. [Google Scholar] [CrossRef]
- Cerón, J.G.; Ramírez, E.; Cerón, R.M.; Carballo, C.; Aguilar, C.; López, U.; Ramírez, A.; Gracia, Y.; Naal, D.; Campero, A.; et al. Diurnal and seasonal variation of BTX in ambient air of one urban site in Carmen City, Campeche, Mexico. J. Environ. Prot. 2013, 4, 40–49. [Google Scholar] [CrossRef]
- National Institute of Statistics and Geography (INEGI). Statistical Yearbook. Nuevo Leon. Mexico. 2011. Available online: http://www.beta.inegi.org.mx/app/biblioteca/ficha.html?upc=702825202019 (accessed on 27 June 2017).
- Secretary of Environment and Natural Resources SEMARNAT. INE. Emissions Inventory of the North Frontier States of Mexico. 2005. Available online: http://www3.cec.org/islandora/es/item/2204a-inventario-de-emisiones-de-los-estados-de-la-frontera-norte-de-mexico-1999-summary-es.pdf (accessed on 5 June 2017).
- Ortínez, A.; Mateos, A. Evaluación Preliminar de las Condiciones Atmosféricas y Climatología Química en el Área Metropolitana de Monterrey. Available online: http://aire.nl.gob.mx/rep_pm2_5.html (accessed on 27 June 2017).
- Statistical Software and Data Analysis Add-on for Excel (XLSTAT), 2016.5 Version. Available online: https://www.xlstat.com/en/ (accessed on 27 March 2017).
- Cerón, J.G.; Cerón, R.M.; Vivas, F.; Barceló, C.; Espinosa, M.L.; Ramírez, E.; Rangel, M.; Montero, J.A.; Rodríguez, A.; Uc, M.P. Characterization and sources of Aromatic Hydrocarbons (BTEX) in the atmosphere of two urban sites located in Yucatan Peninsula in Mexico. Atmosphere 2017, 8, 107. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by High Performance Liquid Chromatography (HPLC). Available online: https://www3.epa.gov/ttnamti1/files/ambient/airtox/to-11ar.pdf (accessed on 21 June 2017).
- Miller, J.C.; Miller, J.N. Estadística Para Química Analítica, 1st ed.; Addison-Wesley Iberoamericana: Wilmington, DE, USA, 1993; ISBN 0-201-60140-0. [Google Scholar]
- Lakes Environmental. WRPLOT View Version 7.0: Wind Rose Plots for Meteorological Data. 2011. Available online: http://www.weblakes.com/products/wrplot/index.html (accessed on 23 July 2017).
- Air Resources Laboratory (ARL). HYSPLIT: Hybrid Single-Particle Lagrangian Integrated Trajectory. Available online: http://www.ready.noaa.gov/READYcmet.php (accessed on 22 July 2017).
- Geng, F.; Cai, C.; Tie, X.; Yu, Q.; An, J.; Peng, L.; Zhou, G.; Xu, J. Analysis of VOC emissions using PCA/APCS receptor model at city Shanghai, China. J. Atmos. Chem. 2009, 62, 229–247. [Google Scholar] [CrossRef]
- Vukovich, F.M. Weekday/Weekend differences in OH reactivity with VOCs and CO in Baltimore, Maryland. J. Air Waste Manag. Assoc. 2000, 50, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (USEPA). Guidelines for Carcinogen Risk Assessment. Risk Assessment Forum. Available online: https://ofmpub.epa.gov/eims/eimscomm.getfile?p_download_id=437005 (accessed on 28 July 2017).
- International Agency for Research on Cancer (IARC). Monographs on the Evaluation of Carcinogenic Risk to Humans. Available online: http://monographs.iarc.fr/ENG/Monographs/vol109/mono109.pdf (accessed on 23 July 2017).
- International Agency for Research on Cancer (IARC). Monographs on the Evaluation of Carcinogenic Risk to Humans, Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol. Available online: http://monographs.iarc.fr/ENG/Monographs/vol88/mono88.pdf (accessed on 23 July 2017).
- Singh, D.; Kumar, A.; Singh, B.P.; Anandam, K.; Singh, M.; Mina, U.; Kumar, K.; Jain, V.K. Spatial and Temporal variability of VOCs and its source estimation during rush/non-rush hours in ambient air of Delhi, India. Air Qual. Atmos. Health 2016, 9, 483–493. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhang, Y.; Lü, S.; Huang, Z.; Huang, X.; Wang, Y. Ambient air benzene at background sites in China’s most developed coastal regions: Exposure levels, source implications and health risks. Sci. Total Environ. 2015, 511, 792–800. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (USEPA). Integrated Risk Information System (IRIS); US Environmental Protection Agency: Washington, DC, USA, 1998.
- United States Environmental Protection Agency (USEPA). Guidelines for Carcinogen Risk Assessment. In Risk Assessment Forum; U.S. Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual Part F, Supplemental Guidance for Inhalation Risk Assessment: Final; Office of Superfund Remediation and Technology Innovation, Environmental Protection Agency: Washington, DC, USA, 2009.
- US Department of Health and Human Services (US DHHS). Public Health Service. In Toxicological Profile for Benzene; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2007. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp3.pdf (accessed on 12 July 2017).
- The Risk Assessment Information System (RAIS). RAIS Toxicity and Properties. Chemical Values for Acetaldehyde. Available online: https://rais.ornl.gov/cgi-bin/tools/TOX_search (accessed on 10 April 2017).
- The Risk Assessment Information System (RAIS). RAIS Toxicity and Properties. Chemical Values for Formaldehyde. Available online: https://rais.ornl.gov/cgi-bin/tools/TOX_search (accessed on 10 April 2017).
- United States Environmental Protection Agency (USEPA). Toxicological Review of Benzene (Noncancer Effects) (CAS No. 71–43–2). In Support of Summary Information on the Integrated Risk Information System (IRIS) EPA/635/R-02/001F; U.S. Environmental Protection Agency: Washington, DC, USA, 2002. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0276tr.pdf (accessed on 23 May 2017).
- United States Environmental Protection Agency (USEPA). Risk Assessment for Carcinogenic Effects. Available online: https://www.epa.gov/fera/risk-assessment-carcinogenic-effects (accessed on 16 May 2017).
- United States Environmental Protection Agency (USEPA). An SAB Report: Formaldehyde Risk Assessment Update—Review of the Office of Toxic Substance’s Draft Formaldehyde Risk Assessment Update by the Environmental Protection Agency. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/9100CAMU.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1991+Thru+1994&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C91thru94%5CTxt%5C00000023%5C9100CAMU.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 6 June 2017).
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans: Allyl Compounds, Aldehydes, Epoxides and Peroxides. Volume 36. World Health Organization, Lyon, 1985. Available online: https://monographs.iarc.fr/ENG/Monographs/vol1–42/mono36.pdf (accessed on 14 June 2017).
- United States Environmental Protection Agency (USEPA). Carcinogenic Effects of Benzene: An Update. EPA/600/P-97/001F April 1998; National Center for Environmental Assessment–Washington Office: Office of Research and Development U.S. Environmental Protection Agency: Washington, DC, USA, 1998. Available online: https://webcache.googleusercontent.com/search?q=cache:yrS9K6L5IQoJ:https://ofmpub.epa.gov/eims/eimscomm.getfile%3Fp_download_id%3D428659+&cd=2&hl=es-419&ct=clnk&gl=mx (accessed on 26 June 2017).
- Menchaca-Torre, H.L.; Mercado-Hernandez, R.; Mendoza-Dominguez, A. Diurnal and seasonal variation of volatile organic compounds in the atmosphere of Monterrey, Mexico. Atmos. Pollut. Res. 2015, 6, 1073–1081. [Google Scholar] [CrossRef]
- Menchaca-Torre, H.L.; Mercado-Hernández, R.; Rodríguez-Rodríguez, J.; Mendoza-Domínguez, A. Diurnal and seasonal variations of carbonyls and their effect on ozone concentrations in the atmosphere of Monterrey, Mexico. J. Air Waste Manag. Assoc. 2015, 65, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Facundo-Torres, D.M.; Ramírez-Lara, E.; Cerón-Bretón, J.G.; Cerón-Bretón, R.M.; García-Vasquez, Y.; Miranda-Guardiola, R.; Rivera de la Rosa, J. Measurement of Carbonyls and its relation with Criteria Pollutants (O3, NO, NO2, NOx, CO and SO2) in an Urban site within the metropolitan area of Monterrey, in Nuevo León, México. Int. J. Energy Environ. 2012, 6, 524–531. [Google Scholar]
- De Andrade, J.B.; Andrade, M.V.; Pinheiro, H.L.C. Atmospheric Levels of Formaldehyde and Acetaldehyde and their Relationship with the Vehicular Fleet Composition in Salvador, Bahia, Brazil. J. Braz. Chem. Soc. 1998, 9, 219–223. [Google Scholar] [CrossRef]
- Marć, M.; Bielawska, M.; Simeonov, V.; Namieśnik, J.; Zabiegala, B. The effect of anthropogenic activity on BTEX, NO2, SO2 and CO concentrations in urban air of the spa city of Sopot and medium-industrialized city of Tczew located in North Poland. Environ. Res. 2016, 147, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Galindo, N.; Varea, M.; Gil-Moltó, J.; Yubero, E. BTX in urban areas of eastern Spain: A focus on time variations and sources. Environ. Sci. Pollut. Res. 2016, 23, 18267–18276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, N.; Wang, N. Characterization and Source identification of ambient VOCs in Jinan, China. Air Qual. Atmos. Health. 2016, 9, 285–291. [Google Scholar] [CrossRef]
- Ho, K.F.; Hang, H.S.S.; Huang, R.J.; Dai, W.T.; Cao, J.J.; Tian, L.; Deng, W.J. Spatiotemporal distribution of carbonyl compounds in China. Environ. Pollut. 2015, 197, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; He, X.; Chen, M.; Tan, J.; Wang, Y. Photochemical production of atmospheric carbonyls in a rural area in Southern China. Arch. Environ. Contam. Toxicol. 2014, 66, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Bretón, J.G.; Cerón-Bretón, R.M.; Kahl, J.D.W.; Ramírez-Lara, E.; Guarnaccia, C.; Aguilar-Ucán, C.; Montalvo-Romero, C.; Anguebes-Franseschi, F.; López-Chuken, U. Diurnal and seasonal variation of BTEX in the air of Monterrey, Mexico: Preliminary study of sources and photochemical ozone pollution. Air Qual. Atmos. Health 2015, 8, 469–482. [Google Scholar] [CrossRef]
- Srivastava, A.; Joseph, A.E.; Devotta, S. Volatile organic compounds in ambient air of Mumbai-India. Atmos. Environ. 2006, 40, 892–903. [Google Scholar] [CrossRef]
- Badol, C.; Locoge, N.; Galloo, J.C. Using a source-receptor approach to characterise VOC behaviour in a French urban area influenced by industrial emissions: Part II: Source contribution assessment using the Chemical Mass Balance (CMB) model. Sci. Total Environ. 2008, 389, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, D.; Williams, E.L., II; Grosjean, E. Peroxyacyl nitrates at southern California mountain forest locations. Environ. Sci. Technol. 1993, 27, 110–121. [Google Scholar] [CrossRef]
- Shepson, P.B.; Hastie, D.R.; Schiff, H.I.; Polizzi, M.; Bottenheim, J.W.; Anlauf, K.; Mackay, G.I.; Karecki, D.R. Atmospheric concentrations and temporal variations of C1–C3 carbonyl compounds at two rural sites in central Ontario. Atmos. Environ. 1991, 25, 2001–2015. [Google Scholar] [CrossRef]
- Jacob, D.J.; Wofsy, S.C. Photochemistry of biogenic emissions over the Amazon forest. J. Geophys. Res. 1988, 93, 1477–1486. [Google Scholar] [CrossRef]
- Lloyd, A.C.; Atkinson, R.; Lurmann, F.W.; Nitta, B. Modelling potential ozone impacts from natural hydrocarbons-I. Development and testing of a chemical mechanism for the NOx-air photooxidations of isoprene and α-pinene under ambient conditions. Atmos. Environ. 1983, 17, 1931–1950. [Google Scholar] [CrossRef]
- Hellén, H.; Hakola, H.; Pirjola, L.; Laurila, T.; Pystynen, K.H. Ambient air concentrations, source profiles and source apportionment of 71 different C2-C10 volatile organic compounds in urban and residential areas of Finland. Environ. Sci. Technol. 2006, 40, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.F.; Lee, S.C.; Louie, P.K.K.; Zou, S.C. Seasonal variation of carbonyl compound concentrations in urban area of Hong Kong. Atmos. Environ. 2002, 36, 1259–1265. [Google Scholar] [CrossRef]
- Okada, Y.; Nakagoshi, A.; Tsurukawa, M.; Matsumura, C.; Eiho, J.; Nakano, T. Environmental risk assessment and concentration trend of atmospheric volatile organic compounds in Hyogo Prefecture, Japan. Environ. Sci. Pollut. Res. 2012, 19, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Báez, A.P.; Padilla, H.; Cervantes, J.; Pereyra, D.; Torres, M.C.; Garcia, R.; Belmont, R. Preliminary study of the determination of ambient carbonyls in Xalapa City, Veracruz, Mexico. Atmos. Environ. 2001, 35, 1813–1819. [Google Scholar] [CrossRef]
- Alves, C.A.; Evtyugina, M.; Cerqueira, M.; Nunes, T.; Duarte, M.; Vicente, E. Volatile organic compounds emitted by the stacks of restaurants. Air Qual. Atmos. Health 2014, 8, 401–412. [Google Scholar] [CrossRef]
- Pang, X.; Mu, X. Seasonal and diurnal variations of carbonyl compounds in Beijing ambient air. Atmos. Environ. 2006, 40, 6313–6320. [Google Scholar] [CrossRef]
- National Institute of Statistics, Geography and Informatics (INEGI). Environmental Statistics for the Metropolitan Area of Monterrey in 2001. Available online: www.inegi.gob.mx (accessed on 17 June 2017).
- Finnlayson-Pitss, B.J.; Pitts, J.N. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications, 1st ed.; Academic Press: San Diego, CA, USA, 1999; ISBN 0–12–257060-x. [Google Scholar]
- Horowitz, A.; Kershner, C.J.; Calvert, J.G. Primary Processes in the Photolysis of Acetaldehyde at 3000 A and 25 °C. J. Phys. Chem. 1982, 86, 3094–3105. [Google Scholar]
- Horowitz, A.; Calvert, J.G. Wavelength Dependence of the Primary Processes in Acetaldehyde Photolysis. Phys. Chem. 1982, 86, 3105–3114. [Google Scholar] [CrossRef]
- Du, Z.; Mo, J.; Zhang, Y. Risk assessment of population inhalation exposure to volatile organic compounds and carbonyls in urban China. Environ. Int. 2014, 73, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Sexton, K.; Linder, S.H.; Marko, D.; Bethel, H.; Lupo, P.J. Commentary Comparative assessment of air pollution-related health risks in Houston. Environ. Health Perspect. 2007, 115, 1388–1393. [Google Scholar] [PubMed]
- Zhang, Y.; Mu, Y.; Liu, J.; Mellouki, A. Levels, sources and health risks of carbonyls and BTEX in the ambient air of Beijing, China. J. Environ. Sci. 2012, 24, 124–130. [Google Scholar] [CrossRef]
- Tunsaringkarn, T.; Siriwong, W.; Prueksasit, T.; Sematong, S.; Zapuang, K.; Rungsiyothin, A. Potential Risk Comparison of Formaldehyde and Acetaldehyde Exposures in Office and Gasoline Station Workers. Int. J. Sci. Res. Pub. 2012, 6, 2250–3153. [Google Scholar]
- Dutta, C.; Som, D.; Chatterjee, A.; Mukherjee, A.K.; Jana, T.K.; Sen, S. Mixing ratios of carbonyls and BTEX in ambient air of Kolkata, India and their associated health risk. Environ. Monit. Assess. 2009, 148, 97–107. [Google Scholar] [CrossRef] [PubMed]
| (a) Toxicity Profiles (USD HHS) [34] | ||||
|---|---|---|---|---|
| Pollutant | CAS No. | RfC a (mg m−3) | Inhalation Cancer Slope Factor (SF) b (kg day/mg) | Carcinogenicity c |
| Formaldehyde | 50,000 | 9.83 × 10−3 | 2.1 × 10−2 | Group B1 d |
| Acetaldehyde | 75,070 | 9 × 10−3 | 1 × 10−2 | Group B2 e |
| Benzene | 71,432 | 3 × 10−2 | 2.89 × 10−2 | Group A f |
| Sampling Site | Characteristics of Study Area | Benzene (µg m−3) | Formaldehyde (µg m−3) | Acetaldehyde (µg m−3) | Author |
|---|---|---|---|---|---|
| San Nicolas de los Garza, N.L., Mexico, 2011 (this study) | Urban and Industrialized Area; Population: 443,273 inhabitants; Surface Area: 86.8 km2; Population density: 5493 inhabitants/km2; Main Sources: Mobile and Area sources | 0.87 (summer); 0.68 (autumn) ;0.51 (winter) | 15.27 (summer); 7.89 (autumn); 3.10 (winter) | 15.91 (summer); 5.70 (autumn); 1.61 (winter) | Ceron et al. (this study) |
| Tcew, North Poland 2013 | Medium size city (Industrialized Area); Population: 60,279 inhabitants; Surface Area: 22.3 km2; Population density: 2700 inhabitants/km2; Main Sources: Diesel engine emissions and low-quality coal burning emissions | 0.75 | Marć et al. 2016 [46] | ||
| Sopot, North Poland 2014 | Medium size city (rural area); Population: 40,000 inhabitants; Surface Area: 17.31 km2; Population density: 2200 inhabitants/km2; Main Sources: Mobile source emissions | 0.53 | Marć et al. 2016 [46] | ||
| Alicante, Spain 2008 | Medium size city (rural area); Population: 330,525 inhabitants; Surface Area: 201.27 km2; Population density: 1642 inhabitants/km2; Main Sources: Mobile source emissions | 1.2 | Galindo et al. 2016 [47] | ||
| Delhi, India | Urban and Industrialized Area; Population: 18,980,000 inhabitants; Surface Area: 1484 km2; Population density: 11,312 inhabitants/km2; Main Sources: Diesel/gasoline internal combustion engines emissions | 26.2 | Singh et al. 2016 [29] | ||
| Jinan, China 2010–2011 | Urban and Industrialized Area; Population: 7,080,000 inhabitants; Surface Area: 8177 km2; Population density: 1400 inhabitants/km2; Main Sources: Vehicular traffic, industry and biomass combustion emissions | 2.42 | Liu et al. 2016 [48] | ||
| Longmen Southern China 2012–2013 | Rural area; Population: 7000 inhabitants; Surface Area: 27.17 km2; Population density: 260 inhabitants/km2; Main Sources: Photochemical activity | 2.40 | 2.46 | Guo et al. 2014 [50] | |
| Center of Monterrey, Mexico (Obispado) 2011–2012 | Urban and Industrialized Area; Population: 1,135,512 inhabitants; Surface Area: 894 km2; Population density: 1170 inhabitants/km2; Main Sources: vehicular traffic, fuel leaks and solvent usage | 1.59 a | 16–42 b | 9–27 b | Menchaca-Torre et al. 2015 [42,43] a, b |
| Monterrey, Nuevo Leon, México 2013 | Urban and Industrialized Area; Population: 1,135,512 inhabitants; Surface Area: 894 km2; Population density: 1170 inhabitants/km2; Main Sources: vehicular traffic, fuel leaks and solvent usage | 21.07 (autumn) | Cerón-Bretón et al. 2015 [51] | ||
| Chengdu, China 2010–2011 | Urban and Industrialized Area; Population: 14,430,000 inhabitants; Surface Area: 14,378 km2; Population density: 11,388 inhabitants/km2; Main Sources: anthropogenic emissions and photochemical reactions | 0.52–21.86 | 0.32–17.33 | Ho et al. 2015 [49] |
| Meteorological Conditions | Summer 2011 | Autumn 2011 | Winter 2011–2012 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B1 | B2 | B3 | B1 | B2 | B3 | |
| Temperature (°C) | 28.9 | 33.7 | 29.6 | 22.1 | 26.3 | 22.8 | 13.07 | 19.26 | 14.3 |
| Relative Humidity (%) | 61.7 | 37.8 | 35.72 | 54.3 | 36.9 | 49.41 | 48.9 | 35.12 | 34.93 |
| Solar Radiation (kW m−2) | 0.9 | 1.3 | 0.7 | 0.72 | 0.84 | 0.63 | 0.4 | 0.48 | 0.18 |
| Wind Direction | NNE | NNE | ENE | NW | NNE | ENE | E | NNE | ENE |
| Wind Speed (km h−1) | 6.8 | 11.1 | 9 | 5.4 | 9.7 | 9 | 5.6 | 8.5 | 8 |
| Air Criteria Pollutants Average Concentrations | Summer 2011 | Autumn 2011 | Winter 2011–2012 | ||||||
| B1 | B2 | B3 | B1 | B2 | B3 | B1 | B2 | B3 | |
| CO (ppm) | 0.62 | 1.52 | 1.53 | 1.26 | 0.90 | 0.99 | 1.13 | 0.79 | 0.78 |
| NOx (ppb) | 11 | 14 | 14 | 38 | 20 | 22 | 74 | 29 | 27 |
| O3 (ppb) | 14 | 52 | 48 | 22 | 35 | 33 | 17 | 34 | 42 |
| SO2 (ppb) | 3 | 4 | 4 | 5 | 5 | 5 | 3 | 6 | 7 |
| NO (ppb) | 4 | 4 | 5 | 20 | 8 | 7 | 51 | 10 | 7 |
| NO2 (ppb) | 8 | 9 | 10 | 19 | 13 | 15 | 24 | 19 | 20 |
| Formaldehyde/Acetaldehyde Concentration Ratios | |||||||
|---|---|---|---|---|---|---|---|
| Climatic Season | Morning Sampling (B1) | Midday Sampling (B2) | Afternoon Sampling (B3) | ||||
| Summer | |||||||
| Range | Mean | Range | Mean | Range | Mean | Range | Mean |
| 0.25–2.79 | 0.96 | 0.54–2.79 | 1.13 | 0.25–1.41 | 0.88 | 0.25–1.5 | 0.92 |
| Autumn | |||||||
| Range | Mean | Range | Mean | Range | Mean | Range | Mean |
| 0.9–2.85 | 1.72 | 1.0–3.35 | 1.94 | 0.94–2.85 | 1.60 | 0.9–2.85 | 1.62 |
| Winter | |||||||
| Range | Mean | Range | Mean | Range | Mean | Range | Mean |
| 0.5–6.15 | 2.26 | 0.7–5.82 | 2.51 | 0.7–6.13 | 2.13 | 0.57–6.15 | 2.06 |
| Mean values | 0.5–5.82 | 1.86 | 0.2–6.13 | 1.54 | 0.2–6.15 | 1.53 | |
| Summer 2011 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | CO | NOx | O3 | SO2 | NO | NO2 | SR | RH | BZ | FOR | ACE | WDIR | TEMP |
| CO | 1 | ||||||||||||
| NOx | 1.000 | 1 | |||||||||||
| O3 | −0.966 | −0.963 | 1 | ||||||||||
| SO2 | 0.416 | 0.406 | −0.638 | 1 | |||||||||
| NO | 0.975 | 0.972 | −0.999 | 0.608 | 1 | ||||||||
| NO2 | 1.000 | 0.999 | −0.972 | 0.439 | 0.980 | 1 | |||||||
| SR | 0.449 | 0.458 | −0.201 | −0.626 | 0.238 | 0.426 | 1 | ||||||
| RH | 0.948 | 0.952 | −0.834 | 0.106 | 0.854 | 0.940 | 0.709 | 1 | |||||
| BZ | 0.876 | 0.881 | −0.721 | −0.075 | 0.746 | 0.863 | 0.824 | 0.984 | 1 | ||||
| FOR | 0.169 | 0.182 | 0.298 | −0.034 | 0.056 | 0.233 | −0.421 | −0.155 | 0.055 | 1 | |||
| ACE | −0.506 | −0.156 | −0.063 | −0.261 | −0.190 | −0.111 | −0.224 | 0.054 | 0.062 | 0.472 | 1 | ||
| WDIR | 0.177 | 0.338 | −0.235 | −0.250 | 0.476 | 0.193 | 0.126 | 0.422 | 0.126 | −0.078 | 0.154 | 1 | |
| TEMP | −0.293 | −0.178 | 0.401 | 0.213 | −0.537 | −0.027 | 0.179 | −0.760 | 0.178 | 0.124 | 0.172 | −0.537 | 1 |
| Autumn 2011 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | CO | NOx | O3 | SO2 | NO | NO2 | SR | RH | BZ | FOR | ACE | WDIR | TEMP |
| CO | 1 | ||||||||||||
| NOx | 0.983 | 1 | |||||||||||
| O3 | −0.994 | −0.997 | 1 | ||||||||||
| SO2 | −0.271 | −0.089 | 0.168 | 1 | |||||||||
| NO | 0.956 | 0.994 | −0.982 | 0.023 | 1 | ||||||||
| NO2 | 0.997 | 0.967 | −0.984 | −0.339 | 0.933 | 1 | |||||||
| SR | −0.129 | 0.056 | 0.023 | 0.096 | 0.167 | −0.199 | 1 | ||||||
| RH | 0.932 | 0.983 | −0.965 | −0.989 | 0.997 | 0.904 | 0.239 | 1 | |||||
| BZ | 0.946 | 0.989 | −0.975 | 0.056 | 0.999 | 0.920 | 0.200 | 0.999 | 1 | ||||
| FOR | 0.960 | −0.292 | 0.419 | −0.250 | −0.274 | −0.321 | 0.858 | 0.178 | 0.311 | 1 | |||
| ACE | 0.619 | −0.369 | 0.531 | −0.288 | −0.377 | −0.443 | 0.728 | 0.127 | 0.319 | 0.941 | 1 | ||
| WDIR | −0.049 | 0.330 | −0.502 | 0.142 | 0.355 | 0.187 | −0.017 | 0.091 | −0.015 | −0.100 | −0.298 | 1 | |
| TEMP | 0.555 | −0.354 | 0.661 | −0.188 | −0.344 | −0.344 | 0.719 | −0.128 | 0.650 | 0.702 | 0.799 | −0.298 | 1 |
| WINTER 2011 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | CO | NOx | O3 | SO2 | NO | NO2 | SR | RH | BZ | FOR | ACE | WDIR | TEMP |
| CO | 1 | ||||||||||||
| NOx | 1.000 | 1 | |||||||||||
| O3 | −0.935 | −0.944 | 1 | ||||||||||
| SO2 | 0.041 | 0.012 | 0.317 | 1 | |||||||||
| NO | 0.998 | 0.999 | −0.954 | −0.019 | 1 | ||||||||
| NO2 | 0.998 | 0.996 | −0.909 | 0.106 | 0.992 | 1 | |||||||
| SR | 0.233 | 0.260 | −0.563 | −0.962 | 0.291 | 0.169 | 1 | ||||||
| RH | 1.000 | 1.000 | −0.939 | 0.028 | 0.999 | 0.997 | 0.246 | 1 | |||||
| BZ | 0.983 | 0.988 | −0.984 | −0.141 | 0.993 | 0.970 | 0.405 | 0.986 | 1 | ||||
| FOR | 0.258 | 0.230 | 0.284 | 0.350 | 0.189 | 0.350 | −0.003 | 0.022 | 0.650 | 1 | |||
| ACE | −0.124 | −0.115 | −0.014 | −0.133 | −0.106 | −0.133 | 0.100 | 0.395 | 0.500 | −0.118 | 1 | ||
| WDIR | −0.099 | −0.041 | −0.333 | −0.298 | 0.026 | −0.298 | 0.347 | 0.299 | 0.025 | 0.347 | 0.028 | 1 | |
| TEMP | 0.225 | 0.118 | 0.745 | 0.524 | 0.010 | 0.524 | 0.440 | −0.643 | 0.400 | 0.421 | −0.170 | 0.513 | 1 |
| Climatic Season | Summer 2011 | Autumn 2011 | Winter 2011–2012 | ||||
|---|---|---|---|---|---|---|---|
| Variables/PCs | PC1 | PC2 | PC1 | PC2 | PC3 | PC1 | PC2 |
| T | - | - | - | 0.834 | - | - | |
| CO | 0.989 | - | 0.942 | - | - | 0.983 | - |
| NOX | 0.990 | - | 0.988 | - | - | 0.988 | - |
| O3 | −0.917 | - | −0.973 | - | - | −0.984 | - |
| SO2 | - | 0.961 | - | 0.988 | - | - | 0.989 |
| NO | 0.931 | - | 0.999 | - | - | 0.992 | - |
| NO2 | 0.985 | - | 0.916 | - | - | 0.968 | - |
| SR | 0.576 | −0.410 | - | - | 0.771 | - | - |
| RH | - | - | −0.978 | - | - | - | |
| BZ | 0.938 | - | 1.000 | - | - | 1.000 | - |
| FOR | 0.837 | - | - | - | 0.809 | 0.635 | - |
| ACE | 0.525 | - | - | - | 0.896 | 0.587 | - |
| WDIR | - | - | - | - | - | - | - |
| Estimated Values for Exposure, Associated Non-Cancer Hazard, and Cancer Risk | ||
|---|---|---|
| Pollutant | Yearly Average Concentration (mg m−3) | E: Daily Average Exposure (mg/kg per Day) |
| Formaldehyde | 9.06 × 10−3 | Adult: 2.77 × 10−3 Children: 2.91 × 10−3 |
| Acetaldehyde | 8.068 × 10−3 | Adult: 2.47 × 10−3 Children: 4.67 × 10−3 |
| Benzene | 0.242 × 10−3 | Adult: 7.42 × 10−5 Children: 1.4 × 10−4 |
| Study Site | HQ | ILTCR | Author | ||||
|---|---|---|---|---|---|---|---|
| San Nicolas de los Garza, Nuevo Leon, Mexico | Formaldehyde | Acetaldehyde | Benzene | Formaldehyde | Acetaldehyde | Benzene | This study |
| 0.9216 | 0.8964 | 0.008 | Adult: 5.82 × 10−5 Children: 6.11 × 10−5 | Adult: 2.47 × 10−5 Children: 4.67 × 10−5 | Adult: 2.14 × 10−6 Children: 4.05 × 10−6 | ||
| Beijing, China (an urban site) | 3.30 | 8.34 | 1.57 | 9.11 × 10−5 | 1.84 × 10−5 | 4.19 × 10−5 | Zhang et al. 2012 [70] |
| Bangkok, Thailand (gasoline station) | 0.16 | 0.05 | - | 1.14 × 10−5 | 1.60 × 10−6 | - | Tunsaringkarn et al. 2012 [71] |
| Kolkata, India (a heavy traffic site) | 0.821 | 0.571 | 0.776 | 2.14 × 10−5 | 2.26 × 10−6 | 3.63 × 10−5 | Dutta et al. 2009 [72] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerón Bretón, J.G.; Cerón Bretón, R.M.; Kahl, J.D.W.; Lara-Severino, R.d.C.; Ramírez Lara, E.; Espinosa Fuentes, M.d.l.L.; Rangel Marrón, M.; Uc Chi, M.P. Atmospheric Levels of Benzene and C1-C2 Carbonyls in San Nicolas de los Garza, Nuevo Leon, Mexico: Source Implications and Health Risk. Atmosphere 2017, 8, 196. https://doi.org/10.3390/atmos8100196
Cerón Bretón JG, Cerón Bretón RM, Kahl JDW, Lara-Severino RdC, Ramírez Lara E, Espinosa Fuentes MdlL, Rangel Marrón M, Uc Chi MP. Atmospheric Levels of Benzene and C1-C2 Carbonyls in San Nicolas de los Garza, Nuevo Leon, Mexico: Source Implications and Health Risk. Atmosphere. 2017; 8(10):196. https://doi.org/10.3390/atmos8100196
Chicago/Turabian StyleCerón Bretón, Julia Griselda, Rosa María Cerón Bretón, Jonathan D.W. Kahl, Reyna del Carmen Lara-Severino, Evangelina Ramírez Lara, María de la Luz Espinosa Fuentes, Marcela Rangel Marrón, and Martha Patricia Uc Chi. 2017. "Atmospheric Levels of Benzene and C1-C2 Carbonyls in San Nicolas de los Garza, Nuevo Leon, Mexico: Source Implications and Health Risk" Atmosphere 8, no. 10: 196. https://doi.org/10.3390/atmos8100196





