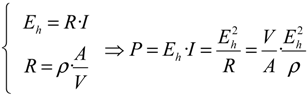1. Introduction
Appliances that electrolyze drinking water for domestic use have been available for several years. These electrolysis machines produce acidic water (EOAW, for Electrolyzed Oxidized Acidic Water) and alkaline water, also sometimes known wrongly as ionized water (ERAW, Electrolyzed Reduced Alkaline Water). Other names for ERAW include: electrolyzed reduced water, Alkali-ionsui water or electrolyzed cathodic water. Acidic water is unsuitable for human consumption but is appropriate for personal body care and hygiene. Alkaline water on the other hand is drinkable and recommended in the commercial and marketing literature as being beneficial in the treatment of gastro-intestinal problems, hypertension, diabetes and cancer.
Originally designed in Japan, water ionizers have become an important and ever growing industry within the country. Numerous manufacturers have grouped together forming an organization called the Association of Alkaline Ionized Water Apparatus. In Japan, the Ministry of Health, Labour and Welfare support this association, but also request a guarantee concerning the security and quality of water produced by water ionizers. Such appliances imported into Europe have obtained a CE (European Commission) label but are not at present considered as medical devices and thus do not have to comply with the strict policies imposed by the health authorities. Today, anybody tempted by the apparent benefits of ERAW described in commercial adverts can easily acquire a water ionizer (1000 €–3000 €). Furthermore, according to the advice contained in the instruction leaflets that accompany the appliance, it is recommended to drink 1.5–2.0 L of water regardless of age, sex and state of health. Perhaps drinking ionized water can be of benefit if it really does contribute to relieving symptoms of patients, supplementing classical treatments, as certain distributers claim. However, it could also become a public health problem if it provokes harmful secondary effects in otherwise healthy beings and even hides the existence of an illness in consumers who are in apparently good health. This report aims to evaluate the issues concerned with the unregulated consumption of ERAW in the absence of medical advice, in order to warn the public authorities against the potential risks involved.
This report presents first the measures taken by the Japanese health authorities concerning the commercialisation of water ionizers and intake of ERAW in response ultimately to a clinical evaluation conducted by academics but limited to the treatment of gastro-intestinal complaints. Next, this report considers the overall scientific results published by research laboratories and teaching clinics mainly from Japan concerning the physico-chemical, biological and therapeutic properties of ERAW, and which, are often exploited by companies that distribute water ionizers in order to increase sales. This report will not deal with EOAW that is used as an antiseptic in many hospitals and hence does not pose the same health risk questions.
2. Industrial Development of Water Ionizers for Domestic Use in Japan and the Administrative Requirements for their Distribution and Use
More than fifty years ago, based on Russian studies performed around 1920, Japanese scientists successfully developed a water-based process involving an electrolysis procedure that consisted of an anode and cathode compartment separated by a membrane permeable to ions, thus allowing separation and recuperation of the individual contents. It was Machisu Suwa, who in collaboration with the Synnhol Electronic Machinery Company, developed the first electrolysis equipment for producing alkaline ionized water called Synnhol Liquid. Subsequently, in collaboration with Professor Akiba from Tokyo University, a Synnhol Electronic Agricultural Machine was constructed for agricultural applications (especially rice cultivation) and livestock farming. In 1950, the Synnhol Medical Science Research Association was created to encourage technology transfer of agriculture applications to the medical sector and the development of water ionizers for domestic use. The resulting Synnohl Electronic Manufacturing Apparatus was approved in accordance with the Pharmaceutical Affairs Law [
1] for the effective treatment of stomach problems, provided that the final pH and calcium concentrations match the mineral content of tap water that varies in different regions of Japan. The use of weakly acid oxidizing water and astringents was reserved for personal hygiene and body care cleaning. From then on, the water for consumption was called Alkaline Ionized Water.
Nevertheless, strong criticism was expressed questioning the validity of a practice that had not been subjected to controlled clinical assessment and for which the long-term impacts were unknown. Furthermore, scientists challenged this designation of ionized water as inappropriate and being what drinking water is naturally, but without interference. For those reasons, in 1993 the Ministry of Health, Labour and Welfare requested the Association of Alkaline Ionized Water Apparatus to provide scientific data guarantying the quality, efficiency and security of ionized water. This task was undertaken by Professor Itokawa of the Medical Department of Kyoto University who led a review committee and released a public report in 1994 confirming the safety of ionised water. This was followed in 1997 by a further report confirming its effectiveness in the treatment of gastro-intestinal illness.
In 1999, at the 25th General Congress of the Japanese Association of Medical Sciences, the report was presented under the title Electrolytic Functional Water in Medical Treatment. It outlined the conclusions of the first ever double-blind clinical tests establishing that ionized water is effective and useful in the treatment of gastro-intestinal illnesses. In 2005, the legislation concerning pharmaceutical sales was revised, qualifying water ionizers as medical equipment approved for domestic use. The Ministry of Health, Labour and Welfare notified (Notification N° 112) that appliances for the generation of alkaline electrolyzed drinking water are useful for improving gastro-intestinal symptoms such as chronic diarrhea, indigestion, abnormal intestinal fermentation activity and stomach acidity [
2].
3. Materials and Methods
In order to quantify the changes in water properties we have recorded as a function of time some physico-chemical parameters before and after electrolysis. Measurements were carried out with a Tae Young model Rettin TY-2505 W known as OSIBA and distributed by Amino-Corp. The machine was equipped with its original prefilters of 0.1 µm given that filtration at 0.01 µm can slightly change the properties of ERAW water produced. The equipment was connected to Erstein (F Bas-Rhin) town water supply that provides a pressure of 5 bars; the flow rate over the ionizer was controlled at 1.2 L min
−1. Temperature, pH, redox potentials
Eh and resistivity ρ of water samples have been measured using standard electrodes. As we are interested in characterizing the anti-oxidative power of a watery medium independently from its
pH-value, it is mandatory to use a mathematical transformation involving the following half-cell reaction occurring in a normal hydrogen electrode (NHE):
Let us introduce chemical potentials µ = µ0 + kBT·ln a; where kB = 1.38 × 10−23 J·K−1 is Boltzmann’s constant; T the absolute temperature in Kelvin and a the thermodynamic activity of the considered chemical substance. Reference states for the solvated proton and the hydrogen gas for the NHE being such that µ(H2) = µ(H+aq) = 0, it comes ∆G0 = µ(H2) − 2µ(H+aq) = kBT·ln a(H2) − 2kBT·ln a(H+aq). Now using Nernst’s law relating free energy changes ∆G° to redox potentials Eh = −∆G0/(n·e), where e = 1.602 × 10−19 C is the elementary charge and introducing pH = −log10 a(H+aq) and rH2 = −log10 a(H2), it comes:
Here,
EB is a constant (
Table 1) that depends on the reference electrode used to measure redox potentials. This origin shift is necessary as the
rH2 parameter measuring the activity of electrons in an aqueous solution was defined relative to the NHE.
Table 1.
EB constant (mV) that should be added to the measured redox potential Eh according to the reference electrode in order to get a value referenced relative to a normal hydrogen electrode.
Table 1.
EB constant (mV) that should be added to the measured redox potential Eh according to the reference electrode in order to get a value referenced relative to a normal hydrogen electrode.
| T/°C | Hg/Hg2Cl2/KCl
saturated | Ag/AgCl/KCl
1 M | Ag/AgCl/KCl
3 M | Ag/AgCl/KCl
3.5 M | Ag/AgCl/KCl
saturated |
|---|
| 5 | 257 | 247 | 221 | 219 | 216 |
| 10 | 254 | 244 | 217 | 215 | 212 |
| 15 | 251 | 242 | 214 | 212 | 207 |
| 20 | 248 | 239 | 211 | 208 | 202 |
| 25 | 244 | 236 | 207 | 204 | 197 |
| 30 | 241 | 233 | 203 | 200 | 192 |
The usefulness of the
rH2-transformation comes from the fact that
rH2 values constitute a good pH-independent measure of the anti-oxidative ability of a given medium. The
rH2 value characterizing pure water may readily be obtained by considering water auto-electrolysis:
For this equilibrium, one may write:
Knowing that for pure water ∆G0 (25 °C, 1 atm) = 474.2 kJ·mol−1 and that a(H2) = 2a(O2) with aW ≈ 1 (dilute solutions), it comes: KE = 8.3 × 10−84 and a(H2) = (2KE)1/3 = 2.6 × 10−28, i.e., rH2 = −log10 a(H2) = 27.6. Redox neutrality is thus observed at rH2 = 28 with rH2 = 0 for the most reducing medium characterized by a(H2) = 1, and rH2 = 42 for the most oxidizing medium characterized by a(O2) = 1, i.e., a(H2) = (KE)1/2. It follows from these considerations that a medium such that 0 < rH2 < 28 has some reductive or anti-oxidant power while a medium with 28 < rH2 < 42 has an oxidative power. It is worth stressing that the rH2 notion is just a mathematical transformation of the redox potential expressing, relative to a hypothetical reference state (H2), the reducing power of an aqueous solution. In particular, it should be clear that a low rH2 value means that some reducing chemical species that are yet to be clearly identified are present in solution. This could be of course molecular dihydrogen gas as in water electrolysis, but also any other compound able to change the redox properties of water.
Another possibility to express water quality is to consider an electrochemical power
P computed according to the following relationship:
Here,
V is the volume of the measurement cell;
A the area of the electrodes in contact with the aqueous solution;
R the electrical resistance of the measuring cell having a resistivity
ρ and crossed by an electrical current of intensity
I. Owing to the relationship between pH and
rH2, it becomes:
For a standard cell characterized by a volume
V = 1 cm
3, equipped with electrodes having an area
A = 1 cm
2, at
T = 25 °C (
i.e.,
uT ≈ 59 mV) one may use the following practical relationship:
This last parameter measures the dissipative power of a given watery medium.
4. Different Types of Alkaline Ionized Water Apparatus and Characteristics of the Electrolyzed Water Produced
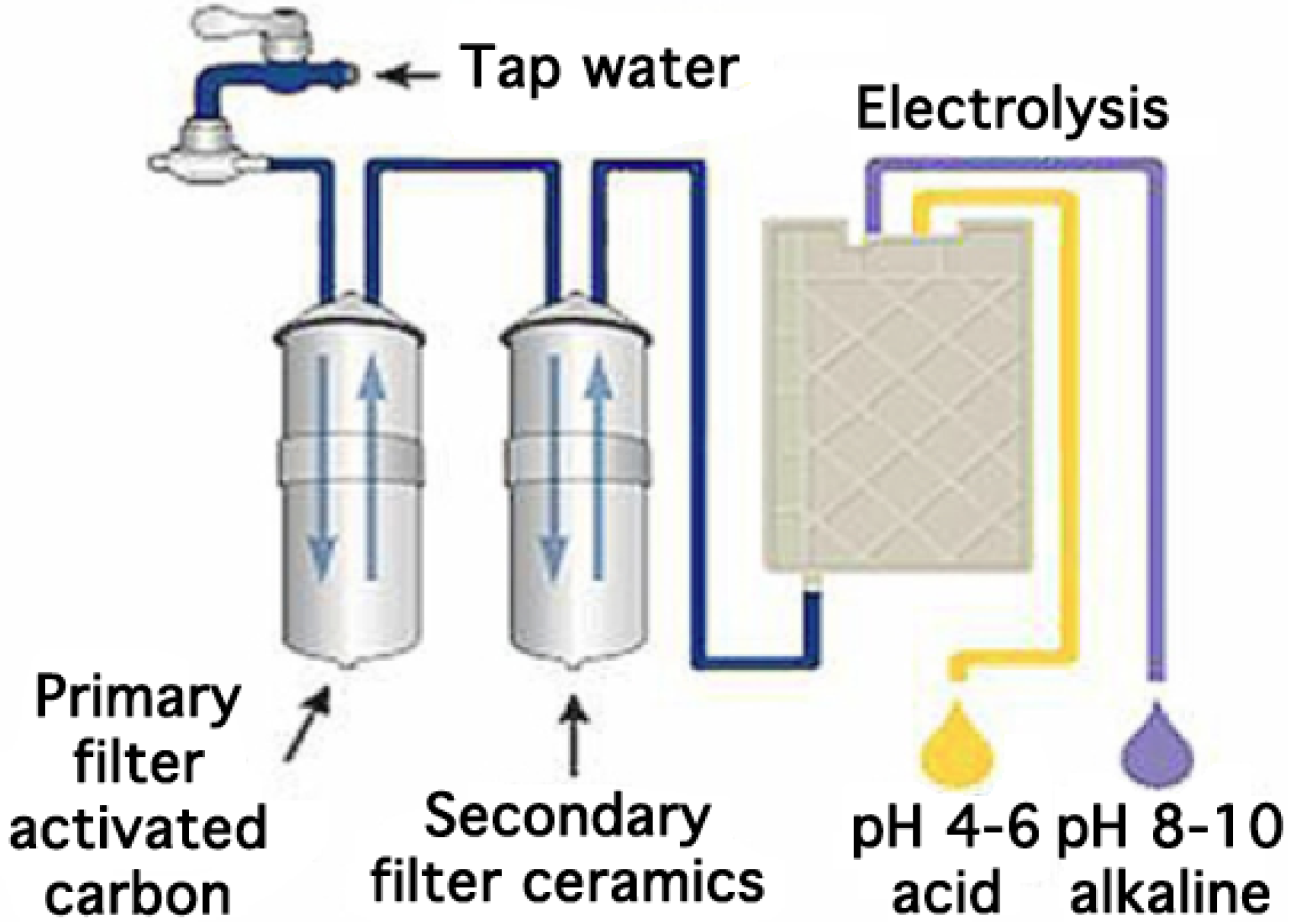
Today, many kinds of more or less sophisticated ionized water generators exist on the market (
Figure 1). Most are manufactured in Japan but more recently the industry has also developed similar products in the United States, Canada and Australia. On average, over 200,000 appliances are sold each year worldwide for a price varying between 600 € and 3000 €. All water ionizers connect to the mains water supply. The water is filtered over at least one activated carbon filter, essential for reducing the chloride level of the tap water and thus avoiding damage to the electrolytic cell (
Figure 2).
The filtered water must have a minimal mineral content of around 50 mg·L
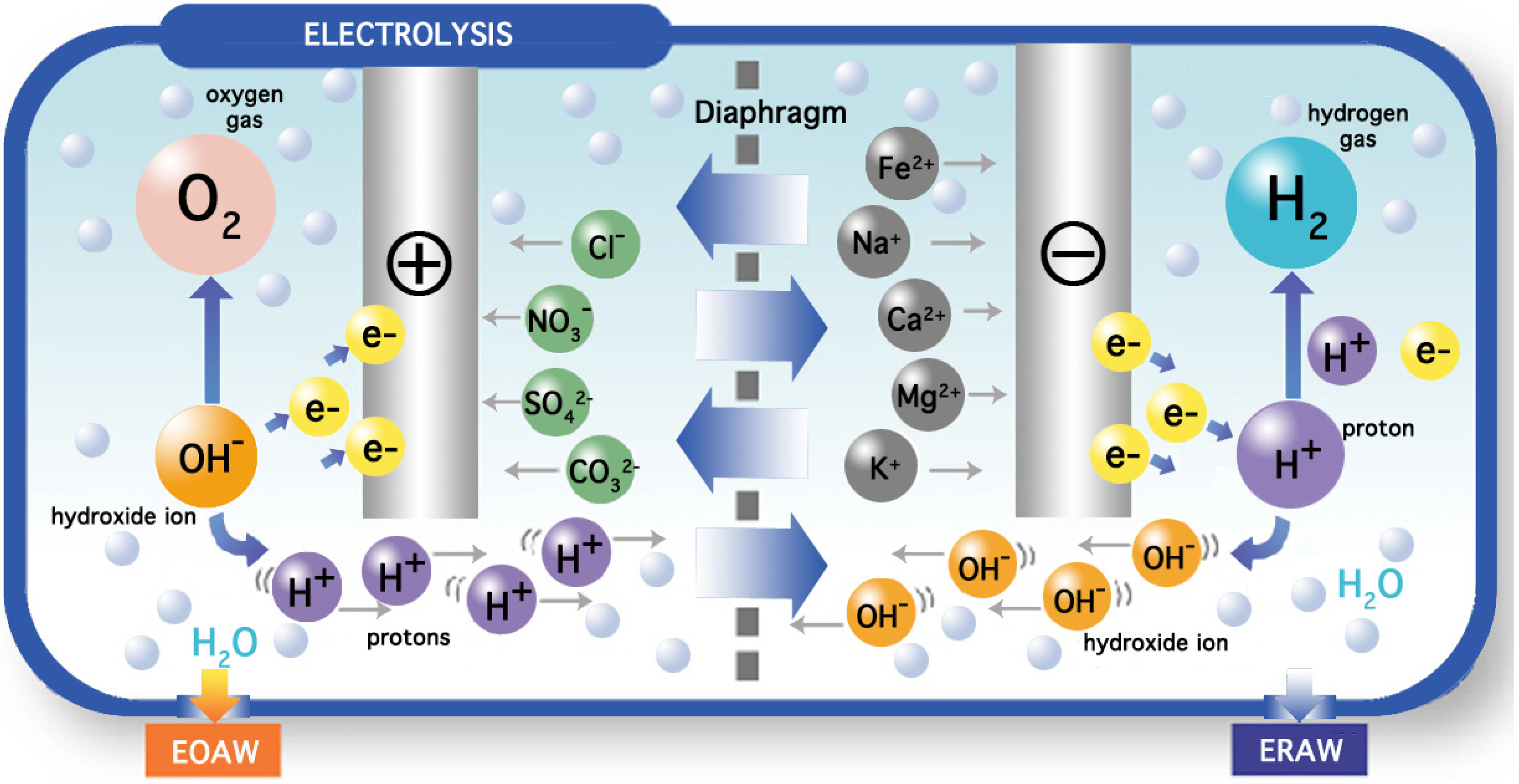
−1 in order to undergo electrolysis in a chamber consisting of an anode and a cathode separated by a semi-permeable diaphragm made of plastic. The flat or mesh-shaped electrodes are made of titanium covered with platinum. The process of electrolysis produces acid and oxidized water at the anode according to the following reaction:
As electrons flow through the electric circuit, the anode compartment accumulates mineral ions (HCO
3−, Cl
−, HSO
4−, NO
3−, …) and as protons and oxygen are liberated, the water acquires a
pH between 4 and 6 and a redox potential that could be as high as +900 mV. On the contrary, reduced alkaline water is produced in the cathode compartment owing to the occurrence of the following reaction:
Here, mineral cations (Na
+, K
+, Ca
2+, Mg
2+, …) accumulate at the cathode and as hydroxyl ions and hydrogen are produced, water changes to a
pH between 8 and 10 and a redox potential as low as −600 mV may be obtained (
Figure 3).
Figure 1.
Some electrolyzed water generators available on the market.
Figure 1.
Some electrolyzed water generators available on the market.
Figure 2.
General overview of a commercial electrolyzed water generator.
Figure 2.
General overview of a commercial electrolyzed water generator.
Figure 3.
General overview of a commercial electrolyzed water generator.
Figure 3.
General overview of a commercial electrolyzed water generator.
The efficiency of the apparatus and the
pH and redox potential values obtained vary greatly, depending on the characteristics of the local water supply, the voltage and current values, water flow rate and temperature.
Table 2 summarizes the results obtained before and after electrolysis, as a function of the three adjustable settings that control alkalinity and two settings that regulate acid, available on any typical machine. One may notice that the main effect of electrolysis is a significant reduction in the
rH2 value of ERAW compared to the original tap water, while the
pH and resistance potential values remain relatively stable. A comparison of these
rH2 values with those of mineral water shows that ionized water is extremely alkaline. Compared to drinking water sources, ERAW has
pH and
Eh values that are rarely encountered in a natural environment. This water should normally therefore be classed in the same category as synthetic waters. With regard to ERAW resistance, one can find a range from 1600 to 1700 Ω·cm that is stable over time and remains within the tolerated official norm of 900–5000 Ω·cm. In terms of
pH, this can range can vary from 6.8 to 8.7 for this type of equipment and also remains relatively stable over time. In comparison, the regulatory standard recommends a
pH between 6.5 and 9 for drinking water. The ionization procedure does not therefore result in abnormal
pH values. Concerning redox potential, values are within the range of −654 to +680 mV with a strong variation over time for negative reduction potentials and a better stability for positive reduction potentials. After correcting for the effect of
pH, the
rH2 (electronic activity) is found to be between 2 and 45, meaning that this type of apparatus can effectively produce water that is extremely oxidising or on the contrary, extremely anti-oxidising.
Table 2.
Physicochemical properties of three types of reduced alkaline electrolyzed water (ERAW) and two types of oxidized acidic electrolyzed water (EOAW). t: duration of electrolysis; Eh: redox potential; r: electrical resistance; P: electrochemical power.
Table 2.
Physicochemical properties of three types of reduced alkaline electrolyzed water (ERAW) and two types of oxidized acidic electrolyzed water (EOAW). t: duration of electrolysis; Eh: redox potential; r: electrical resistance; P: electrochemical power.
| Water | t/min | T/°C | pH | Eh/mV | rH2 | ρ/Ω·cm | P/µW |
|---|
| Start | 0 | 14.6 | 7.26 | +446 | 37.4 | 1684 | 253 |
| Filtered | 0 | 15.7 | 7.28 | +468 | 38.1 | 1651 | 275 |
| ERAW #1 | 10 | 16.3 | 7.70 | −587 | 2.1 | 1682 | 87 |
| ERAW #2 | 10 | 16.4 | 8.24 | −640 | 1.2 | 1690 | 114 |
| ERAW #3 | 10 | 16.7 | 8.73 | −682 | 0.9 | 1725 | 131 |
| EOAW #1 | 10 | 15.5 | 6.89 | +99 | 24.5 | 1692 | 56 |
| EOAW #2 | 10 | 16.5 | 6.71 | +552 | 39.9 | 1654 | 350 |
Since there is no official standard concerning the redox potential or the rH2 of drinking water, one is faced with a situation of great uncertainty. In the natural environment, the most alkaline and anti-oxidising water known is found in a slate mine cave known as Brandholz at Nordenau in Germany with the following characteristics: T = 8–10 °C. pH = 8.01, Eh = −250 mV (rH2 = 14.7) corresponding to an electrochemical power of P = 0.003 µW. Water with similar attributes has also been discovered at Hita Tenryosui in Japan, Tlacote in Mexico and Nadone in India, but is nevertheless of extremely rare occurrence in nature. All of these places are frequented by thousands of people hoping to heal their ailments by hydrotherapy. Biological fluids such as blood, saliva or urine are another example where natural redox potentials span the range −50 to +150 mV, corresponding to a rH2 somewhere between 20 and 26. It is clear however that some of the electrolyzed water produced is completely abnormal biologically, justifying a prerequisite clinical evaluation before any therapeutic usage. The problem is of course the instability of the ERAW over time that must be consumed in the hour following production in order to conserve the redox potential or rH2 levels. A further important concern is that water with a starting pH of 8.24 and a rH2 of 2.9 at T = 17.7 °C will, if the temperature is raised to 22.4 °C on a radiator for example, have an increased rH2 up to 16.9 without any tangible change in pH (8.23). It is very likely therefore that ERAW consumed and heated at human body temperature has a redox potential that will become positive once again.
Likewise, there is no officially accepted norm concerning the electrochemical power
P that can also be extremely variable. Yet, this is the most interesting parameter without any doubt, as it combines three main properties of water, namely acid/alkaline, oxidising/reducing and overall mineral content. The electrochemical power of electrolyzed water can vary from zero to 480 µW, which shows that it is possible with one machine to produce ionized water with an electrochemical power similar to most commercial natural mineral waters, apart from St-Yorre (
Table 3). Note, however, that contrary to mineral water, the electrochemical power is not stable over time and that water with oxidising or reducing properties can have equivalent electrochemical power. From a chemical perspective, ERAW contains the same basic dissolved mineral substances as the starting water used at the outset and a variable amount of dissolved dihydrogen whose concentration depends on the electrolysis conditions and platinum nanoparticles (PtNPs) resulting from degradation of the electrodes, the concentration of which depends on the length of use of the electrodes.
Table 3.
Physicochemical characteristics of some French mineral water at T = 20 °C (RO = reverse osmosis).
Table 3.
Physicochemical characteristics of some French mineral water at T = 20 °C (RO = reverse osmosis).
| Water | resistivity/Ω·cm | pH | rH2 | P/µW |
|---|
| RO water | 30,000 | 6.6 | 22.0 | 2 |
| Mont Roucous | 43,500 | 6.0 | 26.3 | 4 |
| Rosée de la reine | 28,100 | 5.2 | 28.0 | 9 |
| Montcalm | 22,300 | 5.6 | 28.4 | 11 |
| Volvic | 6,000 | 7.0 | 29.6 | 34 |
| Evian | 1,700 | 7.4 | 26.5 | 68 |
| Perrier | 1,370 | 5.5 | 24.3 | 109 |
| Vittel | 825 | 7.6 | 26.9 | 140 |
| Badoit | 519 | 5.9 | 24.9 | 280 |
| Contrex | 431 | 7.2 | 26.6 | 292 |
| Hépar | 383 | 7.3 | 30.0 | 524 |
| St-Yorre | 144 | 6.4 | 25.0 | 874 |
5. Anti-Oxidizing Properties of ERAW
Dr Hidemitsu Hayashi (Director of the Water Institute of Japan) bought an electrolysis apparatus for the Kyowa Medical Clinic as early as 1985. ERAW was subsequently used on a regular basis for the preparation of meals and as drinking water for the patients. He observed a clear amelioration in patients suffering from gastro-intestinal disorders and even a number of other pathologies including diabetes, gout, liver cirrhosis, hepatitis, hypertension and malignant tumours of the liver. To explain these improvements, he proposed a theory in 1995 that ERAW exerts it healing properties primarily due to the presence of activated dihydrogen (H
2) that efficiently scavenges reactive oxygen species (ROS) rather than due to the water’s alkalinity or redox potential [
3]. Even though this hypothesis is flawed by the fact that dehydrogenase enzymes do not exist in the human body and only in bacteria, the claims of H. Hayashi enabled:
- (i)
Development of a cheap alternative method to the water ionizer machine for producing dihydrogen-enriched water displaying alkaline and reducing properties. The method relies on an electrochemical reaction between water and magnesium rods: Mg + 2 H2O → Mg(OH)2 + H2 resulting in what is known as Magnesium Stick Water (MSW);
- (ii)
Stimulated interest in the curative properties of natural reducing waters found in deep underground sites such as at Nordenau in Germany (NA water) or Hita Tenryosui (HT water) or Mount Fuji (IF water) in Japan [
4];
- (iii)
Triggered scientific teams to do more fundamental research in order to understand the physicochemical action and mechanisms behind the reducing properties of electrolysed water.
The first
in vitro research experiments by Shirahata and his group in 1997 demonstrated the ability of ERAW, produced by commercial apparatus (Nihon Trim Co, Osaka, Japan), to destroy reactive oxygen species such as superoxide radicals (O
2−•) and hydrogen peroxide (H
2O
2), similar to the action of superoxide dismutase (SOD) and catalase (CAT) enzymes. In connection with a study of DNA degradation by free radicals, they also indirectly observed elimination of the more aggressive hydroxyl radicals [
5]. Except for the effect upon hydroxyl radicals, these results were later confirmed by other ERAW produced by an apparatus built in the laboratory using water supplemented with 2 mM NaCl [
6,
7]. These types of reactions similar to SOD and CAT enzymes are only possible if dissolved hydrogen is present and activated in its atomic form. For this reason, Shirahata and colleagues proposed that the active hydrogen was stabilized by PtNPs, released through degradation of the electrodes [
8]. To study the validity of this hypothesis that is often used as a commercial argument, an extensive research using an ERAW produced by a commercial apparatus Nihon Trim Co, Osaka, Japan from tap water filtered over ion exchange resin came to the following conclusions [
9]:
- (1)
No trace of platinum was detected in any of the diverse samples studied. In contrast, ionized water prepared from IF or HY water contained vanadium ions;
- (2)
Anti-oxidant activity of ERAW, MSW, HT (deep Water Hita Tenryosi, Japan) and IF (deep water Mount Fuji, Japan) water measured by the inhibition of the oxidation of biological molecules showed little to no difference compared to molecules such as ascorbic acid, tocopherol or polyphenols;
- (3)
The loss of anti-oxidant activity of ERAW and MSW after boiling shows that an unstable transient H
2 element is involved. The concentration of H
2 varies from around 0.5 mg·L
−1 up to 1.5 mg·L
−1 at saturation, but drops off quickly making necessary that the water should be consumed immediately. A recent study [
10] showed that 50% of the H
2 ingested was exhaled while the body tissues took up 45% where it can exert its anti-oxidising activity. For IF and HT water, only a partial loss in their anti-oxidation properties is observed upon boiling depending on the strength of the reducing properties of vanadium ions that are present;
- (4)
ERAW scavenges oxygen O
2−• radicals exclusively and does not seem to scavenge hydroxyl HO• radicals, contrary to the results of Shirahata
et al. [
5] and Ohsawa
et al. [
11]. This is probably due to insufficient sensitivity of electronic resonance spectrometry for detecting other radicals;
- (5)
Ambiguously, the anti-oxidising properties can be explained by chemical analysis but scientific demonstration of the hypothesis of active hydrogen is lacking, even taking into account that PtNPs could have been eliminated by filtration from the samples.
These diverse results confirming the likelihood that ERAW and water enriched naturally or artificially in H2 can scavenge ROS have pushed a number of scientists to engage in vivo experimental research using cellular and animal models of pathologies in which oxidative stress plays a role, such as diabetes, cancer and kidney failure.
6. Experimental Evidence of the Effects Produced by Administration of ERAW and Hydrogen Enriched Water to Animal Models of Pathological Diseases
6.1. Anti-Diabetic Effects of ERAW
As early as 1997, it was shown that administration of ERAW to rats decreases the concentration of blood glucose and lipid peroxide levels by activating hexokinase [
12]. In 2002, the group of S. Shirahata reported on the anti-diabetic effects of ERAW produced by a Nihon Trim Co water ionizer system in comparison with natural NA or HT spring water, renowned for their reducing activity. The study used hamster pancreatic beta-cell line HIT-T15 treated with alloxan. The study shows that the cytotoxic effects induced by alloxane on the cells were inhibited by the all three types of water [
13].
From a biochemical point of view, these waters seem to activate insulin secretion in cells by increasing their sensitivity to glucose, with natural reduced water (NA and HT) having a stronger effect than ERAW. This study also showed that after heating the different waters in an autoclave at 121 °C, they lost those properties and that commercial mineral water does not have anti-diabetic activity. In 2005, the group further demonstrated that the same types of water as used in the previous study actually protect pancreatic cells from apoptosis induced by alloxane [
14]. The effect of dihydrogen-enriched water generated by Alkalogen magnesium sticks (HDr Co, Busan, Korea) on glycaemia and lipidaemia was also studied in OLETF rats, a model of spontaneous type 2 diabetes [
15]. The results showed that dihydrogen-enriched water stimulated growth and that its ingestion during 32 weeks decreased blood glucose, cholesterol and triglyceride concentrations by 10%–20% in comparison to control rats who drank tap water. Moreover, the levels of glutamine-oxaloacetate transaminase (GOT) and glutamate pyruvate transaminase (GPT) secreted by ischemic myocardial cells significantly decreased by 50% in comparison to control animals. Based on these results, the authors suggested that ERAW has a worthwhile prophylactic effect on coronary complications in diabetes.
6.2. Anti-Cancer Effects of ERAW
In 2001, Komatsu
et al. published that A549 human lung adenocarcinoma cells and HT1080 human fibrosarcoma cells when treated with ERAW showed a lower redox potential resulting in reduced growth rate while normal fibroblast cells TIG-1 were not affected [
16]. Shirahata
et al. also showed that A549 or HeLa cervical carcinomas cells when cultivated in medium prepared from ERAW drastically changed their morphology while TIG-1 morphology remained normal. In general, cancer cells owe their immortality to high telomerase activity and the fact that their telomeres do not shorten. In the above study, although telomerase activity remained high, the telomeres became shorter with each consecutive cell division suggesting that ERAW reduced the telomere binding ability of telomerase resulting in telomere shortening [
17]. Nisikawaa
et al., studied the protective effect of ERAW prepared using a TI-200S electrolysis appliance (Nihon Trim Co, Osaka, Japan) on BALB/c-3T3 cells treated with 3-methyl cholantrene (initiator compound) followed by treatment with phorbol-12-myristate-13-acetate (promoter compound) [
18]. They were able to establish that to inhibit the promoter, it was necessary to add PtNPs to ERAW [≈10 parts per million (ppm)] since ERAW alone strongly stimulated malignant transformation. It seems that the PtNPs are incorporated into endothelial cells within the intestine supporting the hypothesis that intracellular molecular dihydrogen is converted into active hydrogen by the PtNPs.
Without the presence of a catalyser such as platinum, chemistry cannot account for the in vitro activity of H2 upon ROS, with the exception of the hydroxyl radical. Thus, it is also important to follow up these in vivo studies in order to elucidate the mechanism of action of H2 at the tissue level and its possible activation by PtNPs that, furthermore, are themselves susceptible to lead to toxicity problems.
7. Experimental Research Concerning the Biological Effects of NPs
7.1. Study of the Anti-Oxidizing Action of Synthetic NPs Supsensions
PtNPs are veritable hydrogen sponges since a 2 nm particle size can absorb up to 12% of hydrogen atoms per atom of platinum under a partial pressure of H
2 not exceeding 0.1 kPa [
19]. PtNPs exert their reducing properties by transferring electrons to hydrogen peroxide and certain free radicals like 2.2ʹ-diphenyl-1-picrylhydrazyl and 2.6-dichlorophenol indophenol [
20]. PtNPs are also capable of eliminating superoxide and hydroxyl radicals [
21]. PtNPs size can vary from 1 to 5 nm with the smallest particles being the most reactive towards superoxide ions [
22]. At a dose of 50 mg
−1, no toxicity is apparent in cultured HeLa cells, and in light of this, is therefore being considered as a new type of anti-oxidant.
Most
in vivo research concerning anti-oxidant activity of PtNPs has primarily been carried out in connection with senescence. Senescence is characterized by a degradation of cellular material due to the production of ROS that augments with age because repair processes diminish [
23]. The free-living model nematode
Caenorhabtidis elegans and strains developed for gerontogenomic studies have been recognised as particularly suitable for assessing the oxidative stress effects on the life span [
24]. In 2008, Kim
et al. showed that a reduction in life span after exposure of the nematode Caenorhabtidis elegans to paraquat, a free radical inducing oxidative stress, happens only at a NP concentration of exactly 0.5 mM (neither more nor less). An increase in life span of nematodes caused by treatment with salen-manganese that stimulates superoxide dismutase and catalase is further reinforced by the presence of NPs [
25]. By fixing the NPs to a peptide sequence that has high affinity for platinum and further linked to a peptide sequence derived from HIV-1TAT and allowing it to pass through the cellular membrane and penetrate cells, the cellular internalization of NPs was increased and the effective dose producing the same effects was now 5 µM, which is 100 times lower [
26].
7.2. Anti-Oxidant Activity of ERAW Contaminated by NPs during the Process of Preparing Electrolyzed Water
The group of Shirahata studied the activity of ERAW on the nematode
Caenorhabtidis elegans [
27]. The authors showed that worms cultivated in medium prepared from ERAW had an extended lifespan and that this protects against the life-shortening effect of paraquat. There are two components of ERAW that can be imputated to explain these results, either dissolved H
2 or the small amounts of PtNPs. Although the concentration of H
2 is maximally 0.9 ppm (0.9 mg·L
−1) at the end of electrolysis, it rapidly decreases to less than 50 parts per billion (ppb) (0.05 ppm or 0.25 µM). The effects of H
2 at such low concentrations have never been investigated. In contrast, an active concentration of NPs at 2.5 ppb is inferior to the concentration of 0.5 mM (95 ppm) but similar to the 5 µM (5 ppb) of particles that are internalized and observed by Kim
et al. [
25]. For this reason, Yan
et al. undertook further experiments to elucidate the respective roles of H
2 and PtNPs [
28]. The authors found again that synthetic NPs significantly augments the life span of nematodes and reduces the accumulation of ROS induced by paraquat. In contrast, hydrogen enriched water had no significant effect on the longevity of the nematode. These results suggest that it is not H
2 but the NPs in ERAW that are responsible for prolonging the lifespan of this particular model (and which cannot be generalized and extrapolated to other models) through ROS scavenging. By referring to earlier work by Kim
et al. that used S Medium, the authors could conclude that a NP concentration of 1.0 ppb would have been internalized into the nematodes. Since this amount is so small, they conclude that it is highly likely that drinking ERAW does not have any side effects [
26]. This explains why toxicology tests have always been negative so far and thus ERAW declared safe to drink [
29]. In their last publication, Shirahata S.
et al. (2012) do not explain the chemical or physical mechanisms that result in electrode degradation and hence production of nanoparticles (NPs) during functioning of the electrolysis apparatus and that probably increases with each daily use [
30]. Instead, the authors explain that the H
2 is stored in the NPs and activated by its catalytic activity. The catalytic activity is actually strengthened because the surface electron density related to the weight of the metal is greater when in the nanocolloidal state than in the solid state. Shirahata
et al. [
29] further assume that the different mineral ions contained in natural drinking water are reduced at the cathode and the atoms released self organize in the form of mineral NPs. Some minerals react with H
2 to form insoluble hydrides. The same is true of NA and HT natural water whose anti-oxidant properties are due to hydrogen produced during anaerobic reaction between basaltic rock and the particularly pure water. The hydrides store hydrogen reserves that can be liberated by hydrolysis. Stevens and Mc Kinley reported evidence for an active anaerobic subsurface microorganism’s life, deriving energy from the geochemically produced H
2, called lithoautotrophic microbial ecosystem, in deep basalt aquifer [
31]. Martin
et al. have reported similar results in submarine hydrothermal vents [
32].
The study of the toxicity of NPs has not been seen as a problem in the context of nanotechnology development [
33]; first, because the sources of contamination are very rare and second because the metal itself is considered as being biologically inert. It is only recently that the question of toxicity has arisen due to the large-scale use of exhaust catalysers that disperse NPs as they age owing to the use of derivatives of platinum for therapeutic use (e.g., cis-platine) or industrial applications. It has now been established that platinum levels in urine are increasing and that these compounds are bio-assimilable. Owing to their dispersion in the atmosphere, toxicity by inhalation has been the subject of most recent scientific studies [
34]. These studies are based on measuring intracellular uptake of NPs in relation to their shape (sphere, flower shaped or multiple spikes) with sizes ranging from 10 to 30 nm, and their ability to induce oxidative stress or inflammation in lung or vascular endothelial cells. Based on markers of oxidative stress and inflammation, toxicity from concentrations of NPs that are produced during pollution of the environment or industrial atmosphere is undetectable. When doses much higher than this are delivered to cells in culture, the biological effects do not exceed 35% inhibition. However, this study depends on toxicity induced by the submicroscopic state of the metal. In contrast, inherent toxicity due to catalytic chemical action of platinum on the oxido-reduction pathways in the body and especially the liver where the particles accumulate has never been shown.
The toxicological study by Saitoh
et al. [
29] and to which Yan
et al. [
28] refer to has concluded that ERAW does not present a risk for human health. Indeed, no sign of intoxication has ever been observed on the basis of clinical examination, values of haematology, blood and urine biochemical parameters or histopathology of the principal organs. However, the contamination of ERAW by NPs and its biological consequences were not addressed compromising the validity of this toxicological study.
8. Experimental Research on Biological and Therapeutic Properties of Dihydrogen and the Cellular Mechanisms Involved
Although the reducing properties of H
2 are well known in chemistry, this gas is considered as non-reactive with tissues under physiological conditions. Nevertheless, its therapeutic potential for cancer was revealed in the USA [
35], for inflammation in England [
36] and in France [
37]. In 2007, experimental work by the research team of Professor Shigeo Ohta reactivated interest in the therapeutic benefits of H
2 and provided convincing evidence supporting the importance of hydrogen to cellular function in the body [
11,
38]. This work stimulated pioneering experimental and clinical research concerning the therapeutic effectiveness of hydrogen available in newly developed dosage forms, competing with the use of ERAW. Ohsawa
et al. demonstrated in disease models that H
2 selectivity eliminates a very reactive form of oxygen: the hydroxyl radical HO• [
11]. However, H
2 does not spontaneously react with superoxide radical anion (O
2−•), hydrogen peroxide (H
2O
2) or nitric oxide radicals (NO•) that play a physiological role in cell signalling and immune defences.
The therapeutic efficacy of H
2 demonstrated in this publication aroused considerable interest by scientists and doctors in Japan, Korea and China. It created intensive study of a number of disease states some of which had already been explored but by administering H
2 using new procedures such as injection, inhalation, eye drops, ingestion of water enriched in hydrogen by bubbling the gas through water, by electrolysis or by electrochemical reaction of magnesium with water and by raising endogenous intestinal H
2 with a diet rich in starchy food, milk, and curry [
38], mannitol, inhibitors of α-glucosidase [
39,
40]. The benefits of administrating hydrogen were again demonstrated on:
- (i)
Experimental models of ischemia followed by reperfusion on brain, heart and lesions of the kidney, lung and intestine and for these last three organs on the outcome of transplantation;
- (ii)
Damage to the central nervous system, hypoxia in newborns and apoptosis, infarction and hemorrhagic transformation after infarctus and hemorrhagic transformation after obstruction of the middle cerebral artery, perinatal asphyxia of newborn guinea pigs, cerebral degeneration and models of Parkinson’s disease, Alzheimer and accelerated ageing;
- (iii)
Inflammation in a variety of models of hepatitis, acute pancreatitis induced by arginine, colitis bought on by the action of sodium dextran sulphate, septicaemia, general inflammation induced by zymosan;
- (iv)
Neurotoxicity induced by cis-platine used in chemotherapy and lesions of acoustic hair cells caused by antimycin;
- (v)
Injury to the brain or spinal cord, damage caused by ionizing radiation at the level of lymphocytes, the gastro-intestinal endothelium, hearing problems and loss of hearing, corneal burns from caustic soda;
- (vi)
Type I and 2 diabetes, intolerance to glucose, potential metabolic syndrome;
- (vii)
Allergies;
- (viii)
Old age (see Beckman
et al. [
23] for a revue about the cause and role of ROS in the process of senescence).
The published articles of Zheng
et al. [
40] and Shirahata
et al. [
30] contain comprehensive bibliographies citing the above research. The hydroxyl radical HO• is the most reactive of the ROS species. During evolution, mammals have lost any endogenous detoxification system for neutralising ROS species. Thanks to hydrogen’s ability to eliminate radical ions, it can exert a cytoprotective effect that prevents the degradation of cellular biopolymers. The action of H
2 also goes beyond just elimination of excess ROS. A number of reports have shown an effect of H
2 on the regulation of gene expression, acting directly on cellular signalling pathways that are stimulated by excess ROS [
18,
41,
42]. However, many of the reactions occurring in the presence of small quantities of H
2 still need to be elucidated [
38]. This recent research activity on the biological consequences of H
2 has certainly had an impact on clinical evaluations that have been done recently. Clinical assessments undertaken in Japan to highlight the appropriateness of ERAW for gastro-intestinal disturbances made it possible for the Ministry of Health, Labour and Welfare to certify the ionizer devices and allow haemodialysis using ERAW (understandably so because haemodialysis requires a continuous flow of dialysis fluid). Almost all the pathological clinical evaluations to demonstrate the beneficial effects of administering H
2 were conducted with water enriched in H
2 by direct contact with the gas or by electrochemical action between magnesium and water (MSW). None however have yet practised administering ERAW, even though studies have shown its effectiveness on experimental models of disease.
9. Clinical Studies of ERAW
9.1. Gastro-Intestinal Problems
The first double-blind clinical studies concerning gastro-intestinal disorders were presented at the 25th General Assembly of the Japanese Medical Conference in April 1999 [
43] and published by Tashiro
et al. [
44]. In this study, volunteers consumed 1 litre of ERAW every day over 12–15 days. Improvements were observed based on various different clinical signs: chronic diarrhea, constipation, intestinal fermentation, hypercholia. It was these tests that allowed the Japanese authorities to authorize the use of domestic electrolysers for the treatment of gastro-intestinal disorders. Other positive results were presented concerning the benefits of ERAW for treating lesions of the gastric and intestinal mucosa by Dr. T. Yoshikazu, in despite of his own self-confessed scepticism and efforts to show negative results.
Haemodialysis
Heamodialysis is often used for cleaning excess toxins or metabolic waste from the blood in patients with severely impaired renal kidney function. However, this procedure can lead to oxidative stress causing cardiovascular disease. A clinical study was performed on 22 men and 15 women submitted to haemodialysis performed with ERAW provided by a commercial apparatus HD-24K (Nihon Trim Co., Osaka, Japan) that was adapted to a AF-150 haemodialyzer equipped with AF-150 membranes (Althane, Althin Medical Inc., Miami, FL, USA) [
45]. The following blood parameters were measured: total anti-oxidant state (TAS), anti-oxidant activity of plasma, level of proteins/oxidised amino acids, lipid peroxidation, interleukin-6 (IL6) levels and the concentration of C-reactive protein. It was shown that haemodialysis with ERAW helps increase the body’s immune defences against oxidative stress by eliminating in particular hydrogen peroxide and the hypochlorite anion. In this example, ERAW seems to decrease changes at the level of the leukocytes, endothelial cells and erythrocytes [
46]. In another study, 21 participants, stabilized by standard haemodialysis, were subjected to a dialysis with ERAW at a rate of three sessions a week [
47]. Water softened and filtered over activated carbon was electrolyzed with a HD-Nihon Trim (Osaka, Japan) system. This was fed into a reverse osmosis MH 500 CJ Water System (Tokyo, Japan) equipment connected to a personal haemodialysis apparatus DBB-22B Nikiso (Tokyo, Japan) delivering a haemodialysis solution with an average dihydrogen concentration of 48 ppb. No secondary effects were observed during the evaluation. The systolic blood pressure dropped in a significant number of patients to a value of 140 mm Hg. The authors think that the hypotensive effect of H
2 is due to its anti-oxidant activity and to elimination of ROS including peroxynitrate, a substance that causes renal hypertension through the reduction of nitrosyl radicals [
48]. In summary, haemodialysis with ERAW improves the inflammatory response and the control of blood pressure in haemodialysis patients.
9.2. Studies of the Therapeutic Action of Neutral Reduced Water Enriched in Dihydrogen
Kajiyama
et al. carried out a randomized, double-blind, placebo-controlled trial concerning the treatment of type 2 diabetes mellitus on 30 patients suffering from diabetes mellitus type 2 (T2DM) and six patients affected with glucose intolerance [
49]. Natural water was first purified by three filtration procedures: filtration by reverse osmosis, by ion-exchanger resins and ultrafiltration over millipore membrane. Neutral reduced water rich in H
2 was produced by direct dissolution of the gas bubbled into the water. The concentration of H
2 was 1.2 mg·L
−1 and the concentration in O
2 was 0.8 mg·L
−1, for a redox potential of −600 mV. Given the quantity of H
2 eliminated by exhalation, an intake of 900 mL of hydrogen water per day during 12 weeks was recommended to maintain a concentration of H
2 sufficient in the tissues and 900 mL of pure water per day for the control group over the same amount of time, followed by a 12 week washout period. All the parameters were determined at the start of the evaluation, at eight weeks and at the end of the evaluation. Serum concentrations of LDL and small dense LDL (sdLDL) decreased significantly after drinking hydrogen water, but no change in the cholesterol level, the concentration of high density lipoprotein (HDL), low density lipoprotein cholesterol (LDLc), RPLc, triglycerides, or non-esterified fatty acids was noted. Similarly, fasting blood glucose and insulin levels and the percentage of HbA1c were not modified. The authors were unable to find a clear explanation for the reduction in concentrations of emLDL, sdLDL and uIsoP other than the protection of membrane lipoproteins by H
2 against oxidation. Finally, four patients amongst six with glucose intolerance were normalized. To explain these results, the authors put forward the hypothesis that the dihydrogen diffuses easily in tissues and protects the DNA against the deleterious effects of ROS, influencing transcription and contributing to the improvement in insulin resistance. In conclusion, the authors indicate that absorption of hydrogen rich water has a beneficial role to play in the prevention of type 2 mellitus and insulin resistance.
9.3. Studies of the Therapeutic Action of MSW
Metabolic syndrome is characterised by a cluster of metabolic risk factors: obesity, elevated insulin levels, high blood pressure and dyslipidemias; therefore, oxidative stress should play a key role in the pathogenesis of this disorder. A pilot study was undertaken at Pittsburgh University in collaboration with KGK Synergic London Ontario Canada in order to evaluate the effectiveness of this type of water on 20 subjects (10 men and 10 women) whose biological status (weight, blood sugar, lipemia and cholesterolemia) was conducive to developing metabolic syndrome [
50]. The MSW was prepared with a magnesium stick (Dr. Suisosui Friendear, Tokyo, Japan) to obtain a concentration of 0.55–0.65 mM. Routine tests of biological markers indicative of this syndrome as well as oxidative stress markers were performed following standard procedures: blood count, creatine, aminotransferase, gamma glutamyltransferase bilirubin, total cholesterol, HDL, LDL, triglycerides, fasting blood glucose, 8-hydroxy-2ʹ-deoxyguanosine, 8 isoprostane, superoxydismutase and thiobarbituric acid. Each patient was required to drink 300–400 mL of water five times per day: the morning before breakfast, one hour after lunch, two hours after the afternoon snack, one hour before supper and half-hour before bedtime. This gives a total consumption of 1.5–2 L maximum per day during eight weeks. Drinking MSW resulted in a 39% increase in the level of SOD, a decrease in thiobarbituric acid of 43% in the urine, an increase by 8% in HDL and a decrease of 13% in total cholesterol. There was no observable change in blood sugar levels. The authors concluded that drinking MSW improves the levels of markers indicative of oxidative stress and strengthens the anti-oxidant activity of the body.
The fatigue associated with an increase in oxidative stress and inflammation due to the formation of ROS by X-radiation spoils the quality of life of patients. Kang
et al. have also published a study about the efficiency of treating this using MSW [
51]. The investigation was conducted on 49 patients suffering from cancer of the liver. The patients (33 men and 13 women) with a mean age of 56.3 years diagnosed with hepatocellular carcinoma were treated by high voltage radiotherapy (6 MV) and received 60–65 Grays. They were separated into two groups by random draw. One of the groups was subjected to drinking 10 times per day 100–300 mL of ERAW making 1.5–2.0 L per day while the second group was given a placebo. The treatment was begun in the first days after starting radiotherapy and continued during six weeks. A questionnaire was filled in by the patients, conforming to the Korean equivalent of the European Organisation for Research and Treatment of Cancer (QLQ-C30 Instrument) with certain modifications that allow a health evaluation with creation of a Quality Of Life Scale (QOLS) that reflects the level of symptoms and their diminution. Within the QOLS, five functional levels are judged, namely: physical state, cognitive function, emotions, social relations with evaluation of three different symptoms: pain, tiredness, nausea or vomiting and six simple items: dyspnea, insomnia, loss of appetite, constipation, diarrhea. The concentration of metabolised oxidized derivatives (dROM) and the biological anti-oxidant powers (BAP) were evaluated from the first to the sixth week of radiotherapy. Measurements of biological parameters including blood, blood count, aspartate aminotransferase, gamma glutamic transpeptidase (γGTP) and cholesterol were also included over the same time period. Drinking MSW improved the quality of life of the patients beginning from the very first week of treatment. However, MSW did not have any effects on gastro-intestinal disturbances. Drinking MSW systematically diminished the concentration of derivatives of reactive oxygen metabolites, oxygenated derivates, maintained the endogenous anti-oxidant activity in the serum that normally deteriorates during radiation therapy and did not alter liver function or blood composition.
10. Conclusions
ERAW was introduced into Japanese pharmacopoeia as early as 1965 with full approval from the health authorities in 1995 for treating sufferers of gastro-intestinal problems including diarrhoea, constipation, acid indigestion, pre-ulcer state and ulcers. Accreditation by the health authorities was founded on a double-blind clinical trial but concerned a limited number of volunteers over a short time period only. The reason for the development of this preventative and palliative therapeutic approach in Japan is probably because these types of complaints are more common due to a diet rich in raw vegetables and fish seasoned with dressing and the existence of a dynamic and innovative industry in this sector. These diseases or disorders are probably alleviated by the alkalinity of ERAW, but the long term detrimental consequences of regularly drinking 1–1.5 L·day−1 of ERAW, which is between pH 9 and 11 and equivalent to a solution of sodium hydroxide at 10−5–10−3 mol·L−1 has not been studied.
More recently, it was suggested that the H
2 supplied in the form of gas or dissolved in a physiological solution is the main active substance in ERAW. Its therapeutic activity was validated in Japan based on
in vitro studies, a number of disease models and by a few clinical assessments of pathologies in which ROS are implicated, such as diabetes, cancer and severe kidney diseases. In fact, it was shown in 2007 that H
2 selectively eliminates hydroxyl HO• radicals against which the body is normally enzymatically defenceless, but H
2 does not have direct action on the superoxide O
2−• and hydrogen peroxide H
2O
2 that are produced during cellular metabolism. ROS are cell signalling molecules and effective bactericidal agents in phagocytes [
52]. Only their excess amount is physiologically eliminated in reactions catalysed by enzymes. O
2−• is transformed by dismutation to H
2O
2 by SOD and H
2O
2 is converted to water by catalase. If O
2−• is in excess, Fe
2+ and Cu
+ that react with H
2O
2 are oxidized to produce HO• [
53]. These reactions that maintain tissue redox homeostasis include contributions from exogenous anti-oxidant molecules such as Vitamins C, A, E, glutathione, flavonoids and polyphenols. These latter molecules are all much more efficient than H
2 dissolved in ERAW. The NPs present in ERAW have a similar effect to superoxide dismutase and catalase, strengthening the anti-oxidising action of dissolved H
2 and are responsible for the elimination of ROS, as shown
in vitro. Even though no clinical study of ERAW has been carried out concerning pathologies caused by oxidative stress, treating with ERAW might be beneficial if (i) a biological control of anti-oxidant status shows that the physiological anti-oxidant mechanisms are exceeded [
54,
55]; and (ii) the potential toxicity of NPs is shown to not cause harmful or intolerable side effects. However, the toxicology of platinum is still unknown and far from being understood. The only recent toxicology data of ERAW, based on a study of NPs mutagenicity and genotoxicity potential and including clinical biology parameters and the histopathology of the main organs concluded that ERAW is safe. However, this investigation did not consider poisoning by NPs.
In conclusion, recent work on the therapeutic benefits of H2 and PtNPs, developed mostly by Japan, show that ERAW should be considered as a potential medicine and the water electrolysis apparatus as medical devices. As such, this equipment is distributed in France without marketing authorization for medical use. The non-restricted purchase of water electrolysers and the free consummation of ERAW (ad libidum) without consulting a doctor might be pointless or harmful in a healthy person and deprive a patient from a genuine therapeutic cure. The health authorities need to be made aware of this and demand that the therapeutic and prophylactic properties or ERAW be firmly established through a clinical trial program in accordance with the protocols and regulations in force. These trials should focus on specific diseases and be compared to the properties of H2 delivered in other appropriate and available galenic forms: water enriched in H2 by gassing and dissolving the gas into the liquid, by electrochemical reaction of magnesium with water, inhalation of hydrogen or naturally reducing spring water.