Effects of Spartina alterniflora Invasion on Soil Carbon, Nitrogen and Phosphorus in Yancheng Coastal Wetlands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Laboratory Analysis
2.3. Data Analysis
3. Results
3.1. Changes in Vegetation Characteristics and Soil Physicochemical Properties of Spartina alterniflora at Different Years of Invasion
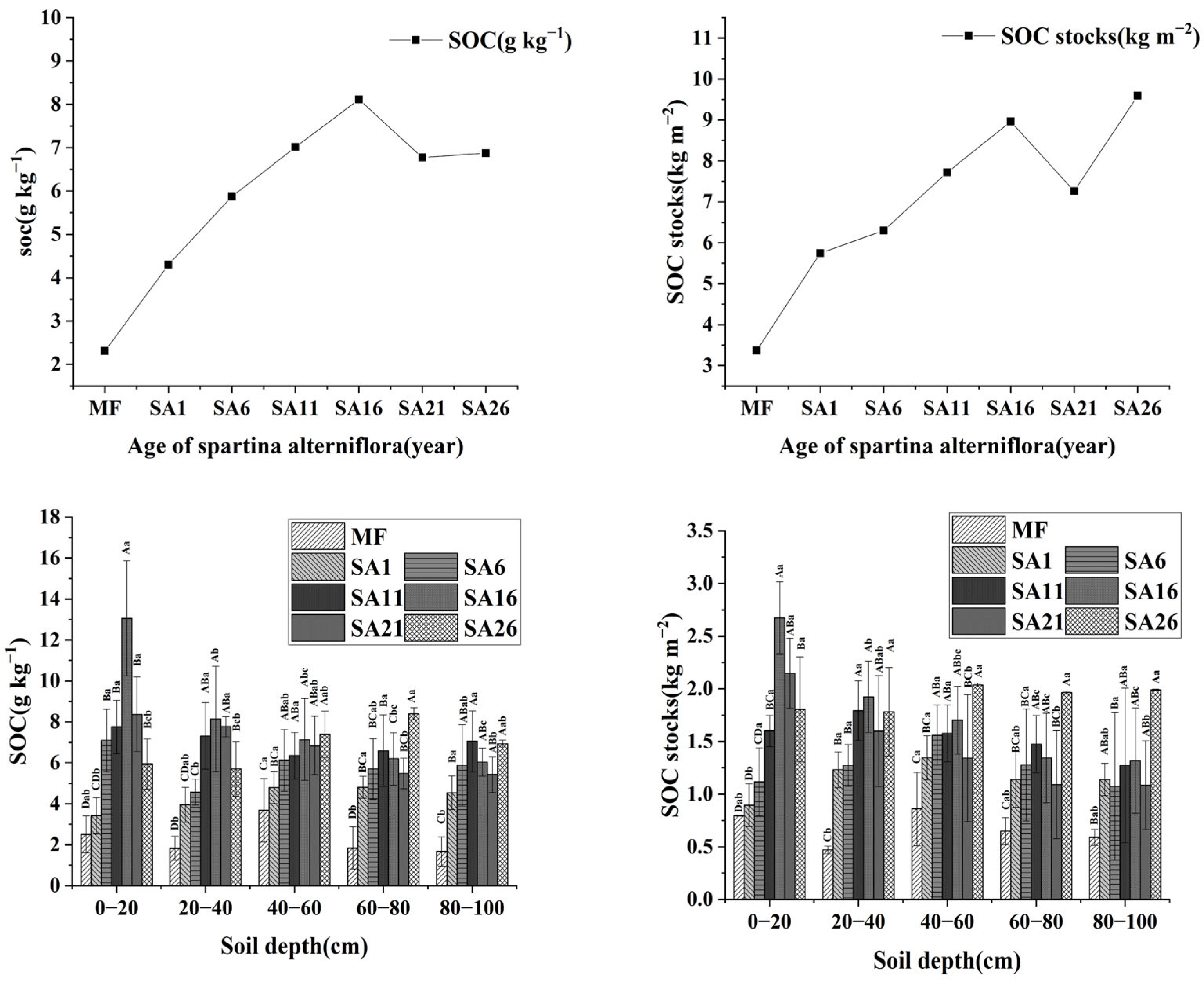
3.2. Changes in SOC at Different Years of Invasion
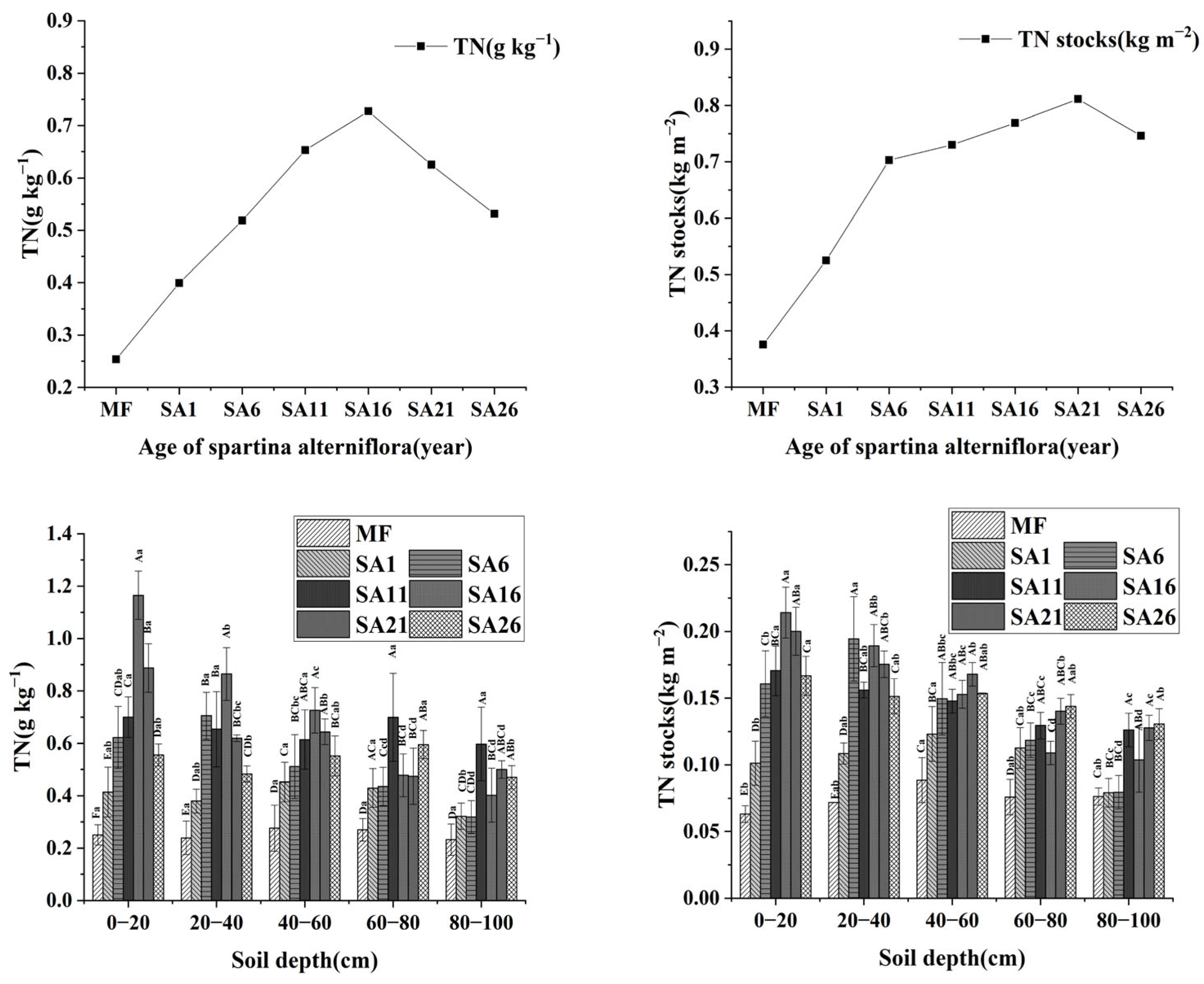
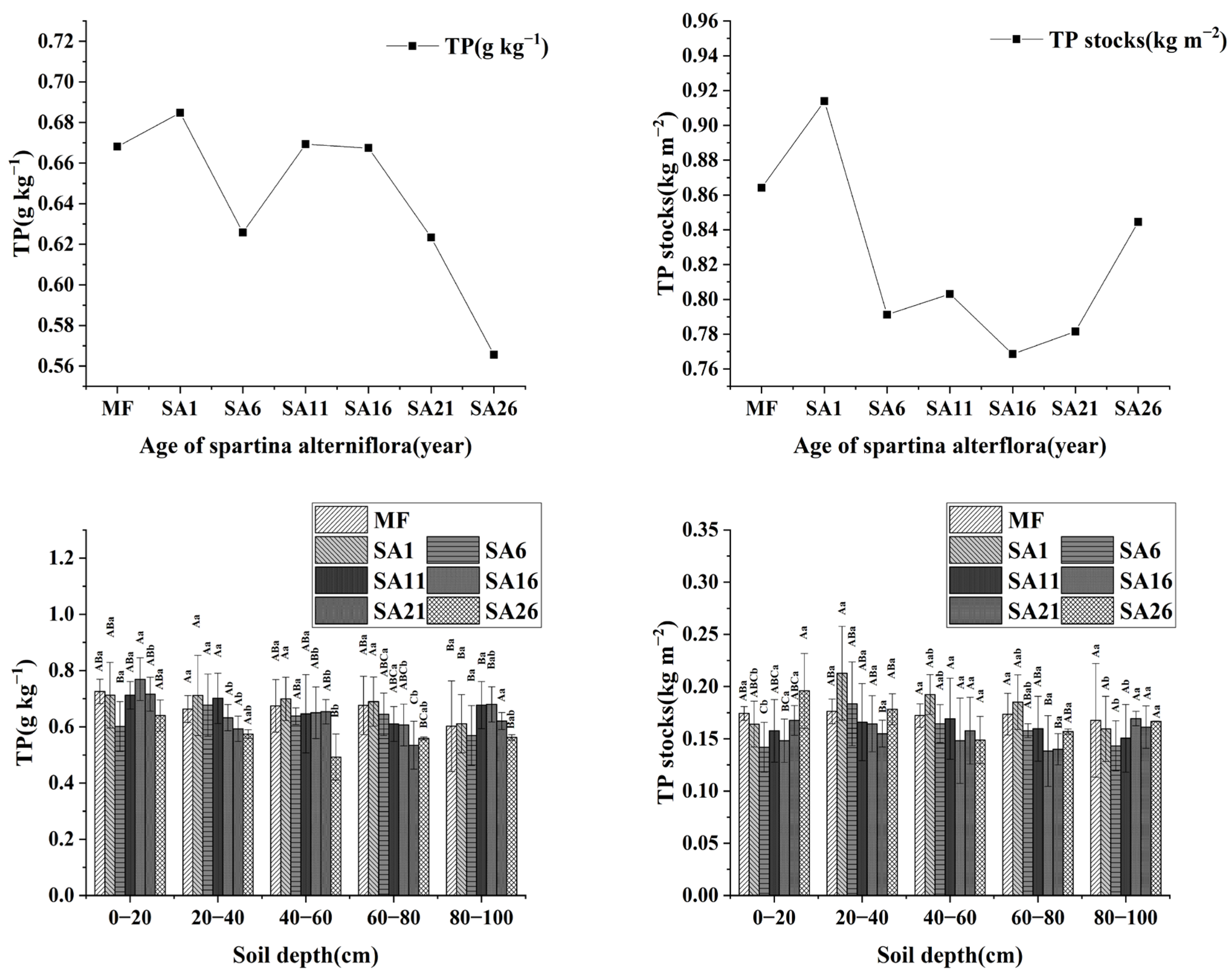
3.3. Changes in TN and TP at Different Years of Invasion
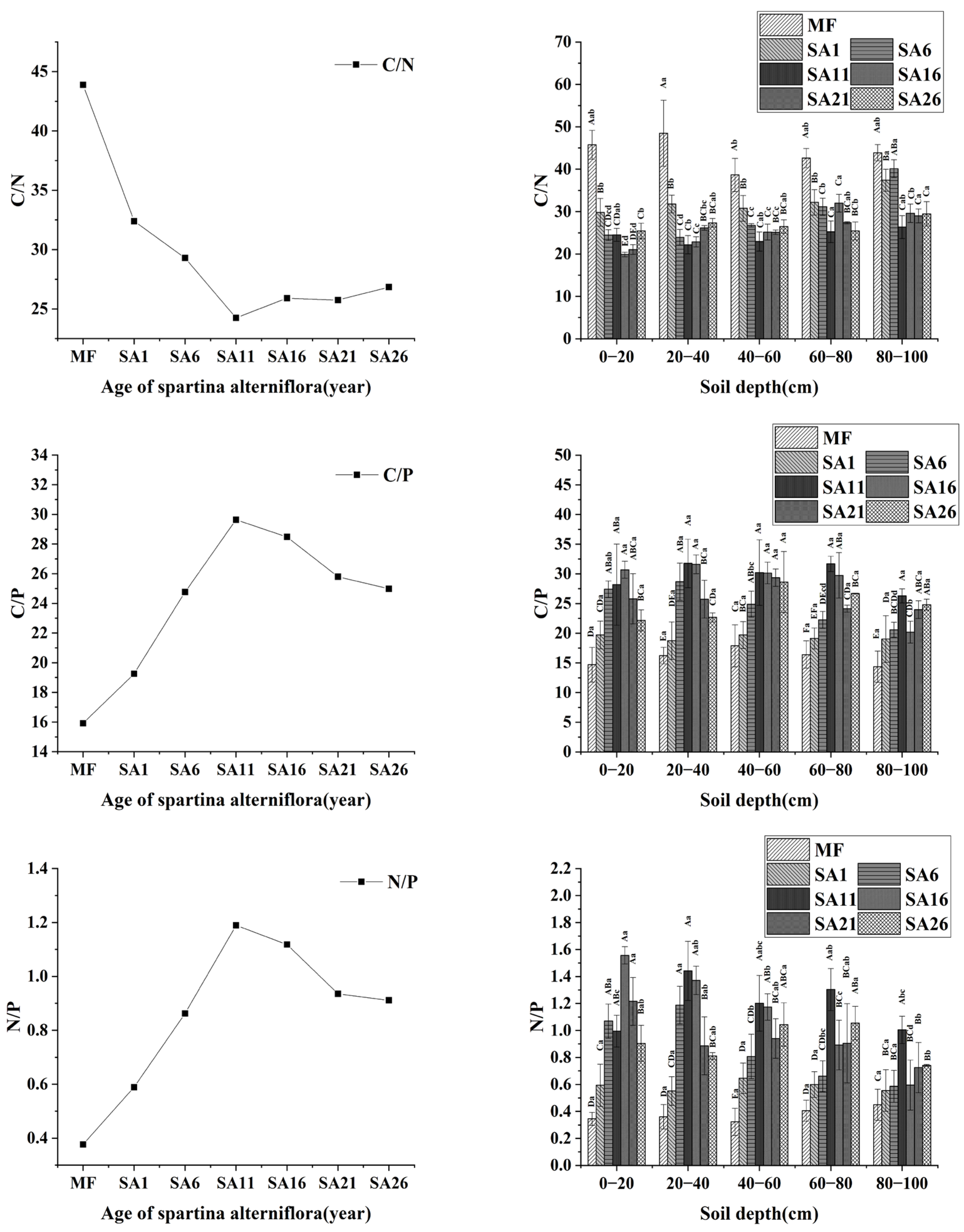
3.4. Ecological Stoichiometric Characteristics of C, N and P in Soils of Different Invasion Years
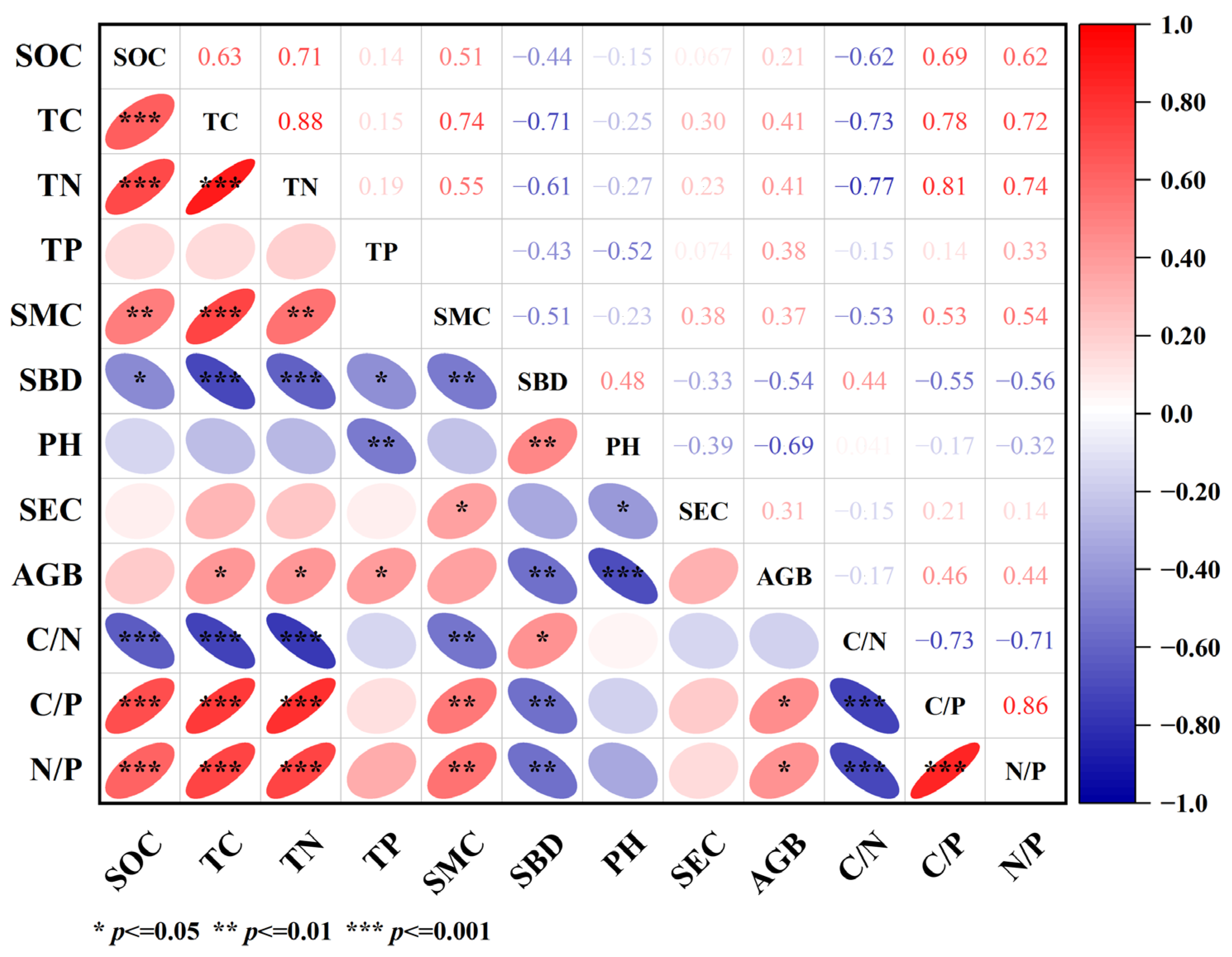
3.5. Correlation of Soil Properties with Soil C, N and P Contents and Their Biogenic Stoichiometric Characteristics
4. Discussion
4.1. Effects of Spartina alterniflora Invasion on Soil C, N and P Contents
4.2. Effects of Spartina alterniflora Invasion on Soil Carbon, Nitrogen and Phosphorus Ecostoichiometry
4.3. Correlation of Soil Properties with Soil C, N and P Content
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Li, W.; Sun, G.; Miao, G.; Noormets, A.; Emanuel, R.; King, J.S. Understanding coastal wetland hydrology with a new regional-scale, process-based hydrological model. Hydrol. Process. 2018, 32, 3158–3173. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Z.; Tian, B.; Huang, Y.; Zhou, Y.; Zhang, T. Impacts of coastal reclamation on wetlands: Loss, resilience, and sustainable management. Estuar. Coast. Shelf Sci. 2018, 210, 153–161. [Google Scholar] [CrossRef]
- Peng, J.; Liu, S.; Lu, W.; Liu, M.; Feng, S.; Cong, P. Continuous Change Mapping to Understand Wetland Quantity and Quality Evolution and Driving Forces: A Case Study in the Liao River Estuary from 1986 to 2018. Remote Sens. 2021, 13, 4900. [Google Scholar] [CrossRef]
- Wu, W.; Zhi, C.; Gao, Y.; Chen, C.; Chen, Z.; Su, H.; Lu, W.; Tian, B. Increasing fragmentation and squeezing of coastal wetlands: Status, drivers, and sustainable protection from the perspective of remote sensing. Sci. Total Environ. 2022, 811, 152339. [Google Scholar] [CrossRef]
- Lu, W.; Xiao, J.; Liu, F.; Zhang, Y.; Liu, C.; Lin, G. Contrasting ecosystem CO2 fluxes of inland and coastal wetlands: A meta-analysis of eddy covariance data. Glob. Chang. Biol. 2017, 23, 1180–1198. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiu, J.; Li, Z.; Li, Y. Assessment of Blue Carbon Storage Loss in Coastal Wetlands under Rapid Reclamation. Sustainability 2018, 10, 2818. [Google Scholar] [CrossRef] [Green Version]
- Hansen, V.D.; Nestlerode, J.A. Carbon sequestration in wetland soils of the northern Gulf of Mexico coastal region. Wetl. Ecol. Manag. 2014, 22, 289–303. [Google Scholar] [CrossRef]
- Song, J.; Xu, G.; Zhang, Y.; Lv, Y. Phosphorus Storage Capacity and Loss Risk in Coastal Reed Wetland Surrounding Bohai Sea. Environ. Sci. 2020, 41, 728–733. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Ecosystem Consequences of Biological Invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Zhou, Y.; Staver, A.C. Enhanced activity of soil nutrient-releasing enzymes after plant invasion: A meta-analysis. Ecology 2019, 100, e2830. [Google Scholar] [CrossRef]
- Vourlitis, G.L.; Lobo, F.D.; Biudes, M.S.; Ortíz, C.E.R.; Nogueira, J.D. Spatial Variations in Soil Chemistry and Organic Matter Content across a Vochysia divergens Invasion Front in the Brazilian Pantanal. Soil Sci. Soc. Am. J. 2011, 75, 1554–1561. [Google Scholar] [CrossRef]
- Sun, J.; Rutherford, S.; Saif Ullah, M.; Ullah, I.; Javed, Q.; Rasool, G.; Ajmal, M.; Azeem, A. Plant–soil feedback during biological invasions: Effect of litter decompositionfrom an invasive plant (Sphagneticola trilobata) on its native congener (S. calendulacea). J. Plant Ecol. 2022, 15, 610–624. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Wren, I.F.; Herman, D.J.; Firestone, M.K. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol. Lett. 2005, 8, 976–985. [Google Scholar] [CrossRef]
- Custer, G.E.; van Diepen, L.T.A. Plant Invasion Has Limited Impact on Soil Microbial α-Diversity: A Meta-Analysis. Diversity 2020, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Belnap, J.; Phillips, S.L.; Sherrod, S.K.; Moldenke, A. Soil biota can change after exotic plant invasion: Does this affect ecosystem processes? Ecology 2005, 86, 3007–3017. [Google Scholar] [CrossRef]
- Wilcox, J.; Beck, C.W. Effects of Ligustrum sinense Lour. (Chinese privet) on Abundance and Diversity of Songbirds and Native Plants in a Southeastern Nature Preserve. Southeast. Nat. 2007, 6, 535–550. [Google Scholar] [CrossRef]
- Houston, W.A.; Duivenvoorden, L.J. Replacement of littoral native vegetation with the ponded pasture grass Hymenachne amplexicaulis: Effects on plant, macroinvertebrate and fish biodiversity of backwaters in the Fitzroy River, Central Queensland, Australia. Mar. Freshw. Res. 2002, 53, 1235–1244. [Google Scholar] [CrossRef]
- Effah, E.; Barrett, D.P.; Peterson, P.G.; Potter, M.A.; Holopainen, J.K.; McCormick, A.C. Effects of Two Invasive Weeds on Arthropod Community Structure on the Central Plateau of New Zealand. Plants 2020, 9, 919. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, Y.; Liu, M.; Chang, Y.; Yan, X.; Bu, R.; Zhao, D.; Li, Z. Introduction and Spread of an Exotic Plant, Spartina alterniflora, Along Coastal Marshes of China. Wetlands 2017, 37, 1181–1193. [Google Scholar] [CrossRef]
- Lee, A.K.; Ayres, D.R.; Pakenham-Walsh, M.R.; Strong, D.R. Responses to salinity of Spartina hybrids formed in San Francisco Bay, California (S. alterniflora × foliosa and S. densiflora × foliosa). Biol. Invasions. 2016, 18, 2207–2219. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, D.L.; White, D.S.; Howes, B.L. Replacement of Phragmites australis by Spartina alterniflora: The Role of Competition and Salinity. Wetlands 2013, 33, 421–430. [Google Scholar] [CrossRef]
- Wang, Q.; An, S.; Ma, Z.; Zhao, B.; Chen, J.; Li, B. Invasive Spartina alterniflora: Biology, ecology and management. Acta Phytotaxon. Sinica. 2006, 44, 559. [Google Scholar] [CrossRef]
- Roberts, P.D.; Pullin, A.S. The effectiveness of management interventions for the control of Spartina species: A systematic review and meta-analysis. Aquat. Conserv. 2008, 18, 592–618. [Google Scholar] [CrossRef]
- Zhao, Z.; Cheng, L.; He, C.; Wang, F.; Liu, J.; Li, Y.; Chen, X.; Liu, X.; Lv, G.; Wang, D. Spartina alterniflora Invaded Coastal Wetlands by Raising Soil Sulfur Contents: A Meta-Analysis. Water 2022, 14, 1633. [Google Scholar] [CrossRef]
- Bu, N.; Qu, J.; Li, Z.; Li, G.; Zhao, H.; Zhao, B.; Li, B.; Chen, J.; Fang, C. Effects of Spartina alterniflora Invasion on Soil Respiration in the Yangtze River Estuary, China. PLoS ONE 2015, 10, 121571. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Sha, C.; Chen, J. Spartina alterniflora invasion affects soil carbon in a C3 plant-dominated tidal marsh. Sci. Rep. 2018, 8, 628. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Zhao, H.; Chen, X.; Yin, S.; Cheng, X.; An, S. Consequences of short-term C4 plant Spartina alterniflora invasions for soil organic carbon dynamics in a coastal wetland of Eastern China. Ecol. Eng. 2013, 61, 50–57. [Google Scholar] [CrossRef]
- Yang, W.; An, S.; Zhao, H.; Xu, L.; Qiao, Y.; Cheng, X. Impacts of Spartina alterniflora invasion on soil organic carbon and nitrogen pools sizes, stability, and turnover in a coastal salt marsh of eastern China. Ecol. Eng. 2016, 86, 174–182. [Google Scholar] [CrossRef]
- Huang, X.; Duan, Y.; Tao, Y.; Wang, X.; Long, H.; Luo, C.; Lai, Y. Effects of Spartina alterniflora Invasion on Soil Organic Carbon Storage in the Beihai Coastal Wetlands of China. Front. Mar. Sci. 2022, 9, 890811. [Google Scholar] [CrossRef]
- Wan, S.; Liu, X.; Mou, X.; Zhao, Y. Comparison of Carbon, Nitrogen, and Sulfur in Coastal Wetlands Dominated by Native and Invasive Plants in the Yancheng National Nature Reserve, China. Chin. Geogr. Sci. 2020, 30, 202–216. [Google Scholar] [CrossRef]
- González, A.L.; Kominoski, J.S.; Danger, M.; Ishida, S.; Iwai, N.; Rubach, A. Can ecological stoichiometry help explain patterns of biological invasions? Oikos 2010, 119, 779–790. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, D.; Chen, G. Ecological stoichiometry: A science to explore the complexity of living systems. Chin. J. Plant Ecol. 2005, 29, 141–153. [Google Scholar] [CrossRef]
- Zhi, Y.; Li, H.; An, S.; Zhao, L.; Zhou, C.; Deng, Z. Inter-specific competition: Spartina alterniflora is replacing Spartina anglica in coastal China. Estuar. Coast. Shelf Sci. 2007, 74, 437–448. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, Y.; Chen, J.; Li, B. Ecophysiological characteristics of invasive Spartina alterniflora and native species in salt marshes of Yangtze River estuary, China. Estuar. Coast. Shelf Sci. 2009, 81, 74–82. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, W.; Xia, L.; Qiao, Y.; Xiao, Y.; Cheng, X.; An, S. Nitrogen-Enriched Eutrophication Promotes the Invasion of Spartina alterniflora in Coastal China. CLEAN-Soil Air Water 2015, 43, 244–250. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Liang, J.; Gao, J.; Xu, X.; Yu, F. High nitrogen uptake and utilization contribute to the dominance of invasive Spartina alterniflora over native Phragmites australis. Biol. Fertil. Soils. 2021, 57, 1007–1013. [Google Scholar] [CrossRef]
- Li, J.; Lai, Y.; Xie, R.; Ding, X.; Wu, C. Sediment phosphorus speciation and retention process affected by invasion time of Spartina alterniflora in a subtropical coastal wetland of China. Environ. Sci. Pollut. Res. 2018, 25, 35365–35375. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Y.; Shi, Y.; Liu, Y.; Wang, L.; Yan, N. Comparison of phosphorus fractions and phosphatase activities in coastal wetland soils along vegetation zones of Yancheng National Nature Reserve, China. Estuar. Coast. Shelf Sci. 2015, 157, 93–98. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Sardans, J.; Zeng, C.; Tong, C.; Peñuelas, J. Plant invasive success associated with higher N-use efficiency and stoichiometric shifts in the soil–plant system in the Minjiang River tidal estuarine wetlands of China. Wetl. Ecol. Manag. 2015, 23, 865–880. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Zhou, J.; Wang, L.; Cui, X.; Ning, C.; Wu, H.; Zhu, X.; Lin, G. Effects of short-term invasion of Spartina alterniflora and the subsequent restoration of native mangroves on the soil organic carbon, nitrogen and phosphorus stock. Chemosphere 2017, 184, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wei, S.; Chen, H.; Li, B.; Nie, M. Effects of Spartina invasion on the soil organic carbon content in salt marsh and mangrove ecosystems in China. J. Appl. Ecol. 2022, 59, 1937–1946. [Google Scholar] [CrossRef]
- Xia, S.; Wang, W.; Song, Z.; Kuzyakov, Y.; Guo, L.; Van Zwieten, L.; Li, Q.; Hartley, I.P.; Yang, Y.; Wang, Y.; et al. Spartina alterniflora invasion controls organic carbon stocks in coastal marsh and mangrove soils across tropics and subtropics. Glob. Chang. Biol. 2021, 27, 1627–1644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Bai, J.; Jia, J.; Wang, X.; Wang, W.; Zhao, Q.; Zhang, S. Soil Organic Carbon Contents and Stocks in Coastal Salt Marshes with Spartina alterniflora Following an Invasion Chronosequence in the Yellow River Delta, China. Chin. Geogr. Sci. 2018, 28, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Xie, R.; Zhu, Y.; Li, J.; Liang, Q. Changes in sediment nutrients following Spartina alterniflora invasion in a subtropical estuarine wetland, China. Catena 2019, 180, 16–23. [Google Scholar] [CrossRef]
- Lu, X.; Lin, Y.; Wu, Y.; Gu, Y.; Zhao, Q.; Zhang, X. Spatial Distribution Characteristics of Soil Physical and Chemical Properties in Milu National Nature Reserve of Coastal Wetland. J. Oceanol. Limnol. 2018, 4, 74–81. [Google Scholar] [CrossRef]
- Yan, D.; Li, J.; Yao, X.; Luan, Z. Quantifying the Long-Term Expansion and Dieback of Spartina Alterniflora Using Google Earth Engine and Object-Based Hierarchical Random Forest Classification. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2021, 14, 9781–9793. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and A Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Yang, W.; An, S.; Zhao, H.; Fang, S.; Xia, L.; Xiao, Y.; Qiao, Y.; Cheng, X. Labile and Recalcitrant Soil Carbon and Nitrogen Pools in Tidal Salt Marshes of the Eastern Chinese Coast as Affected by Short-Term C-4 Plant Spartina alterniflora Invasion. CLEAN-Soil Air Water 2015, 43, 872–880. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Cheng, W.; Zhou, L.; Zhang, G.; Zhou, G.; Liu, R.; Shao, J.; Zhu, K.; Cheng, W. Linking Improvement of Soil Structure to Soil Carbon Storage Following Invasion by a C4 Plant Spartina alterniflora. Ecosystems 2019, 22, 859–872. [Google Scholar] [CrossRef]
- Yu, J.; Dong, H.; Li, Y.; Wu, H.; Guan, B.; Gao, Y.; Zhou, D.; Wang, Y. Spatiotemporal Distribution Characteristics of Soil Organic Carbon in Newborn Coastal Wetlands of the Yellow River Delta Estuary. CLEAN-Soil Air Water 2014, 42, 311–318. [Google Scholar] [CrossRef]
- Liao, C.; Luo, Y.; Jiang, L.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Invasion of Spartina alterniflora Enhanced Ecosystem Carbon and Nitrogen Stocks in the Yangtze Estuary, China. Ecosystems 2007, 10, 1351–1361. [Google Scholar] [CrossRef]
- Cheng, X.; Luo, Y.; Chen, J.; Lin, G.; Chen, J.; Li, B. Short-term C4 plant Spartina alterniflora invasions change the soil carbon in C3 plant-dominated tidal wetlands on a growing estuarine Island. Soil Biol. Biochem. 2006, 38, 3380–3386. [Google Scholar] [CrossRef]
- Windham, L.; Ehrenfeld, J.G. Net Impact of a Plant Invasion on Nitrogen-Cycling Processes within a Brackish Tidal Marsh. Ecol. Appl. 2003, 13, 883–896. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Zhao, Q.; Jia, J.; Wang, W.; Wang, X. Bacterial Succession in Salt Marsh Soils Along a Short-term Invasion Chronosequence of Spartina alterniflora in the Yellow River Estuary, China. Microb. Ecol. 2020, 79, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sardans, J.; Wang, C.; Zeng, C.; Tong, C.; Chen, G.; Huang, J.; Pan, H.; Peguero, G.; Vallicrosa, H.; et al. The response of stocks of C, N, and P to plant invasion in the coastal wetlands of China. Glob. Chang. Biol. 2018, 25, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Yang, J.; Liu, L.; Tian, Y.; Yu, Z. Effects of Spartina alterniflora invasion on biogenic elements in a subtropical coastal mangrove wetland. Environ. Sci. Pollut. R. 2015, 22, 3107–3115. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, W.; Luo, J.; Donnison, A. Changes in soil organic carbon dynamics in an Eastern Chinese coastal wetland following invasion by a C4 plant Spartina alterniflora. Soil Biol. Biochem. 2010, 42, 1712–1720. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, H.; Leng, X.; Cheng, X.; An, S. Soil organic carbon and nitrogen dynamics following Spartina alterniflora invasion in a coastal wetland of eastern China. Catena 2017, 156, 281–289. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Hou, Y.; Zhu, S.; Wang, L.; Shi, X.; Li, P.; Han, G.; Xie, B. Effects of Spartins alterniflora Invasion on Soil Carbon, Nitrogen, Phosphorus and Their Ecostoichiometric Characteristics in the Yellow River Estuary. Ecol. Environ. Sci. 2022, 31, 1360–1369. [Google Scholar] [CrossRef]
- Jin, B.; Yan, H.; Wang, W.; Zeng, C. Changes of soil carbon, nitrogen and phosphorus and stoichionmetry characteristics in marsh invaded by Spartina alterniflora. Chin. J. Appl. Ecol. 2017, 28, 1541–1549. [Google Scholar] [CrossRef]
- Huang, X.; Liang, S.; Tao, Y.; Wang, X.; Duan, Y. Distribution characteristics of organic carbon stocks of Spartina alterniflora in Dafeng River Estuary, Beibu Gulf. Guihaia 2021, 41, 853–861. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, X.; Xue, Z.; Liu, X. Is There a Redfieid-Type C:N:P Ratio in Chinese Wetland Soils? Acta Pedol. Sinica. 2016, 53, 1160–1169. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Liu, D.; Qi, X.; Xie, H. Characteristics and determining factors for ecological stoichiometry of soil carbon, nitrogen, and phosphorus in different marsh wetlands. Arid. Land Geogr. 2020, 37, 618–626. [Google Scholar] [CrossRef]
- Murphy, N.; Waterhouse, H.; Dahlke, H. Influence of agricultural managed aquifer recharge on nitrate transport: The role of soil texture and flooding frequency. Vadose Zone J. 2021, 20, e20150. [Google Scholar] [CrossRef]
- He, D.; He, D.; Yan, J.; Wang, R.; Cai, J.; Wang, P.; You, W.; Chen, X. Effect of Invasive Spartina Alterniflora on Soil Organic Carbon in Eastern Fujian Coastal Wetlands. Oceanol. Et. Limnol. Sinica. 2016, 68–76. [Google Scholar] [CrossRef]
- Yang, R.; Chen, L. Spartina alterniflora invasion alters soil bulk density in coastal wetlands of China. Land Degrad. Dev. 2021, 32, 1993–1999. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, J.; Zhang, G.; Jia, J.; Wang, W.; Wang, X. Effects of water and salinity regulation measures on soil carbon sequestration in coastal wetlands of the Yellow River Delta. Geoderma 2018, 319, 219–229. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, H.; Ran, S.; Liu, J.; Su, H.; Yu, P. Effects of Nitrogen and Phosphorus Addition on Photosynthesized Carbon Allocation in Spartina Altern. J. Ecol. Rural. Environ. 2019, 35, 627–633. [Google Scholar] [CrossRef]


| Sample | Aboveground Biomass (AGB, g m−2) | Plant Height (cm) | Plant Density (n m−2) |
|---|---|---|---|
| SA1 | 1816.92 ± 373.78 b | 157.71 ± 11.70 b | 394.44 ± 65.88 b |
| SA6 | 2321.08 ± 350.51 b | 169.71 ± 13.00 b | 354.17 ± 81.27 b |
| SA11 | 3178.08 ± 527.66 a | 194.78 ± 14.64 a | 338.89 ± 76.15 b |
| SA16 | 2339.5 ± 591.03 b | 134.33 ± 4.72 c | 587.50 ± 72.02 a |
| SA21 | 1737.56 ± 335.84 b | 117.56 ± 9.61 d | 535.71 ± 65.92 a |
| SA26 | 541.67 ± 149.31 c | 44.00 ± 3.61 e | 625.00 ± 139.36 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Y.; Luan, Z.; Yan, D.; Li, J.; Xie, S.; Liu, Y.; Chen, L.; Li, M.; Wu, C. Effects of Spartina alterniflora Invasion on Soil Carbon, Nitrogen and Phosphorus in Yancheng Coastal Wetlands. Land 2022, 11, 2218. https://doi.org/10.3390/land11122218
Sheng Y, Luan Z, Yan D, Li J, Xie S, Liu Y, Chen L, Li M, Wu C. Effects of Spartina alterniflora Invasion on Soil Carbon, Nitrogen and Phosphorus in Yancheng Coastal Wetlands. Land. 2022; 11(12):2218. https://doi.org/10.3390/land11122218
Chicago/Turabian StyleSheng, Yufeng, Zhaoqing Luan, Dandan Yan, Jingtai Li, Siying Xie, Yao Liu, Li Chen, Min Li, and Cuiling Wu. 2022. "Effects of Spartina alterniflora Invasion on Soil Carbon, Nitrogen and Phosphorus in Yancheng Coastal Wetlands" Land 11, no. 12: 2218. https://doi.org/10.3390/land11122218







