Toward the Integrated Framework Analysis of Linkages among Agrobiodiversity, Livelihood Diversification, Ecological Systems, and Sustainability amid Global Change
Abstract
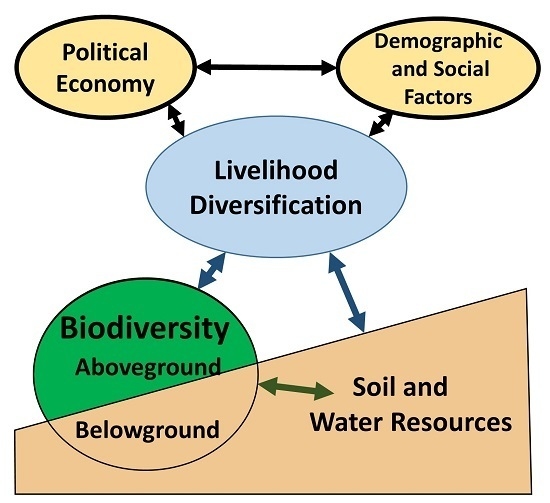
:1. Introduction: Integrating the Analysis of Livelihoods, Agrobiodiversity, and Ecological Interactions
2. Empirical Focus and Research Methodology: Global Smallholders and Nested Meta-Analysis
3. Results: Livelihood Diversification and Environmental Linkages
4. Results: Ecological Linkages within Agrobiodiversity Systems
5. Discussion: Hypothesizing Pathways and the Spatial-Geographic Dynamics of Livelihoods and Agrobiodiversity amid Global Change
6. Conclusion: Interactions of Agrobiodiversity and Smallholder Livelihood Diversification in the Context of Global Change
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vandermeer, J.; van Noordwijk, M.; Anderson, J.; Ong, C.; Perfecto, I. Global change and multi-species agroecosystems: Concepts and issues. Agric. Ecosyst. Environ. 1998, 67, 1–22. [Google Scholar] [CrossRef]
- Zimmerer, K.S. Biological diversity in agriculture and global change. Annu. Rev. Environ. Resour. 2010, 35, 137–166. [Google Scholar] [CrossRef]
- Garnett, T.; Appleby, M.C.; Balmford, A.; Bateman, I.J.; Benton, T.G.; Bloomer, P.; Godfray, H.C.J. Sustainable intensification in agriculture: Premises and policies. Science 2013, 341, 33–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Brookfield, H.C. Exploring Agrodiversity; Columbia University Press: New York, NY, USA, 2001. [Google Scholar]
- Brush, S.B. Farmers’ Bounty: Locating Crop Diversity in the Contemporary World; Yale University Press: New Haven, CT, USA, 2004. [Google Scholar]
- Zimmerer, K.S. Changing Fortunes: Peasant Livelihood and Biodiversity in the Peruvian Andes; University of California Press: Los Angeles, Berkeley, CA, USA, 1996. [Google Scholar]
- Zimmerer, K.S. Understanding agrobiodiversity and the rise of resilience: Analytic category, boundary concept, or meta-level transition? Resil. Int. Policies Pract. Discourses 2015, 3, 1–16. [Google Scholar] [CrossRef]
- Jackson, L.E.; Pascual, U.; Hodgkin, T. Utilizing and conserving agrobiodiversity in agricultural landscapes. Agric. Ecosyst. Environ. 2007, 121, 196–210. [Google Scholar] [CrossRef]
- Jarvis, D.I.; Padoch, C.; Cooper, H.D. Managing Biodiversity in Agricultural Ecosystems; Columbia University Press: New York, NY, USA, 2007. [Google Scholar]
- Tscharntke, T.; Clough, Y.; Wanger, T.C.; Jackson, L.; Motzke, I.; Perfecto, I.; Vandermeer, J.; Whitbread, A. Global food security, biodiversity conservation and the future of agricultural intensification. Biol. Conserv. 2012, 151, 53–59. [Google Scholar] [CrossRef]
- Nabhan, G.P. Where Our Food Comes from: Retracing Nikolay Vavilov's Quest to End Famine; Island Press: Washington, DC, USA, 2008. [Google Scholar]
- Zimmerer, K.S. Woodlands and agrobiodiversity in irrigation landscapes amidst global change: Bolivia, 1990–2002. Prof. Geogr. 2010, 62, 335–356. [Google Scholar] [CrossRef]
- Zimmerer, K.S. The compatibility of agricultural intensification in a global hotspot of smallholder agrobiodiversity (Bolivia). Proc. Natl. Acad. Sci. USA 2013, 110, 2769–2774. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Brush, S.B. In situ conservation of landraces in centers of crop diversity. Crop Sci. 1995, 35, 346–354. [Google Scholar] [CrossRef]
- Brush, S.B.; Perales, H.R. A maize landscape: Ethnicity and agro-biodiversity in Chiapas Mexico. Agric. Ecosyst. Environ. 2007, 121, 211–221. [Google Scholar] [CrossRef]
- Benin, S.; Smale, M.; Pender, J.; Gebremedhin, B.; Ehui, S. The economic determinants of cereal crop diversity on farms in the Ethiopian highlands. Agric. Econ. 2004, 31, 197–208. [Google Scholar] [CrossRef]
- Bezabih, M.; Sarr, M. Risk preferences and environmental uncertainty: Implications for crop diversification decisions in Ethiopia. Environ. Resour. Econ. 2012, 53, 483–505. [Google Scholar] [CrossRef]
- Chavas, J.P.; Di Falco, S. On the productive value of crop biodiversity: Evidence from the highlands of Ethiopia. Land Econ. 2012, 88, 58–74. [Google Scholar] [CrossRef]
- Samberg, L.H.; Shennan, C.; Zavaleta, E.S. Human and environmental factors affect patterns of crop diversity in an Ethiopian Highland agroecosystem. Prof. Geogr. 2010, 62, 395–408. [Google Scholar] [CrossRef]
- Almekinders, C.J.; Fresco, L.O.; Struik, P.C. The need to study and manage variation in agro-ecosystems. NJAS Wagening J. Life Sci. 1995, 43, 127–142. [Google Scholar]
- Bellon, M.R.; Hodson, D.; Hellin, J. Assessing the vulnerability of traditional maize seed systems in Mexico to climate change. Proc. Natl. Acad. Sci. USA 2011, 108, 13432–13437. [Google Scholar] [CrossRef] [PubMed]
- Hellin, J.; Bellon, M.R.; Hearne, S.J. Maize landraces and adaptation to climate change in Mexico. J. Crop Improv. 2014, 28, 484–501. [Google Scholar] [CrossRef]
- Lin, B.B.; Perfecto, I.; Vandermeer, J. Synergies between agricultural intensification and climate change could create surprising vulnerabilities for crops. BioScience 2008, 58, 847–854. [Google Scholar] [CrossRef]
- Lin, B.B. Resilience in agriculture through crop diversification: Adaptive management for environmental change. BioScience 2011, 61, 183–193. [Google Scholar] [CrossRef]
- McCord, P.F.; Cox, M.; Schmitt-Harsh, M.; Evans, T. Crop diversification as a smallholder livelihood strategy within semi-arid agricultural systems near Mount Kenya. Land Use Policy 2015, 42, 738–750. [Google Scholar] [CrossRef]
- Brussaard, L.; Caron, P.; Campbell, B.; Lipper, L.; Mainka, S.; Rabbinge, R.; Babin, D.; Pulleman, M. Reconciling biodiversity conservation and food security: Scientific challenges for a new agriculture. Curr. Opin. Environ. Sustain. 2010, 2, 34–42. [Google Scholar] [CrossRef]
- De Haan, S.; Núñez, J.; Bonierbale, M.; Ghislain, M. Multilevel agrobiodiversity and conservation of Andean potatoes in Central Peru: Species, morphological, genetic, and spatial diversity. Mt. Res. Dev. 2010, 30, 222–231. [Google Scholar] [CrossRef]
- Jackson, L.E.; Pulleman, M.M.; Brussaard, L.; Bawa, K.S.; Brown, G.G.; Cardoso, I.M.; De Ruiter, P.C.; García-Barrios, L.; Hollander, A.D.; Lavelle, P.; et al. Social-ecological and regional adaptation of agrobiodiversity management across a global set of research regions. Glob. Environ. Chang. 2012, 22, 623–639. [Google Scholar] [CrossRef]
- Bellon, M.R. Conceptualizing interventions to support on-farm genetic resource conservation. World Dev. 2004, 32, 159–172. [Google Scholar] [CrossRef]
- Smale, M.; Bellon, M.R.; Jarvis, D.; Sthapit, B. Economic concepts for designing policies to conserve crop genetic resources on farms. Genet. Res. Crop Evol. 2004, 51, 121–135. [Google Scholar] [CrossRef]
- Keleman, A.; Hellin, J. Specialty maize varieties in Mexico: A case study in market-driven agro-biodiversity conservation. J. Lat. Am. Geogr. 2009, 8, 147–174. [Google Scholar] [CrossRef]
- Keleman, A.; Hellin, J.; Flores, D. Diverse varieties and diverse markets: Scale-related maize “profitability crossover” in the central Mexican highlands. Hum. Ecol. 2013, 41, 683–705. [Google Scholar] [CrossRef]
- Jones, A.D.; Shrinivas, A.; Bezner-Kerr, R. Farm production diversity is associated with greater household dietary diversity in Malawi: Findings from nationally representative data. Food Policy 2014, 46, 1–12. [Google Scholar] [CrossRef]
- Skarbø, K. The cooked is the kept: Factors shaping the maintenance of agrobiodiversity in the Andes. Hum. Ecol. 2014, 42, 711–726. [Google Scholar] [CrossRef] [Green Version]
- Johns, T. Agrobiodiversity, diet, and human health. In Managing Biodiversity in Agricultural Ecosystems; Jarvis, D.I., Padoch, C., Cooper, H.D., Eds.; Columbia University Press: New York, NY, USA, 2007; pp. 382–406. [Google Scholar]
- Johns, T.; Eyzaguirre, P.B. Linking biodiversity, diet and health in policy and practice. Proc. Nutr. Soc. 2006, 65, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Oyarzun, P.J.; Borja, R.M.; Sherwood, S.; Parra, V. Making sense of agrobiodiversity, diet, and intensification of smallholder family farming in the Highland Andes of Ecuador. Ecol. Food Nutr. 2013, 52, 515–541. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, D.I.; Brown, A.H.D.; Cuong, P.H.; Collado-Panduro, L.; Latournerie-Moreno, L.; Gyawali, S.; Tanto, T.; Sawadogo, M.; Mar, I.; Sadiki, M.; et al. A global perspective of the richness and evenness of traditional crop-variety diversity maintained by farming communities. Proc. Natl. Acad. Sci. USA 2008, 105, 5326–5331. [Google Scholar] [CrossRef] [PubMed]
- Zimmerer, K.S. Cultural ecology: Placing households in human-environment studies—The cases of tropical forest transitions and agrobiodiversity change. Prog. Hum. Geogr. 2004, 28, 795–806. [Google Scholar] [CrossRef]
- Folke, C. Resilience: The emergence of a perspective for social-ecological systems analyses. Glob. Environ. Chang. 2006, 16, 253–267. [Google Scholar] [CrossRef]
- Ostrom, E. A general framework for analyzing sustainability of social-ecological systems. Science 2009, 325, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Zimmerer, K.S. Retrospective on nature-society geography: Tracing trajectories (1911–2010) and reflecting on translations. Ann. Assoc. Am. Geogr. 2010, 100, 1076–1094. [Google Scholar] [CrossRef]
- Moran, E. Environmental Social Science: Human-Environment Interactions and Sustainability; John Wiley & Sons: New York, NY, USA, 2010. [Google Scholar]
- Moran, E.F.; Ostrom, E. Seeing the Forest and the Trees. Human-Environment Interactions in Forest Ecosystems; MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Liu, J.; Dietz, T.; Carpenter, S.R.; Alberti, M.; Folke, C.; Moran, E.; Pell, A.N.; Deadman, P.; Kratz, T.; Lubchenko, J.; et al. Complexity of coupled human and natural systems. Science 2007, 317, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Brondízio, E.S.; Chowdhury, R.R. Human-environment research: Past trends, current challenges, and future directions. In Human-Environment Interactions: Current and Future Directions; Brondizio, E.S., Moran, E.F., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 391–400. [Google Scholar]
- Zimmerer, K.S. Geographic approaches to LTSER: Principal themes and concepts with a case study of Andes-Amazon watersheds. In Long Term Socio-Ecological Research; Singh, S.J., Haberl, H., Chertow, M., Mirtl, M., Schmid, M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 163–187. [Google Scholar]
- Brannstrom, C.; Vadjunec, J.M. Land Change Science, Political Ecology and Sustainability: Synergies and Divergences; Routledge: London, UK, 2014. [Google Scholar]
- Watts, N.; Scales, I.R. Seeds, agricultural systems and socio-natures: Towards an actor–network theory informed political ecology of agriculture. Geogr. Compass 2015, 9, 225–236. [Google Scholar] [CrossRef]
- Zimmerer, K.; Carney, J.A.; Vanek, S.J. Sustainable smallholder intensification in global change? Pivotal spatial interactions, gendered livelihoods, and agrobiodiversity. Curr. Opin. Environ. Sustain. 2015, 14, 49–60. [Google Scholar] [CrossRef]
- Rocheleau, D.E. Gender and biodiversity: A feminist political ecology perspective. IDS Bull. 1995, 26, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Rocheleau, D.; Thomas-Slayter, B.; Wangari, E. Feminist Political Ecology: Global Issues and Local Experience; Routledge: New York, NY, USA, 1996. [Google Scholar]
- Hecht, S. The new rurality: Globalization, peasants and the paradoxes of landscapes. Land Use Policy 2010, 27, 161–169. [Google Scholar] [CrossRef]
- Kay, C. Reflections on Latin American rural studies in the neoliberal globalization period: A new rurality? Dev. Chang. 2008, 39, 915–943. [Google Scholar] [CrossRef]
- Postma-Blaauw, M.B.; de Goede, R.G.M.; Bloem, J.; Faber, J.H.; Brussaard, L. Soil biota community structure and abundance under agricultural intensification and extensification. Ecology 2010, 91, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, L.; de Ruiter, P.C.; Brown, G.G. Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 2007, 121, 233–244. [Google Scholar] [CrossRef]
- Hooper, D.U.; Bignell, D.E.; Brown, V.K.; Brussaard, L.; Dangerfield, J.M.; Wall, D.H.; Wardle, D.A.; Coleman, D.C.; Giller, K.E.; Lavelle, P.; et al. Interactions between aboveground and belowground biodiversity in terrestrial ecosystems: Patterns, mechanisms, and feedbacks. BioScience 2000, 50, 1049–1061. [Google Scholar] [CrossRef]
- Barrett, C.; Constas, M. Toward a theory of resilience for international development applications. Proc. Natl. Acad. Sci. USA 2014, 111, 14625–14630. [Google Scholar] [CrossRef] [PubMed]
- Reardon, T.; Vosti, S.A. Links between rural poverty and the environment in developing countries: Asset categories and investment poverty. World Dev. 1995, 23, 1495–1506. [Google Scholar] [CrossRef]
- Cassman, K.G. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. USA 1999, 96, 5952–5959. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.; Pascual, U.; Russell, N. A theoretical model of agrobiodiversity as a supporting service for sustainable agricultural intensification. Ecol. Econ. 2010, 69, 1926–1933. [Google Scholar] [CrossRef]
- Pretty, J. Agricultural sustainability: Concepts, principles and evidence. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2008, 363, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Snapp, S.S.; Blackie, M.J.; Gilbert, R.A.; Bezner-Kerr, R.; Kanyama-Phiri, G.Y. Biodiversity can support a greener revolution in Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 20840–20845. [Google Scholar] [CrossRef] [PubMed]
- Zimmerer, K.S.; Rojas Vaca, H.L. Fine-grain spatial patterning and dynamics of land use and agrobiodiversity amid global changes in the Bolivian Andes. Reg. Environ. Change 2016. [Google Scholar] [CrossRef]
- McMichael, P. Food system sustainability: Questions of environmental governance in the new world (dis) order. Glob. Environ. Chang. 2011, 21, 804–812. [Google Scholar] [CrossRef]
- Thompson, J.; Scoones, I. Addressing the dynamics of agri-food systems: An emerging agenda for social science research. Environ. Sci. Policy 2009, 12, 386–397. [Google Scholar] [CrossRef]
- Turner, B.L. The sustainability principle in global agendas: Implications for understanding land-use/cover change. Geogr. J. 1997, 163, 133–140. [Google Scholar]
- Turner, B.L.; Lambin, E.F.; Reenberg, A. The emergence of land change science for global environmental change and sustainability. Proc. Natl. Acad. Sci. USA 2007, 104, 20666–20671. [Google Scholar] [CrossRef] [PubMed]
- Scherr, S.J.; McNeely, J.A. Biodiversity conservation and agricultural sustainability: Towards a new paradigm of “ecoagriculture” landscapes. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2008, 363, 477–494. [Google Scholar] [CrossRef] [PubMed]
- International Fund for Agricultural Development (IFAD). Smallholders, Food Security, and the Environment; International Fund for Agricultural Development, U.N. Environment Programme: Rome, Italy, 2013. [Google Scholar]
- DeFries, R.; Rosenzweig, C. Toward a whole-landscape approach for sustainable land use in the tropics. Proc. Natl. Acad. Sci. USA 2010, 107, 19627–19632. [Google Scholar] [CrossRef] [PubMed]
- Keys, E.; McConnell, W.J. Global change and the intensification of agriculture in the tropics. Glob. Environ. Chang. 2005, 15, 320–337. [Google Scholar] [CrossRef]
- Rudel, T.K. Meta-analyses of case studies: A method for studying regional and global environmental change. Glob. Environ. Chang. 2008, 18, 18–25. [Google Scholar] [CrossRef]
- Magliocca, N.R.; Rudel, T.K.; Verburg, P.H.; McConnell, W.J.; Mertz, O.; Gerstner, K.; Ellis, E.C. Synthesis in land change science: Methodological patterns, challenges, and guidelines. Reg. Environ. Chang. 2015, 15, 211–226. [Google Scholar] [CrossRef] [PubMed]
- High Level Panel of Experts on Food Security and Nutrition (HLPE). Investing in Smallholder Agriculture for Food Security; High Level Panel of Experts on Food Security and Nutrition of the Committee on World Food Security: Rome, Italy, 2013; pp. 25–28. [Google Scholar]
- DeFries, R.S.; Foley, J.A.; Asner, G.P. Land-use choices: Balancing human needs and ecosystem function. Front. Ecol. Environ. 2004, 2, 249–257. [Google Scholar] [CrossRef]
- Aide, T.M.; Grau, H.R. Globalization, migration, and Latin American ecosystems. Science 2004, 305, 1915–1916. [Google Scholar] [CrossRef] [PubMed]
- Batterbury, S. Landscapes of diversity: A local political ecology of livelihood diversification in south-western Niger. Cult. Geogr. 2001, 8, 437–464. [Google Scholar] [CrossRef]
- Bilsborrow, R.E. Rural Poverty, Migration, and the Environment in Developing Countries: Three Case Studies; World Bank Publications: Washington, DC, USA, 1992; Volume 1017. [Google Scholar]
- Garcia-Barrio, R.; García-Barrios, L. Environmental and technological degradation in peasant agriculture: A consequence of development in Mexico. World Dev. 1990, 18, 1569–1585. [Google Scholar] [CrossRef]
- Bilsborrow, R.E.; DeLargy, P.F. Land use, migration, and natural resource deterioration: The experience of Guatemala and the Sudan. Popul. Dev. Rev. 1990, 16, 125–147. [Google Scholar] [CrossRef]
- Carr, D. Rural migration: The driving force behind tropical deforestation on the settlement frontier. Prog. Hum. Geogr. 2009, 33, 355–378. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.J.; Momsen, J.H. From the kitchen and the field: Gender and maize diversity in the Bajío region of Mexico. Singap. J. Trop. Geogr. 2007, 28, 39–56. [Google Scholar] [CrossRef]
- Chen, X.; Frank, K.A.; Dietz, T.; Liu, J. Weak ties, labor migration, and environmental impacts: Toward a sociology of sustainability. Organ. Environ. 2012, 25, 3–24. [Google Scholar] [CrossRef]
- Collins, J.L. Labor scarcity and ecological change. In Lands at Risk in the Third World: Local-Level Perspectives; Little, P.D., Horowitz, M.M., Eds.; Westview: Boulder, CO, USA, 1987; pp. 19–37. [Google Scholar]
- Collins, J.L. Unseasonal Migrations: The Effects of Rural Labor Scarcity in Peru; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Davis, J.; Lopez-Carr, D. The effects of migrant remittances on population–environment dynamics in migrant origin areas: International migration, fertility, and consumption in highland Guatemala. Popul. Environ. 2010, 32, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Lopez-Carr, D. Migration, remittances and smallholder decision-making: Implications for land use and livelihood change in Central America. Land Use Policy 2014, 36, 319–329. [Google Scholar] [CrossRef] [PubMed]
- European Commission. The Impact of European Water Policy on the Water Cultural Heritage; European Policy Brief; Mediterranean Mountainous Landscapes (MEMOLA): Granada, Spain, 2015. [Google Scholar]
- Grau, H.R.; Aide, T.M. Are rural-urban migration and sustainable development compatible in mountain systems? Mt. Res. Dev. 2007, 27, 119–123. [Google Scholar] [CrossRef]
- Gray, C.L. Rural out-migration and smallholder agriculture in the southern Ecuadorian Andes. Popul. Environ. 2009, 30, 193–217. [Google Scholar] [CrossRef]
- Gray, C.L.; Bilsborrow, R.E. Consequences of out-migration for land use in rural Ecuador. Land Use Policy 2014, 36, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.C. What kind of intensification? Agricultural practice, soil fertility and socioeconomic differentiation in rural Burkina Faso. Geogr. J. 2005, 171, 70–82. [Google Scholar] [CrossRef]
- Heartherington, T. Tasting cultural ecology: Foodscapes of sustainability in the Mediterranean. Gastr. J. Crit. Food Stud. 2014, 14, 16–26. [Google Scholar] [CrossRef]
- Hecht, S.B.; Kandel, S.; Gomes, I.; Cuellar, N.; Rosa, H. Globalization, forest resurgence, and environmental politics in El Salvador. World Dev. 2006, 34, 308–323. [Google Scholar] [CrossRef]
- Huijun, G.; Padoch, C.; Coffey, K.; Aiguo, C.; Yongneng, F. Economic development, land use and biodiversity change in the tropical mountains of Xishuangbanna, Yunnan, Southwest China. Environ. Sci. Policy 2002, 5, 471–479. [Google Scholar] [CrossRef]
- Iniesta-Arandia, I.; del Amo, D.G.; García-Nieto, A.P.; Piñeiro, C.; Montes, C.; Martín-López, B. Factors influencing local ecological knowledge maintenance in Mediterranean watersheds: Insights for environmental policies. AMBIO 2015, 44, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Jewitt, S. Unequal knowledges in Jharkhand, India: De-romanticizing women’s agroecological expertise. Dev. Chang. 2000, 31, 961–985. [Google Scholar] [CrossRef]
- Jiménez Olivencia, Y. Consecuencias del abandono del regadío den la montaña mediterránea. In El Agua Domesticada, Los Paisajes del Regadíos de Montaña en Andalucía; Guzmán Álvarez, J.R., Navarro Cerrillo, R.M., Eds.; Consejería de Medio Ambiente de la Junta de Andalucía: Sevilla, Spain, 2010; pp. 508–513. [Google Scholar]
- Jiménez Olivencia, Y.; Porcel Rodríguez, L.; Caballero Calvo, A. A half-century of landscape evolution in the Sierra Nevada (Spain). Bol. Asoc. Geógr. Españoles 2015, 68, 497–502. [Google Scholar]
- Jokisch, B.D. Migration and agricultural change: The case of smallholder agriculture in Highland Ecuador. Hum. Ecol. 2002, 30, 523–550. [Google Scholar] [CrossRef]
- Lerner, A.M.; Eakin, H.; Sweeney, S. Understanding peri-urban maize production through an examination of household livelihoods in the Toluca Metropolitan Area, Mexico. J. Rural Stud. 2013, 30, 52–63. [Google Scholar] [CrossRef]
- Li, Y.; López-Carr, D.; Chen, W. Factors affecting migration intentions in ecological restoration areas and their implications for the sustainability of ecological migration policy in arid northwest China. Sustainability 2014, 6, 8639–8660. [Google Scholar] [CrossRef]
- López, E.; Bocco, G.; Mendoza, M.; Velázquez, A.; Aguirre-Rivera, J.R. Peasant emigration and land-use change at the watershed level: A GIS-based approach in Central Mexico. Agric. Syst. 2006, 90, 62–78. [Google Scholar] [CrossRef]
- Mutersbaugh, T. Certifying biodiversity: Conservation networks, landscape connectivity, and certified agriculture in Southern Mexico. In Globalization and New Geographies of Conservation; Zimmerer, K.S., Ed.; University of Chicago Press: Chicago, IL, USA, 2006; pp. 49–70. [Google Scholar]
- Nuijten, E. Gender and management of crop diversity in The Gambia. J. Political Ecol. 2010, 17, 42–58. [Google Scholar]
- Preston, D.; Macklin, M.; Warburton, J. Fewer people, less erosion: The twentieth century in southern Bolivia. Geogr. J. 1997, 198–205. [Google Scholar] [CrossRef]
- Qin, H. Rural-to-urban labor migration, household livelihoods, and the rural environment in Chongqing Municipality, Southwest China. Hum. Ecol. 2010, 38, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Radel, C.; Schmook, B. Male transnational migration and its linkages to land-use change in a southern Campeche ejido. J. Lat. Am. Geogr. 2008, 7, 59–84. [Google Scholar] [CrossRef]
- Radel, C.; Schmook, B.; McEvoy, J.; Mendez, C.; Petrzelka, P. Labour migration and gendered agricultural relations: The feminization of agriculture in the Ejidal sector of Calakmul, Mexico. J. Agrar. Chang. 2012, 12, 98–119. [Google Scholar] [CrossRef]
- Reardon, T.; Berdegue, J.; Escobar, G. Rural nonfarm employment and incomes in Latin America: Overview and policy implications. World Dev. 2001, 29, 395–409. [Google Scholar] [CrossRef]
- Rudel, T.K.; Bates, D.; Machinguiashi, R. A tropical forest transition? Agricultural change, out-migration, and secondary forests in the Ecuadorian Amazon. Ann. Assoc. Am. Geogr. 2002, 92, 87–102. [Google Scholar] [CrossRef]
- Schmook, B.; van Vliet, N.; Radel, C.; de Jesus Manzon-Che, M.; McCandless, S. Persistence of Swidden cultivation in the face of globalization: A case study from communities in Calakmul, Mexico. Hum. Ecol. 2013, 41, 93–107. [Google Scholar] [CrossRef]
- Turner, M.D. Labor process and the environment: The effects of labor availability and compensation on the quality of herding in the Sahel. Hum. Ecol. 1999, 27, 267–296. [Google Scholar] [CrossRef]
- Zimmerer, K.S. Labor shortages and crop diversity in the southern Peruvian sierra. Geogr. Rev. 1991, 81, 414–432. [Google Scholar] [CrossRef]
- Zimmerer, K.S. Soil erosion and labor shortages in the Andes with special reference to Bolivia, 1953–1991: Implications for “conservation-with-development”. World Dev. 1993, 21, 1659–1675. [Google Scholar] [CrossRef]
- Zimmerer, K.S. Conserving agrobiodiversity amid global change, migration, and nontraditional livelihood networks: The dynamic uses of cultural landscape knowledge. Ecol. Soc. 2014, 19, 1. [Google Scholar]
- Acosta-Martínez, V.; Bell, C.W.; Morris, B.E.L.; Zak, J.; Allen, V.G. Long-term soil microbial community and enzyme activity responses to an integrated cropping-livestock system in a semi-arid region. Agric. Ecosyst. Environ. 2010, 137, 231–240. [Google Scholar] [CrossRef]
- Alvey, S.; Bagayoko, M.; Neumann, G.; Buerkert, A. Cereal/legume rotations affect chemical properties and biological activities in two West African soils. Plant Soil 2001, 231, 45–54. [Google Scholar] [CrossRef]
- Asgharipour, M.R.; Rafiei, M. The effects of land use on biomass and catabolic diversity of soil microbial communities. Afr. J. Agric. Res. 2011, 6, 4607–4612. [Google Scholar]
- Bach, E.M.; Baer, S.G.; Six, J. Plant and soil responses to high and low diversity grassland restoration practices. Environ. Manag. 2012, 49, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Berthrong, S.T.; Schadt, C.W.; Pineiro, G.; Jackson, R.B. Afforestation alters the composition of functional genes in soil and biogeochemical processes in South American grasslands. Appl. Environ. Microbiol. 2009, 75, 6240–6248. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D.H.; Schmidt, T.M. The structure of microbial communities in soil and the lasting impact of cultivation. Microb. Ecol. 2001, 42, 11–21. [Google Scholar] [PubMed]
- Duchicela, J.; Sullivan, T.S.; Bontti, E.; Bever, J.D. Soil aggregate stability increase is strongly related to fungal community succession along an abandoned agricultural field chronosequence in the Bolivian Altiplano. J. Appl. Ecol. 2013, 50, 1266–1273. [Google Scholar] [CrossRef]
- Garbeva, P.; Postma, J.; van Veen, J.A.; van Elsas, J.D. Effect of aboveground plant species on soil microbial community structure and its impact on suppression of Rhizoctonia solani AG3. Environ. Microbiol. 2006, 8, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.R.L.; Nahas, E. Microbial populations and the activity of the soil under agricultural and agricultural–pastoral systems. Arch. Agron. Soil Sci. 2012, 58, 511–525. [Google Scholar] [CrossRef]
- Gomez, E.V.; Garland, J.L.; Roberts, M.S. Microbial structural diversity estimated by dilution–extinction of phenotypic traits and T-RFLP analysis along a land-use intensification gradient. FEMS Microbiol. Ecol. 2004, 49, 253–259. [Google Scholar] [CrossRef] [PubMed]
- González-Chávez, M.A.; Aitkenhead-Peterson, J.A.; Gentry, T.J.; Zuberer, D.; Hons, F.; Loeppert, R. Soil microbial community, C, N, and P responses to long-term tillage and crop rotation. Soil Tillage Res. 2010, 106, 285–293. [Google Scholar] [CrossRef]
- Jiang, D.; Cao, C.; Zhang, Y.; Cui, Z.; Han, X. Plantations of native shrub species restore soil microbial diversity in the Horqin Sandy Land, northeastern China. J. Arid Land 2014, 6, 445–453. [Google Scholar] [CrossRef]
- Johnson, D.; Anderson, I.C.; Williams, A.; Whitlock, R.; Grime, J.P. Plant genotypic diversity does not beget root-fungal species diversity. Plant Soil 2010, 336, 107–111. [Google Scholar] [CrossRef]
- Larkin, R.P.; Honeycutt, C.W. Effects of different 3-year cropping systems on soil microbial communities and Rhizoctonia diseases of potato. Phytopathology 2006, 96, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Lienhard, P.; Terrat, S.; Prévost-Bouré, N.C.; Nowak, V.; Régnier, T.; Sayphoummie, S.; Panyasiri, K.; Tivet, F.; Mathieu, O.; Levêque, J.; et al. Pyrosequencing evidences the impact of cropping on soil bacterial and fungal diversity in Laos tropical grassland. Agron. Sustain. Dev. 2014, 34, 525–533. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Tiemann, L.K.; Grandy, A.S. Does agricultural crop diversity enhance soil microbial biomass and organic matter dynamics? A meta-analysis. Ecol. Appl. 2014, 24, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Meriles, J.M.; Vargas Gil, S.; Conforto, C.; Figoni, G.; Lovera, E.; March, G.J.; Guzmán, C.A. Soil microbial communities under different soybean cropping systems: Characterization of microbial population dynamics, soil microbial activity, microbial biomass, and fatty acid profiles. Soil Tillage Res. 2009, 103, 271–281. [Google Scholar] [CrossRef]
- Millard, P.; Singh, B.K. Does grassland vegetation drive soil microbial diversity? Nutr. Cycl. Agroecosyst. 2010, 88, 147–158. [Google Scholar] [CrossRef]
- Nayyar, A.; Hamel, C.; Lafond, G.; Gossen, B.D.; Hanson, K.; Germida, J. Soil microbial quality associated with yield reduction in continuous-pea. Appl. Soil Ecol. 2009, 43, 115–121. [Google Scholar] [CrossRef]
- Nurulita, Y.; Adetutu, E.M.; Kadali, K.K.; Zul, D.; Mansur, A.A.; Ball, A.S. The assessment of the impact of oil palm and rubber plantations on the biotic and abiotic properties of tropical peat swamp soil in Indonesia. Int. J. Agric. Sustain. 2015, 13, 150–166. [Google Scholar] [CrossRef]
- Shen, W.; Lin, X.; Gao, N.; Zhang, H.; Yin, R.; Shi, W.; Duan, Z. Land use intensification affects soil microbial populations, functional diversity and related suppressiveness of cucumber Fusarium wilt in China’s Yangtze River Delta. Plant Soil 2008, 306, 117–127. [Google Scholar] [CrossRef]
- Sheng, R.; Meng, D.; Wu, M.; Di, H.; Qin, H.; Wei, W. Effect of agricultural land use change on community composition of bacteria and ammonia oxidizers. J. Soil Sediments 2013, 13, 1246–1256. [Google Scholar] [CrossRef]
- Vasileiadis, S.; Puglisi, E.; Arena, M.; Cappa, F.; van Veen, J.A.; Cocconcelli, P.S.; Trevisan, M. Soil microbial diversity patterns of a lowland spring environment. FEMS Microbiol. Ecol. 2013, 86, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Bowman, D.; Shi, W. Soil microbial community structure and diversity in a turfgrass chronosequence: Land-use change versus turfgrass management. Appl. Soil Ecol. 2006, 34, 209–218. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, W.; Yin, Y.; Zhang, J.; Cai, Z.; Zhong, W. Response of soil microbial diversity to land-use conversion of natural forests to plantations in a subtropical mountainous area of southern China. Soil Sci. Plant Nutr. 2012, 58, 450–461. [Google Scholar] [CrossRef]
- Almaca, A.; Ortas, I. Growth response of maize plants (Zea mays L.) to wheat and lentil pre-cropping and to indigenous mycorrhizae in field soil. Span. J. Agric. Res. 2010, 8, 131–136. [Google Scholar] [CrossRef]
- Bedini, S.; Avio, L.; Sbrana, C.; Turrini, A.; Migliorini, P.; Vazzana, C.; Giovannetti, M. Mycorrhizal activity and diversity in a long-term organic Mediterranean agroecosystem. Biol. Fertil. Soils 2013, 49, 781–790. [Google Scholar] [CrossRef]
- Carpenter, F.L.; Mayorga, S.P.; Quintero, E.G.; Schroeder, M. Land-use and erosion of a Costa Rican Ultisol affect soil chemistry, mycorrhizal fungi and early regeneration. For. Ecol. Manag. 2001, 144, 1–17. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Mehta, C.M.; Singh, S.; Sharma, A.K. Host influences Arbuscular mycorrhizal fungal diversity. J. Mycol. Plant Pathol. 2009, 39, 124. [Google Scholar]
- Dai, M.; Bainard, L.D.; Hamel, C.; Gan, Y.; Lynch, D. Impact of land use on arbuscular mycorrhizal fungal communities in Rural Canada. Appl. Environ. Microbiol. 2013, 79, 6719–6729. [Google Scholar] [CrossRef] [PubMed]
- Gavito, M.E.; Miller, M.H. Changes in mycorrhiza development in maize induced by crop management practices. Plant Soil 1998, 198, 185–192. [Google Scholar] [CrossRef]
- Hendrix, J.W.; Guo, B.Z.; An, Z.Q. Divergence of mycorrhizal fungal communities in crop production systems. Plant Soil 1995, 170, 131–140. [Google Scholar] [CrossRef]
- Higo, M.; Isobe, K.; Drijber, R.A.; Kondo, T.; Yamaguchi, M.; Takeyama, S.; Suzuki, Y.; Niijima, D.; Matsuda, Y.; Ishii, R.; et al. Impact of a 5-year winter cover crop rotational system on the molecular diversity of arbuscular mycorrhizal fungi colonizing roots of subsequent soybean. Biol. Fertil. Soils 2014, 50, 913–926. [Google Scholar] [CrossRef]
- Hijri, I.; SýKorová, Z.; Oehl, F.; Ineichen, K.; Mäder, P.; Wiemken, A.; Redecker, D. Communities of arbuscular mycorrhizal fungi in arable soils are not necessarily low in diversity. Mol. Ecol. 2006, 15, 2277–2289. [Google Scholar] [CrossRef] [PubMed]
- Lehman, R.M.; Taheri, W.I.; Osborne, S.L.; Buyer, J.S.; Douds, D.D. Fall cover cropping can increase arbuscular mycorrhizae in soils supporting intensive agricultural production. Appl. Soil Ecol. 2012, 61, 300–304. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T.; Twomlow, S.J. Effect of agricultural management practices on arbuscular mycorrhizal fungal abundance in low-input cropping systems of southern Africa: A case study from Zimbabwe. Biol. Fertil. Soils 2008, 44, 917–923. [Google Scholar] [CrossRef]
- Melo, C.D.; Walker, C.; Rodríguez-Echeverría, S.; Borges, P.A.; Freitas, H. Species composition of arbuscular mycorrhizal fungi differ in semi-natural and intensively managed pastures in an isolated oceanic island (Terceira, Azores). Symbiosis 2014, 64, 73–85. [Google Scholar] [CrossRef]
- Muchane, M.N. Effect of land use system on Arbuscular Mycorrhiza fungi in Maasai Mara ecosystem, Kenya. Afr. J. Microbiol. Res. 2012, 6, 3904–3916. [Google Scholar]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Mader, P.; Boller, T.; Wiemken, A. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 2003, 69, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Tarafdar, J.C.; Sharma, S.K.; Kumar, P.; Aggarwal, R.K. Influence of cropping systems on soil biochemical properties in an arid rain-fed environment. J. Arid Environ. 1995, 31, 237–244. [Google Scholar] [CrossRef]
- Säle, V.; Aguilera, P.; Laczko, E.; Mäder, P.; Berner, A.; Zihlmann, U.; van der Heijden, M.G.A.; Oehl, F. Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2015, 84, 38–52. [Google Scholar] [CrossRef]
- Stürmer, S.L.; Siqueira, J.O. Species richness and spore abundance of arbuscular mycorrhizal fungi across distinct land uses in Western Brazilian Amazon. Mycorrhiza 2011, 21, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Tchabi, A.; Coyne, D.; Hountondji, F.; Lawouin, L.; Wiemken, A.; Oehl, F. Arbuscular mycorrhizal fungal communities in sub-Saharan Savannas of Benin, West Africa, as affected by agricultural land use intensity and ecological zone. Mycorrhiza 2008, 18, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Drijber, R.A.; Zhang, J.L.; Li, X.L. Impact of long-term nitrogen fertilization and rotation with soybean on the diversity and phosphorus metabolism of indigenous arbuscular mycorrhizal fungi within the roots of maize (Zea mays L.). Agric. Ecosyst. Environ. 2013, 164, 53–61. [Google Scholar] [CrossRef]
- Verbruggen, E.; Röling, W.F.M.; Gamper, H.A.; Kowalchuk, G.A.; Verhoef, H.A.; van der Heijden, M.G.A. Positive effects of organic farming on belowground mutualists: Large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol. 2010, 186, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Vestberg, M.; Kahiluoto, H.; Wallius, E. Arbuscular mycorrhizal fungal diversity and species dominance in a temperate soil with long-term conventional and low-input cropping systems. Mycorrhiza 2011, 21, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, A.; Bedano, J.C.; Becker, A.R. Negative effects of no-till on soil macrofauna and litter decomposition in Argentina as compared with natural grasslands. Soil Tillage Res. 2010, 110, 51–59. [Google Scholar] [CrossRef]
- Hulugalle, N.R.; de Bruyn, L.A.L.; Entwistle, P. Residual effects of tillage and crop rotation on soil properties, soil invertebrate numbers and nutrient uptake in an irrigated Vertisol sown to cotton. Appl. Soil Ecol. 1997, 7, 11–30. [Google Scholar] [CrossRef]
- Maria de Aquino, A.; Ferreira da Silva, R.; Mercante, F.M.; Fernandes Correia, M.E.; de Fátima Guimarães, M.; Lavelle, P. Invertebrate soil macrofauna under different ground cover plants in the no-till system in the Cerrado. Eur. J. Soil Biol. 2008, 44, 191–197. [Google Scholar] [CrossRef]
- Sileshi, G.; Mafongoya, P.; Chintu, R.; Akinnifesi, F. Mixed-species legume fallows affect faunal abundance and richness and N cycling compared to single species in maize-fallow rotations. Soil Biol. Biochem. 2008, 40, 3065–3075. [Google Scholar] [CrossRef]
- Hunter, L.M.; Luna, J.K.; Norton, R.M. Environmental dimensions of migration. Ann. Rev. Sociol. 2015, 41, 377–397. [Google Scholar] [CrossRef]
- Carr, D.L.; Lopez, A.C.; Bilsborrow, R.E. The population, agriculture, and environment nexus in Latin America: Country-level evidence from the latter half of the twentieth century. Popul. Environ. 2009, 30, 222–246. [Google Scholar] [CrossRef]
- Paulson, S. Gendered practices and landscapes in the Andes: The shape of asymmetrical exchanges. Hum. Organ. 2003, 62, 242–254. [Google Scholar] [CrossRef]
- Radel, C.; Schmook, B.; McCandless, S. Environment, transnational labor migration, and gender: Case studies from southern Yucatan, Mexico and Vermont, USA. Popul. Environ. 2010, 32, 177–197. [Google Scholar] [CrossRef]
- Barrett, C.B.; Reardon, T.; Webb, P. Nonfarm income diversification and household livelihood strategies in rural Africa: Concepts, dynamics, and policy implications. Food Policy 2001, 26, 315–331. [Google Scholar] [CrossRef]
- Barrett, C.B.; Travis, A.J.; Dasgupta, P. On biodiversity conservation and poverty traps. Proc. Natl. Acad. Sci. USA 2011, 108, 13907–13912. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.L. Environment, land, and rural out-migration in the Southern Ecuadorian Andes. World Dev. 2009, 37, 457–468. [Google Scholar] [CrossRef]
- Gray, C.L. Soil quality and human migration in Kenya and Uganda. Glob. Environ. Chang. 2011, 21, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.L.; Bilsborrow, R.E. Environmental influences on human migration in rural Ecuador. Demography 2013, 50, 1217–1241. [Google Scholar] [CrossRef] [PubMed]
- López-Carr, D. Agro-ecological drivers of rural out-migration to the Maya Biosphere Reserve, Guatemala. Environ. Res. Lett. 2012, 7, 045603. [Google Scholar] [CrossRef] [PubMed]
- Zimmerer, K.S. The landscape technology of spate irrigation amid development changes: Assembling the links to resources, livelihoods, and agrobiodiversity-food in the Bolivian Andes. Glob. Environ. Chang. 2011, 21, 917–934. [Google Scholar] [CrossRef]
- Rudel, T.K.; Schneider, L.; Uriarte, M.; Turner, B.L.; DeFries, R.; Lawrence, D.; Geoghegan, J.; Hecht, S.; Ickowitz, A.; Lambin, E.F.; et al. Agricultural intensification and changes in cultivated areas, 1970–2005. Proc. Natl. Acad. Sci. USA 2009, 106, 20675–20680. [Google Scholar] [CrossRef] [PubMed]
- Rudel, T.K.; Defries, R.; Asner, G.P.; Laurance, W.F. Changing drivers of deforestation and new opportunities for conservation. Conserv. Biol. 2009, 23, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- López-Carr, D.; Burgdorfer, J. Deforestation drivers: Population, migration, and tropical land use. Environment 2013, 55, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.A. Peasant women and economic transformation in The Gambia. Dev. Chang. 1992, 23, 67–90. [Google Scholar] [CrossRef]
- Carney, J.A. The bitter harvest of Gambian rice policies. Globalizations 2008, 5, 129–142. [Google Scholar] [CrossRef]
- Deere, C.D. The division of labor by sex in agriculture: A Peruvian case study. Econ. Dev. Cult. Chang. 1982, 30, 795–811. [Google Scholar] [CrossRef]
- Deere, C.D.; León de Leal, M. Women in Andean Agriculture: Peasant Production and Rural Wage Employment in Colombia and Peru; International Labor Office: Geneva, Switzerland, 1982. [Google Scholar]
- Momsen, J.H. Gender and agrobiodiversity: Introduction to the Special Issue. Singap. J. Trop. Geogr. 2007, 28, 1–6. [Google Scholar] [CrossRef]
- Sachs, C. Reconsidering diversity in agriculture and food systems: An ecofeminist approach. Agric. Hum. Values 1992, 9, 4–10. [Google Scholar] [CrossRef]
- Sachs, C. Gendered Fields: Rural Women, Agriculture, and Environment; Westview Press: Boulder, CO, USA, 1996. [Google Scholar]
- Sachs, C. Women Working in the Environment: Resourceful Natures; Taylor & Francis: New York, NY, USA, 2014. [Google Scholar]
- Sachs, C.; Gajurel, K.; Bianco, M. Gender, seeds, and biodiversity. In Women Working in the Environment; Sachs, C., Ed.; Taylor and Francis: Washington, DC, USA, 1996; pp. 177–192. [Google Scholar]
- Kerr, R.B. Lost and found crops: Agrobiodiversity, indigenous knowledge, and a feminist political ecology of sorghum and finger millet in Northern Malawi. Ann. Assoc. Am. Geogr. 2014, 104, 577–593. [Google Scholar] [CrossRef]
- Giller, K.E.; Tittonell, P.; Rufino, M.C.; van Wijk, M.T.; Zingore, S.; Mapfumo, P.; Adjei-Nsiah, S.; Herrero, M.; Chikowo, R.; Corbeels, M.; et al. Communicating complexity: Integrated assessment of trade-offs concerning soil fertility management within African farming systems to support innovation and development. Agric. Syst. 2011, 104, 191–203. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; Corbeels, M.; Tittonell, P. Conservation agriculture and smallholder farming in Africa: The heretics’ view. Field Crops Res. 2009, 114, 23–34. [Google Scholar] [CrossRef]
- Radel, C. Gendered livelihoods and the politics of socio-environmental identity: Women’s participation in conservation projects in Calakmul, Mexico. Gend. Place Cult. 2012, 19, 61–82. [Google Scholar] [CrossRef]
- Schmook, B.; Radel, C. International labor migration from a tropical development frontier: Globalizing households and an incipient forest transition. Hum. Ecol. 2008, 36, 891–908. [Google Scholar] [CrossRef]
- Kröhnert, S.; Klingholz, R. Not am Mann: Von Helden der Arbeit zur Neuen Unterschicht? Berlin-Institut für Bevölkerung und Entwicklung: Berlin, Germany, 2007. [Google Scholar]
- Bilsborrow, R.E.; Ogendo, H.W. Population-driven changes in land use in developing countries. AMBIO 1992, 21, 37–45. [Google Scholar]
- Barrios, E. Soil biota, ecosystem services and land productivity. Ecol. Econ. 2007, 64, 269–285. [Google Scholar] [CrossRef]
- Culman, S.W.; Young-Mathews, A.; Hollander, A.D.; Ferris, H.; Sánchez-Moreno, S.; O’Geen, A.T.; Jackson, L.E. Biodiversity is associated with indicators of soil ecosystem functions over a landscape gradient of agricultural intensification. Landsc. Ecol. 2010, 25, 1333–1348. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Moreira, F.M.S.; Siqueira, J.O.; Brussaard, L. Soil organisms in tropical ecosystems: A key role for Brazil in the global quest for the conservation and sustainable use of biodiversity. In Soil Biodiversity in Amazonian and Other Brazilian Ecosystems; Moreira, F.M.S., Siqueira, J.O., Brussaard, L., Eds.; CABI: Wallingford, UK, 2006; pp. 1–12. [Google Scholar]
- Reynolds, H.L.; Packer, A.; Bever, J.D.; Clay, K. Grassroots ecology: Plant-microbe-soil interactions as drivers of plant community structure and dynamics. Ecology 2003, 84, 2281–2291. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Buma, D.S.; de Boer, W.; Klinkhamer, P.G.; van Veen, J.A. Effects of aboveground plant species composition and diversity on the diversity of soil-borne microorganisms. Antonie Van Leeuwenhoek 2002, 81, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Berthrong, S.T.; Buckley, D.H.; Drinkwater, L.E. Agricultural management and labile carbon additions affect soil microbial community structure and interact with carbon and nitrogen cycling. Microb. Ecol. 2013, 66, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Witter, E.; Mcgrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Lavelle, P.; Bignell, D.E.; Austen, M.C.; Brown, V.K.; Behan-Pelletier, V.; Garey, J.R.; Giller, P.S.; Hawkins, S.J.; Brown, G.G.; St. John, M.; et al. Connecting soil and sediment biodiversity: The role of scale and implications for management. In Sustaining Biodiversity and Ecosystem Services in Soils and Sediments; Wall, D.H., Ed.; Island Press: Washington, DC, USA, 2004; pp. 193–224. [Google Scholar]
- Liu, A.; Hamel, C.; Begna, S.H.; Ma, B.L.; Smith, D.L. Soil phosphorus depletion capacity of arbuscular mycorrhizae formed by maize hybrids. Can. J. Soil Sci. 2003, 83, 337–342. [Google Scholar] [CrossRef]
- Martinez, T.N.; Johnson, N.C. Agricultural management influences propagule densities and functioning of arbuscular mycorrhizas in low- and high-input agroecosystems in arid environments. Appl. Soil Ecol. 2010, 46, 300–306. [Google Scholar] [CrossRef]
- Evans, L.T. Crop Evolution, Adaptation and Yield; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Kates, R.W.; Parris, T.M. Long-term trends and a sustainability transition. Proc. Natl. Acad. Sci. USA 2003, 100, 8062–8067. [Google Scholar] [CrossRef] [PubMed]
- Lambin, E.F.; Meyfroidt, P. Global land use change, economic globalization, and the looming land scarcity. Proc. Natl. Acad. Sci. USA 2011, 108, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.C.; Reenberg, A.; Boone, C.G.; Fragkias, M.; Haase, D.; Langanke, T.; Simon, D. Urban land teleconnections and sustainability. Proc. Natl. Acad. Sci. USA 2012, 109, 7687–7692. [Google Scholar] [CrossRef] [PubMed]
- Greiner, C.; Sakdapolrak, P. Rural–urban migration, agrarian change, and the environment in Kenya: A critical review of the literature. Popul. Environ. 2013, 34, 524–553. [Google Scholar] [CrossRef]
- Neumann, K.; Hilderink, H. Opportunities and challenges for investigating the environment-migration nexus. Hum. Ecol. 2015, 43, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.; Gramig, G.G.; Hendrickson, J.R.; Archer, D.W.; Forcella, F.; Liebig, M.A. Crop species diversity changes in the United States: 1978–2012. PLoS ONE 2015, 10, e0136580. [Google Scholar] [CrossRef] [PubMed]
- Tsing, A.L. Friction: An Ethnography of Global Connection; Princeton University Press: Princeton, NJ, USA, 2005. [Google Scholar]
- Sundberg, J. Decolonizing posthumanist geographies. Cult. Geogr. 2014, 21, 33–47. [Google Scholar] [CrossRef]
- De Janvry, A. The Agrarian Question and Reformism in Latin America; Johns Hopkins University Press: Baltimore, MD, USA, 1981. [Google Scholar]



| Search terms consulted (Web of Science database) |
| (“Livelihood diversification” OR “migration” OR “development”) AND (“agrobiodiversity” OR “crop diversity” OR “soil” OR “water” OR “biodiversity” OR “secondary forest transition”) |
| Authors and publication year of sources with numbered reference |
| Aide, and Grau 2004 [79], Batterbury 2001 [80], Bilsborrow 1992 [81], Garcia-Barrio and García-Barrios 1990 [82], Bilsborrow and DeLargy 1990 [83], Carr 2009 [84], Chambers and Momsen 2007 [85], Chen et al. 2012 [86], Collins 1987, 1988 [87,88], Davis and López-Carr 2010, 2014 [89,90], European Commission 2015 [91], Grau and Aide 2007 [92], Gray 2009a [93] Gray and Bilsborrow 2014 [94], Gray 2005 [95], Heartherington 2014 [96], Hecht et al., 2006 [97], Huijun et al. 2002 [98], Iniesta-Arandia et al., 2015 [99], Jewitt 2000 [100], Jiménez Olivencia 2010 [101], Jiménez Olivencia et al., 2015 [102], Jokisch 2002 [103], Lerner et al., 2013 [104], Li et al. 2014 [105], López 2006 [106], McCord et al. 2015 [27], Mutersbaugh 2006 [107]; Nuijten 2010 [108], Preston, D 1997 [109], Qin 2010 [110], Radel and Schmook 2008 [111], Radel et al. 2012 [112], Reardon et al. 2001 [113], Rudel et al. 2002 [114], Schmook et al. 2013 [115], Turner 1999 [116], Zimmerer 1991 [117], 1993 [118], 1996 [7], 2014 [119]. |
| Search terms consulted (Web of Science database) | |
| (“Land use” OR “crop rotation” OR “crop diversity” OR “cropping system“) AND (“microbial diversity” OR “microbial functional diversity” OR “macrofaunal diversity” OR “macrofauna” OR “mycorrhiza” OR “fungal diversity” OR “bacterial diversity”) | |
| Domain of soil biota | Authors and publication year of sources with numbered reference |
| Whole community: e.g., soil bacteria, archaea, and/or fungal communities | Acosta-Martínez et al. 2010 [120], Alvey et al. 2001 [121], Asgharipour et al. 2011 [122], Bach et al. 2012 [123], Berthrong et al. 2009 [124], Buckley and Schmidt 2003 [125], Duchicela 2013 [126], Garbeva et al. 2006 [127], Garcia et al. 2012 [128], Gomez et al. 2004 [129], Gonzalez- Chavez et al. 2010 [130], Jiang et al. 2014 [131], Johnson et al. 2010 [132], Larkin and Honeycutt 2006 [133], Lienhard et al. 2014 [134], McDaniel et al. 2014 [135], Meriles et al. 2009 [136], Millard and Singh 2010 [137], Nayyar et al. 2009c[138], Nurulita et al. 2015 [139], Shen et al. 2008 [140], Sheng et al. 2013 [141], Vasileiadis 2013 [142], Yao et al. 2006 [143], Yu et al. 2012 [144] |
| Mycorrhizae | Almaca and Ortas 2010 [145], Bedini et al. 2013 [146], Carpenter et al. 2001 [147], Chaturvedi et al. 2009 [148], Dai et al. 2013 [149], Gavito and Miller 1998 [150], Hendrix et al. 1995 [151], Higo et al. 2014 [152], Hijri et al. 2006 [153], Lehman et al. 2012 [154], Lekberg et al. 2008 [155], Melo et al. 2014 [156], Muchane 2012 [157], Oehl et al. 2003 [158], Rao et al. 1995 [159], Säle et al. 2015 [160], Sturmer et al. 2011 [161], Tchabi et al. 2008 [162], Tian et al. 2013 [163], Verbruggen et al. 2010 [164], Vestberg et al. 2011 [165] |
| Macrofauna | Dominguez et al. 2010 [166], Hulugalle et al. 1997 [167], Maria De Aquino et al. 2008 [168], Sileshi et al. 2008 [169] |
| 3.1. All studies: association of livelihood diversification with change vs. no change in agrobiodiversity, soil, and water resource factors | |||||
| N (Studies Reporting) | Change in One or More Factors | No Change (in Any Factor) | |||
| 42 | 93% | 7% | |||
| 3.2. All studies: changes in food plant agrobiodiversity richness (species or varieties) and the environmental quality of soil and water resources | |||||
| N (Number of Comparisons) | Increase | No Change or Mixed | Decrease | Observations and Main Tendencies | |
| Changes in agrobiodiversity | N = 12 | 8% | 58% | 33% | Change interpreted as a mixed outcome shows both increase and decrease occurring as local variation among households and local places as well as regionally. |
| Changes in soil quality | N = 27 | 26% | 15% | 59% | |
| Changes in water quality | N = 17 | 35% | 12% | 53% | |
| Changes in wild biodiversity | N = 18 | 39% | 22% | 39% | |
| 3.3. Studies grouped by predominant type of livelihood diversification | |||||
| (a) Studies that focus primarily on migration as the major form of livelihood diversification | |||||
| Changes in agrobiodiversity | N = 6 | 17% | 50% | 33% | Livelihood diversification studies with a migration emphasis tend to incorporate a focus on the abandonment of soil and water infrastructure (e.g., historic irrigation systems) |
| Changes in soil quality | N = 20 | 25% | 15% | 65% | |
| Changes in water quality | N = 12 | 25% | 8% | 67% | |
| Changes in wild biodiversity | N = 12 | 42% | 25% | 33% | |
| (b) Studies with emphasis primarily on-farm livelihood diversification (without migration emphasis) | |||||
| Changes in agrobiodiversity | N = 6 | 0% | 67% | 33% | Livelihood diversification studies (without migration emphasis) tend to focus on local land-use capacity, including increased infrastructure for soil and water management (e.g., irrigation) |
| Changes in soil quality | N = 7 | 33% | 17% | 50% | |
| Changes in water quality | N = 5 | 60% | 20% | 20% | |
| Changes in wild biodiversity | N = 6 | 33% | 16% | 50% | |
| 3.4. All studies: gender analysis in diversified livelihood interactions with food plant agrobiodiversity, soil, and water resources | |||||
| N (Number of Comparisons) | Frequency | ||||
| Contains analysis of gendering | N = 23 | 55% | Level of gender analysis varies widely in these studies | ||
| Women’s increased role in resource use | N = 22 | 96% | |||
| Men’s increased role in resource use | N = 8 | 35% | Associated principally with livelihood diversification and relatively minor role of migration and, in some cases, recent increased migration among rural women (e.g., Spain and Andean countries) | ||
| 4.1. All studies: changes in soil biotic community structure | |||||
| N (Number of Comparisons) | Change | No change | |||
| N = 53 | 92% | 8% | |||
| 4.2. All studies: changes in soil biological diversity, abundance, and biological function under increasing aboveground biodiversity | |||||
| N (Number of Comparisons) | Increase | No Change | Decrease | Observations and Main Tendencies | |
| Changes in soil biotic diversity | N = 53 | 55% | 34% | 9% | |
| Changes in biotic abundance or biomass | N = 26 | 69% | 23% | 4% | |
| Changes in soil biological function (e.g., enzyme activity, mycorrhizal colonization) | N = 28 | 79% | 14% | 7% | |
| 4.3. Studies grouped by land management types that alter aboveground biodiversity: | |||||
| Studies that examined changes in land use (forest conversion, succession, agroforestry vs. cropping) | |||||
| Changes in soil biotic diversity | N = 25 | 64% | 28% | 8% | Differences in soil organic matter accumulation, disturbance, and species type that drive soil biotic differences are especially strong between long-term perennial uses as a group (e.g., forest) versus cropping systems as a group. |
| Changes in biotic abundance or biomass | N = 9 | 78% | 11% | 11% | |
| Changes in soil biological function | N = 8 | 100% | 0% | 0% | |
| Studies that examined changes in agricultural and livestock management within agricultural land uses (among crop rotations, fallows, pastures) | |||||
| Changes in soil biotic diversity | N = 34 | 41% | 47% | 9% | Management accompanying crop diversity is important, e.g., increased tillage under continuous monoculture vs. diverse rotations with fallows and variation in tillage regimes. |
| Changes in biotic abundance or biomass | N = 16 | 69% | 31% | 0% | |
| Changes in soil biological function | N = 17 | 65% | 24% | 12% | |
| 4.4. Studies grouped by soil biota type impacted by increasing aboveground biodiversity: | |||||
| N (Number of Comparisons) | Increase | No Change | Decrease | Observations and Main Tendencies | |
| Analyses of soil whole microbial communities (using e.g., bacteria, archaea, fungi using whole metagenome analysis) | |||||
| Increases in soil biotic diversity | N = 20 | 50% | 40% | 10% | Long lag times: decadal “imprint” of management based on previous land use. High degree of functional diversity and redundancy in bacterial and archaeal communities that creates temporal stability/hysteresis in community composition. Less well-understood aboveground/belowground relationships than for symbiont communities or macrofauna. In addition the fungal: bacterial ratio of various biomass measures (e.g., PLFA) often increases with the amount of recalcitrant residues in systems and decreases under soil disturbance (e.g., grassland has a higher fungal: bacterial ratio than an intensified vegetable rotation.) |
| Increases in biotic abundance or biomass | N = 9 | 78% | 22% | 0% | |
| Analyses of arbuscular mycorrhizal communities (using spore classification and counts and fingerprinting using mycorrhizal DNA primers) | |||||
| Increases in soil biotic diversity | N = 34 | 59% | 35% | 6% | Strong influence of whether aboveground species that add biodiversity are mycorrhizal or non-mycorrhizal, and specialist vs. generalist mycorrhizal fungal species. |
| Increases in biotic abundance or biomass | N = 12 | 67% | 25% | 8% | |
| Analyses of macrofaunal communities (using field methods and visual classification of macrofauna) | |||||
| Increases in soil biotic diversity | N = 4 | 75% | 25% | 0% | Mediation by plant residue quantity and quality accompanying aboveground species diversity and associated management is especially strong. |
| Increases in biotic abundance or biomass | N = 3 | 100% | 0% | 0% | |
| Regional Agri-Food and Livelihood Diversification Space | Predicted Social-Ecological Characterization | Predicted Agricultural Intensification Level | Predicted Livelihood Diversification Level | Anticipated Environmental Change Pathway 1 | Above-ground Agrobiodiversity | Belowground Agrobiodiversity | Examples (United States and Peru) |
|---|---|---|---|---|---|---|---|
| Prime Agricultural | Productivist, corporate industrial; smallholders nominal | High | Low | Predominant agrochemical substitution (LR) | Low, non-local seed and breed systems | Low, limited by low soil organic matter and residue returns | Central Valley, California, US; major valleys, Peru |
| Marginal-Land and Remote Rural Food Growing | Low-input land use for local and regional food; smallholder significance high | Low-Medium | Low | Locally varied; includes ecological disintensification (UL) and intensification (UR) | High, labor-saving local seed and breed systems | High/varied, both crop and “wild” managed ecosystem components | California coastal range, US; Andean uplands |
| Remote Rural Specialized Livestock or Cropping | Transition from food-growing; smallholder significance medium | Low-Medium | Low | Specialized intensification (LR) and disintensification (LL) | Low, non-local seed and breed systems | Medium/varied, limited by low residue inputs. | Great Plains, US; Altiplano, Peru 2 |
| Peri-Urban, Ex-Urban | Multi-purpose; smallholder significance medium | Medium | Low | Locally varied; includes ecological intensification (UR) | High, local seed and breed systems | High, managed to foster agroecosystem services | Bay Area, US; Huancayo, Peru |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmerer, K.S.; Vanek, S.J. Toward the Integrated Framework Analysis of Linkages among Agrobiodiversity, Livelihood Diversification, Ecological Systems, and Sustainability amid Global Change. Land 2016, 5, 10. https://doi.org/10.3390/land5020010
Zimmerer KS, Vanek SJ. Toward the Integrated Framework Analysis of Linkages among Agrobiodiversity, Livelihood Diversification, Ecological Systems, and Sustainability amid Global Change. Land. 2016; 5(2):10. https://doi.org/10.3390/land5020010
Chicago/Turabian StyleZimmerer, Karl S., and Steven J. Vanek. 2016. "Toward the Integrated Framework Analysis of Linkages among Agrobiodiversity, Livelihood Diversification, Ecological Systems, and Sustainability amid Global Change" Land 5, no. 2: 10. https://doi.org/10.3390/land5020010