Immunochemical Analysis of the Antimalarial Drugs Artemisinin and Artesunate
Abstract
:1. Introduction

2. Establishment of icELISA Using Anti-AM mAb
2.1. Production and Characterization of Anti-AM mAb
2.2. Evaluation and Application of icELISA Using Anti-AM mAb
| Sample | Content (µg/mg dry wt.) |
|---|---|
| Chinese wormwood 1 | 3.21 ± 0.09 |
| Chinese wormwood 2 | 1.02 ±0.23 |
| Aerial part of regenerated A. annua | 1.07 ± 0.05 |
3. Production of Recombinant Fab against AM and AS
3.1. Cloning of cDNAs Encoding Fab and Bacterial Expression of Fab against AM and AS
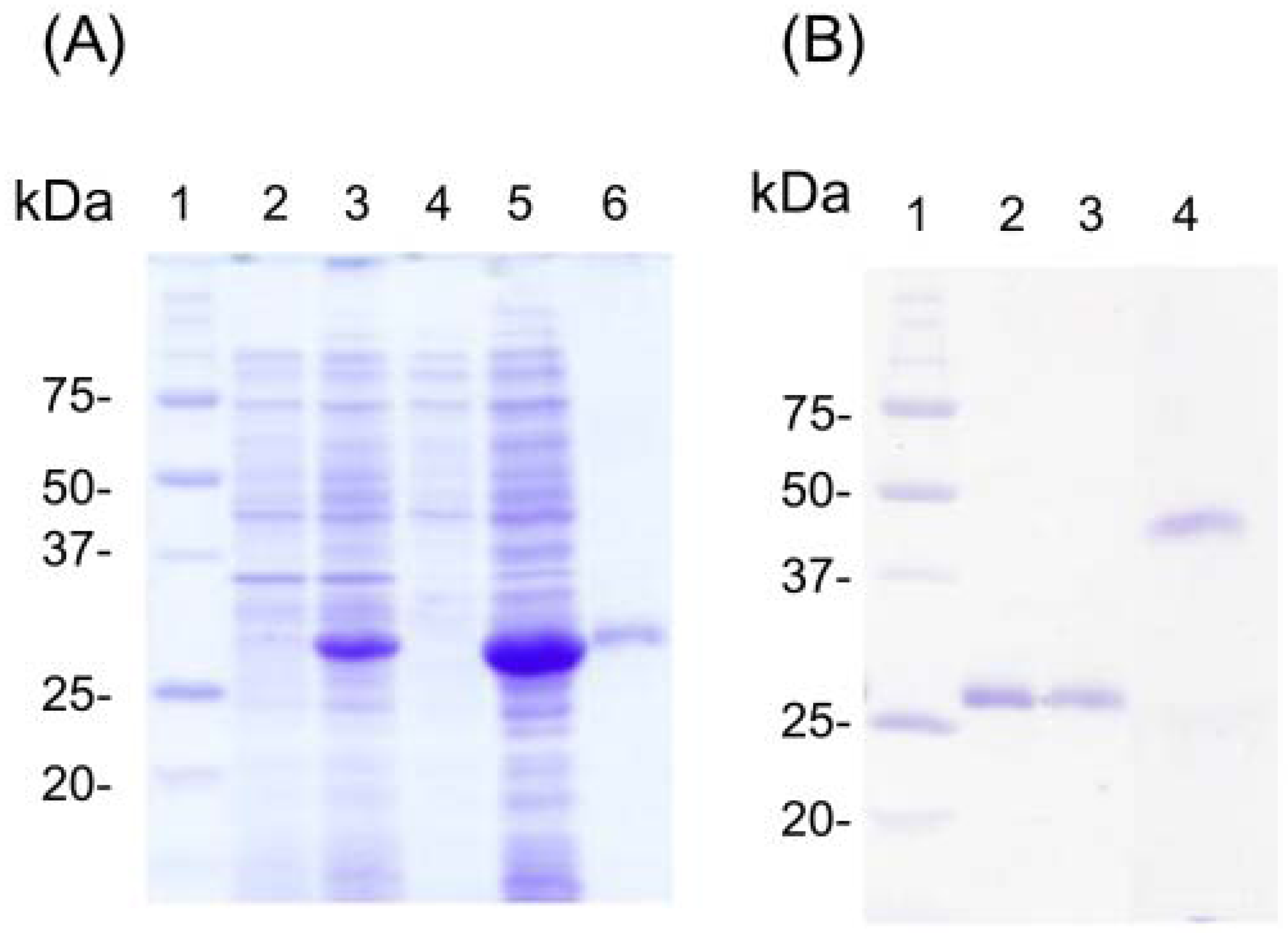
3.2. Refolding and Reactivity Tests for Fab against AM and AS
3.3. Development of icELISA Using Recombinant Fab against AM and AS
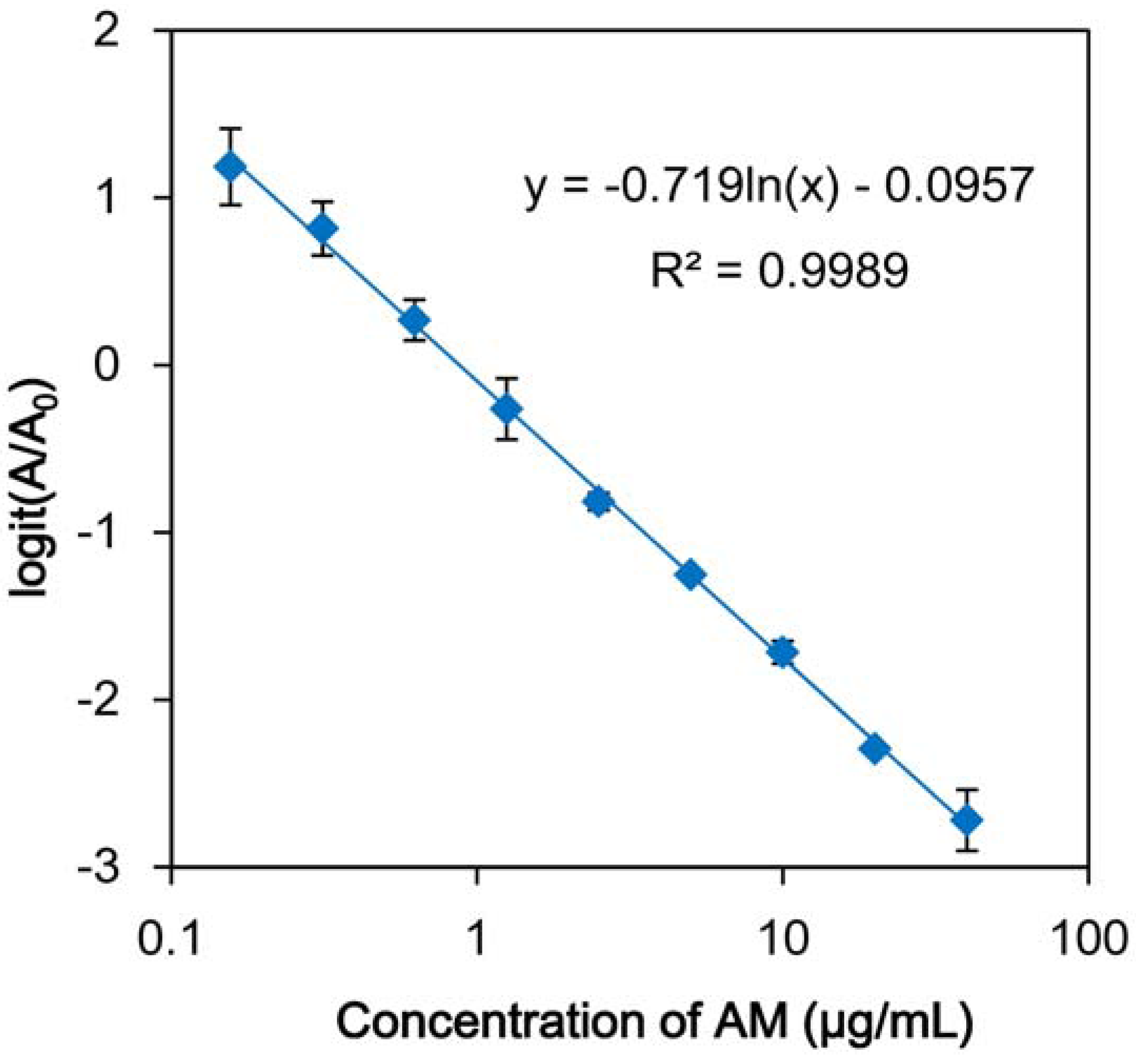
| Compound | IC50 (µg/mL) |
|---|---|
| Artemisinin | 1.58 |
| Artesunate | 0.022 |
| Saikosaponin a | >100 |
| Ginsenoside Rb1 | >100 |
| Baicalin | >100 |
| Quinine | >100 |
| Berberine | >100 |
| Arbutin | >100 |
| Amygdalin | >100 |
| Swertiamarin | >100 |
| Digitonin | >100 |
4. Conclusions
Acknowledgments
References
- Klayman, D.L. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049–1055. [Google Scholar]
- Eckstein-Ludwig, U.; Webb, R.J.; van Goethem, I.D.A.; East, J.M.; Lee, A.G.; Kimura, M.; O’Neill, P.M.; Bray, P.G.; Ward, S.A.; Krishna, S. Artemisinins target the SERCA of Plasmodium falciparum. Nature 2003, 424, 957–961. [Google Scholar] [CrossRef]
- Miller, L.H.; Su, X. Artemisin: Discovery from the Chinese Herbal Garden. Cell 2011, 146, 855–858. [Google Scholar] [CrossRef]
- Smithuis, F.; Kyaw, M.K.; Phe, O.; Aye, K.Z.; Htet, L.; Barends, M.; Lindegardh, N.; Singtoroj, T.; Ashley, E.; Lwin, S.; et al. Efficacy and effectiveness of dihydroartemisinin-piperaquine versus artesunate-mefloquine in falciparum malaria: An open-label randomised comparison. Lancet 2006, 367, 2075–2085. [Google Scholar]
- Zongo, I.; Dorsey, G.; Rouamba, N.; Tinto, H.; Dokomajilar, C.; Guiguemde, R.T.; Rosenthal, P.J.; Ouedraogo, J.B. Artemether-lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: A randomised non-inferiority trial. Lancet 2007, 369, 491–498. [Google Scholar] [CrossRef]
- Tshefu, A.K.; Gaye, O.; Kayentao, K.; Thompson, R.; Bhatt, K.M.; Sesay, S.S.; Bustos, D.G.; Tjitra, E.; Bedu-Addo, G.; Borghini-Fuhrer, I.; et al. Efficacy and safety of a fixed-dose oral combination of pyronaridine-artesunate compared with artemether-lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: A randomised non-inferiority trial. Lancet 2010, 375, 1468–1481. [Google Scholar]
- Crawley, J.; Chu, C.; Mtove, G.; Nosten, F. Malaria in children. Lancet 2010, 375, 1457–1467. [Google Scholar] [CrossRef]
- Poespoprodjo, J.R.; Fobia, W.; Kenangalem, E.; Hasanuddin, A.; Sugiarto, P.; Tjitra, E.; Anstey, N.M.; Price, R.N. Highly effective therapy for maternal malaria associated with a lower risk of vertical transmission. J. Infect. Dis. 2011, 204, 1613–1619. [Google Scholar] [CrossRef]
- Ravindranathan, T.; Kumar, M.A.; Menon, R.B.; Hiremath, S.V. Stereoselective synthesis of artemisinin. Tetrahedron Lett. 1990, 31, 755–758. [Google Scholar] [CrossRef]
- Avery, M.A.; Chong, W.K.M.; Jenningswhite, C. Stereoselective total synthesis of (+)-artemisinin, the antimalarial constituent of Artemisia annua L. J. Am. Chem. Soc. 1992, 114, 974–979. [Google Scholar] [CrossRef]
- Ro, D.K.; Paradise, E.M.; Ouellet, M.; Fisher, K.J.; Newman, K.L.; Ndungu, J.M.; Ho, K.A.; Eachus, R.A.; Ham, T.S.; Kirby, J.; et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943. [Google Scholar] [CrossRef]
- Woerdenbag, H.J.; Moskal, T.A.; Pras, N.; Malingre, T.M.; Elferaly, F.S.; Kampinga, H.H.; Konings, A.W.T. Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor-cells. J. Nat. Prod. 1993, 56, 849–856. [Google Scholar] [CrossRef]
- Mercer, A.E.; Copple, I.M.; Maggs, J.L.; O’Neill, P.M.; Park, B.K. The role of heme and the mitochondrion in the chemical and molecular mechanisms of mammalian cell death induced by the artemisinin antimalarials. J. Biol. Chem. 2011, 286, 987–996. [Google Scholar]
- Liu, W.M.; Gravett, A.M.; Dalgleish, A.G. The antimalarial agent artesunate possesses anticancer properties that can be enhanced by combination strategies. Int. J. Cancer 2011, 128, 1471–1480. [Google Scholar] [CrossRef]
- Disbrow, G.L.; Baege, A.C.; Kierpiec, K.A.; Yuan, H.; Centeno, J.A.; Thibodeaux, C.A.; Hartmann, D.; Schlegel, R. Dihydroartemisinin is cytotoxic to papillomavirus- expressing epithelial cells in vitro and in vivo. Cancer Res. 2005, 65, 10854–10861. [Google Scholar] [CrossRef]
- Hou, J.; Wang, D.; Zhang, R.; Wang, H. Experimental therapy of hepatoma with artemisinin and its derivatives: In vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin. Cancer Res. 2008, 14, 5519–5530. [Google Scholar] [CrossRef]
- Posner, G.H.; McRiner, A.J.; Paik, I.H.; Sur, S.; Borstnik, K.; Xie, S.J.; Shapiro, T.A.; Alagbala, A.; Foster, B. Anticancer and antimalarial efficacy and safety of artemisinin-derived trioxane dimers in rodents. J. Med. Chem. 2004, 47, 1299–1301. [Google Scholar] [CrossRef]
- Jeyadevan, J.P.; Bray, P.G.; Chadwick, J.; Mercer, A.E.; Byrne, A.; Ward, S.A.; Park, B.K.; Williams, D.P.; Cosstick, R.; Davies, J.; et al. Antimalarial and antitumor evaluation of novel C-10 non-acetal dimers of 10beta-(2-hydroxyethyl)deoxoartemisinin. J. Med. Chem. 2004, 47, 1290–1298. [Google Scholar] [CrossRef]
- Galal, A.M.; Ross, S.A.; ElSohly, M.A.; ElSohly, H.N.; El-Feraly, F.S.; Ahmed, M.S.; McPhail, A.T. Deoxyartemisinin derivatives from photooxygenation of anhydrodeoxydihydroartemisinin and their cytotoxic evaluation. J. Nat. Prod. 2002, 65, 184–188. [Google Scholar] [CrossRef]
- Li, Y.; Shan, F.; Wu, J.M.; Wu, G.S.; Ding, J.; Xiao, D.; Yang, W.Y.; Atassi, G.; Leonce, S.; Caignard, D.H.; et al. Novel antitumor artemisinin derivatives targeting. G1 phase of the cell cycle. Bioorg. Med. Chem. Lett. 2001, 11, 5–8. [Google Scholar] [CrossRef]
- Jaziri, M.; Diallo, B.; Vanhaelen, M.; Homes, J.; Yoshimatsu, K.; Shimomura, K. Immunodetection of artemisinin in Artemisia annua cultivated in hydroponic conditions. Phytochemistry 1993, 33, 821–826. [Google Scholar]
- Ferreira, J.F.; Janick, J. Immunoquantitative analysis of artemisinin from Artemisia annua using polyclonal antibodies. Phytochemistry 1996, 41, 97–104. [Google Scholar] [CrossRef]
- Eggelte, T.A.; van Agtmael, M.A.; Vuong, T.D.; van Boxtel, C.J. The development of an immunoassay for the detection of artemisinin compounds in urine. Am. J. Trop. Med. Hyg. 1999, 61, 449–456. [Google Scholar]
- He, S.P.; Tan, G.Y.; Li, G.; Tan, W.M.; Nan, T.G.; Wang, B.M.; Li, Z.H.; Li, Q.X. Development of a sensitive monoclonal antibody-based enzyme-linked immunosorbent assay for the antimalaria active ingredient artemisinin in the Chinese herb Artemisia annua L. Anal. Bioanal. Chem. 2009, 393, 1297–1303. [Google Scholar] [CrossRef]
- Yanagihara, H.; Sakata, R.; Minami, H.; Tanaka, H.; Shoyama, Y.; Murakami, H. Immunoaffinity column chromatography against forskolin using an anti-forskolin monoclonal antibody and its application. Anal. Chim. Acta 1996, 335, 63–70. [Google Scholar] [CrossRef]
- Shan, S.J.; Tanaka, H.; Shoyama, Y. Enzyme-linked immunosorbent assayfor glycyrrhizin using anti-glycyrrhizin monoclonalantibody and an eastern blottingtechnique for glucuronides of glycyrrhetic acid. Anal. Chem. 2001, 73, 5784–5790. [Google Scholar] [CrossRef]
- Kim, J.S.; Tanaka, H.; Shoyama, Y. Immunoquantitative analysis for berberine and its related compounds using monoclonal antibodies in herbal medicines. Analyst 2004, 129, 87–91. [Google Scholar] [CrossRef]
- Phrompittayarat, W.; Putalun, W.; Tanaka, H.; Wittaya-Areekul, S.; Jetiyanon, K.; Ingkaninan, K. An enzyme-linked immunosorbant assay using polyclonal antibodies against bacopaside I. Anal. Chim. Acta 2007, 584, 1–6. [Google Scholar] [CrossRef]
- Sakamoto, S.; Putalun, W.; Tsuchihashi, R.; Morimoto, S.; Kinjo, J.; Tanaka, H. Development of an enzyme-linked immunosorbent assay (ELISA) using highly-specific monoclonal antibodies against plumbagin. Anal. Chim. Acta 2008, 607, 100–105. [Google Scholar] [CrossRef]
- Kido, K.; Edakuni, K.; Morinaga, O.; Tanaka, H.; Shoyama, Y. An enzyme-linked immunosorbent assay for aconitine-type alkaloids using an anti-aconitine monoclonal antibody. Anal. Chim. Acta 2008, 616, 109–114. [Google Scholar] [CrossRef]
- Juengwatanatrakul, T.; Sritularak, B.; Amornnopparattanakul, P.; Tassanawat, P.; Putalun, W.; Tanaka, H.; Morimoto, S. Preparation of a specificmonoclonal antibody to asiaticoside for the development of an enzyme-linked immunosorbent assay. Analyst 2011, 136, 1013–1017. [Google Scholar] [CrossRef]
- Kido, K.; Morinaga, O.; Shoyama, Y.; Tanaka, H. Quick analysis of baicalin in Scutellariae Radix by enzyme-linked immunosorbent assay using a monoclonal antibody. Talanta 2008, 77, 346–350. [Google Scholar] [CrossRef]
- Paudel, M.K.; Putalun, W.; Sritularak, B.; Morinaga, O.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Development pf a combined technique using a rapid one-step immunochromatographic assay and indirect competitive ELISA for the rapid detection of bacalin. Anal. Chim. Acta 2011, 701, 189–193. [Google Scholar] [CrossRef]
- Tanaka, H.; Putalun, W.; De-Eknamkul, W.; Matangkasombut, O.; Shoyama, Y. Preparation of a novel monoclonal antibody against the antimalarial drugs artemisinin and artesunate. Planta Med. 2007, 73, 1127–1132. [Google Scholar] [CrossRef]
- Paudel, K.M.; Takei, A.; Sakoda, J.; Juengwatanatrakul, T.; Sasaki-Tabata, K.; Putalun, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Preparation of a single-chain variable fragment and a recombinant antigen-binding Fragment against the anti-malarial drugs, artemisinin and artesunate, and their application in an ELISA. Anal. Chem. 2012, 84, 2002–2008. [Google Scholar] [CrossRef]
- Weiler, E.W.; Zenk, M.H. Radioimmunoassay for determination of digoxin and related compounds in Digitalis lanata. Phytochemistry 1976, 15, 1537–1545. [Google Scholar] [CrossRef]
- Peng, C.A.; Ferreira, J.F.S.; Wood, A.J. Direct analysis of artemisinin from Artemisia annua L. using high-performance liquid chromatography with evaporative light scattering detector and gas chromatography with flame ionization detector. J. Chromatogr. A 2006, 1133, 254–258. [Google Scholar] [CrossRef]
- Liu, C.Z.; Zhou, H.Y.; Zhao, Y. An effective method for fast determination of artemisinin in Artemisia annua L. by high performance liquid chromatography with evaporative light scattering detection. Anal. Chin. Acta 2007, 581, 298–302. [Google Scholar] [CrossRef]
- Kabat, E.A.; Wu, T.T. Identical V-region amino-acid sequences and segments of sequences in antibodies of VH and VL genes, minigenes, and complementarity-determining regions to binding of antibody-combining sites. J. Immun. 1991, 147, 1709–1719. [Google Scholar]
- Chothia, C.; Lesk, A.M.; Tramontano, A.; Levitt, M.; Smithgill, S.L.; Air, G.; Sheriff, S.; Padlan, E.A.; Davies, D.; Tulip, W.R.; et al. Conformations of immunoglobulin hypervariable regions. Nature 1989, 342, 877–8833. [Google Scholar] [CrossRef]
- Umetsu, M.; Tsumoto, K.; Hara, M.; Ashish, K.; Goda, S.; Adschiri, T.; Kumagai, I. How additives influence the refolding of immunoglobulin-folded proteins in a stepwise dialysis system. Spectroscopic evidence for highly efficient refolding of a single-chain Fv fragment. J. Biol. Chem. 2003, 278, 8979–8987. [Google Scholar]
- Abdul, M.; Ibrar, A.; Waheed, A.; Muhammad, F.A.; Rizwana, A.Q.; Izhar, H.; Bushra, M. Survey of artemisinin production by diverse Artemisia species in northern Pakistan. Malaria J. 2010, 9, 310. [Google Scholar] [CrossRef]
- Marchand, E.; Atemnkeng, M.A.; Vanermen, S.; Plaizier-Vercammen. Development and validation of a simple thin layer chromatographic method for the analysis of artemisinin in Artemisia annua L. plant extracts. J. Biomed. Chromatogr. 2008, 22, 454–459. [Google Scholar] [CrossRef]
- Koobkokkruad, T.; Chochai, A.; Kerdmanee, C.; De-Eknamkul, W. TLC-Densitometric analysis of artemisinin for the rapid screening of high-producing plantlets of Artemisia annua L. Phytochem. Anal. 2007, 18, 229–234. [Google Scholar] [CrossRef]
- Shishan, Z.; Mei-Yi, Z. Application of precolumn reaction to high-performance liquid chromatography of qinghaosu in animal plasma. Anal. Chem. 1986, 58, 289–292. [Google Scholar] [CrossRef]
- Lapkin, A.A.; Walker, A.; Sullivan, N.; Khambay, B.; Mlambo, B.; Chemat, S. Development of HPLC analytical protocols for quantification of artemisinin in biomass and extracts. J. Pharm. Biomed. Anal. 2009, 49, 908–915. [Google Scholar] [CrossRef]
- Diawara, H.Z.; Gbaguidi, F.; Evrard, B.; Leclercq, J.Q.; Moudachirou, M.; Debrus, B.; Hubert, P.; Rozet, E. Validation, transfer and measurement uncertainty estimation of an HPLC-UV method for the quantification of artemisinin in hydro alcoholic extracts of Artemisia annua L. J. Pharm. Biomed. Anal. 2011, 56, 7–15. [Google Scholar] [CrossRef]
- Lai, C.S.; Nair, N.K.; Mansor, S.M.; Olliaro, P.L.; Navaratnam, V. An analytical method with a single extraction procedure and two separate high performance liquid chromatographic systems for the determination of artesunate, dihydroartemisinin and mefloquine in human plasma for application in clinical pharmacological studies of the drug combination. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2007, 857, 308–314. [Google Scholar] [CrossRef]
- Hodel, E.M.; Zanolari, B.; Mercier, T.; Biollaz, J.; Keiser, J.; Olliaro, P.; Genton, B.; Decosterd, L.A. A single LC-tandem mass spectrometry method for the simultaneous determination of 14 antimalarial drugs and their metabolites in human plasma. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2009, 877, 867–886. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tanaka, H.; Paudel, M.K.; Takei, A.; Sakoda, J.; Juengwatanatrakul, T.; Sasaki-Tabata, K.; Putalun, W.; De-Eknamkul, W.; Matangkasombut, O.; Shoyama, Y.; et al. Immunochemical Analysis of the Antimalarial Drugs Artemisinin and Artesunate. Antibodies 2012, 1, 273-283. https://doi.org/10.3390/antib1030273
Tanaka H, Paudel MK, Takei A, Sakoda J, Juengwatanatrakul T, Sasaki-Tabata K, Putalun W, De-Eknamkul W, Matangkasombut O, Shoyama Y, et al. Immunochemical Analysis of the Antimalarial Drugs Artemisinin and Artesunate. Antibodies. 2012; 1(3):273-283. https://doi.org/10.3390/antib1030273
Chicago/Turabian StyleTanaka, Hiroyuki, Madan K. Paudel, Ayako Takei, Junichi Sakoda, Thaweesak Juengwatanatrakul, Kaori Sasaki-Tabata, Waraporn Putalun, Wanchai De-Eknamkul, Oraphan Matangkasombut, Yukihiro Shoyama, and et al. 2012. "Immunochemical Analysis of the Antimalarial Drugs Artemisinin and Artesunate" Antibodies 1, no. 3: 273-283. https://doi.org/10.3390/antib1030273




