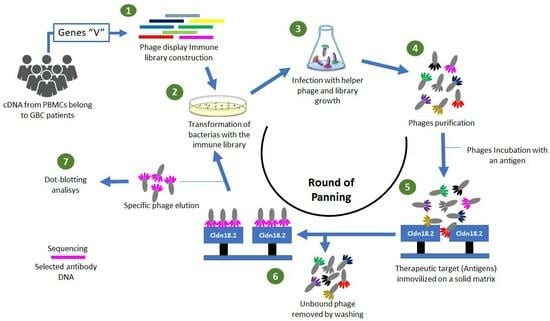
Construction of a Human Immune Library from Gallbladder Cancer Patients for the Single-Chain Fragment Variable (scFv) Antibody Selection against Claudin 18.2 via Phage Display
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples, RNA Extraction, and cDNA Synthesis
2.2. Amplification of Variable Genes (VH and VL) from Immunoglobulins
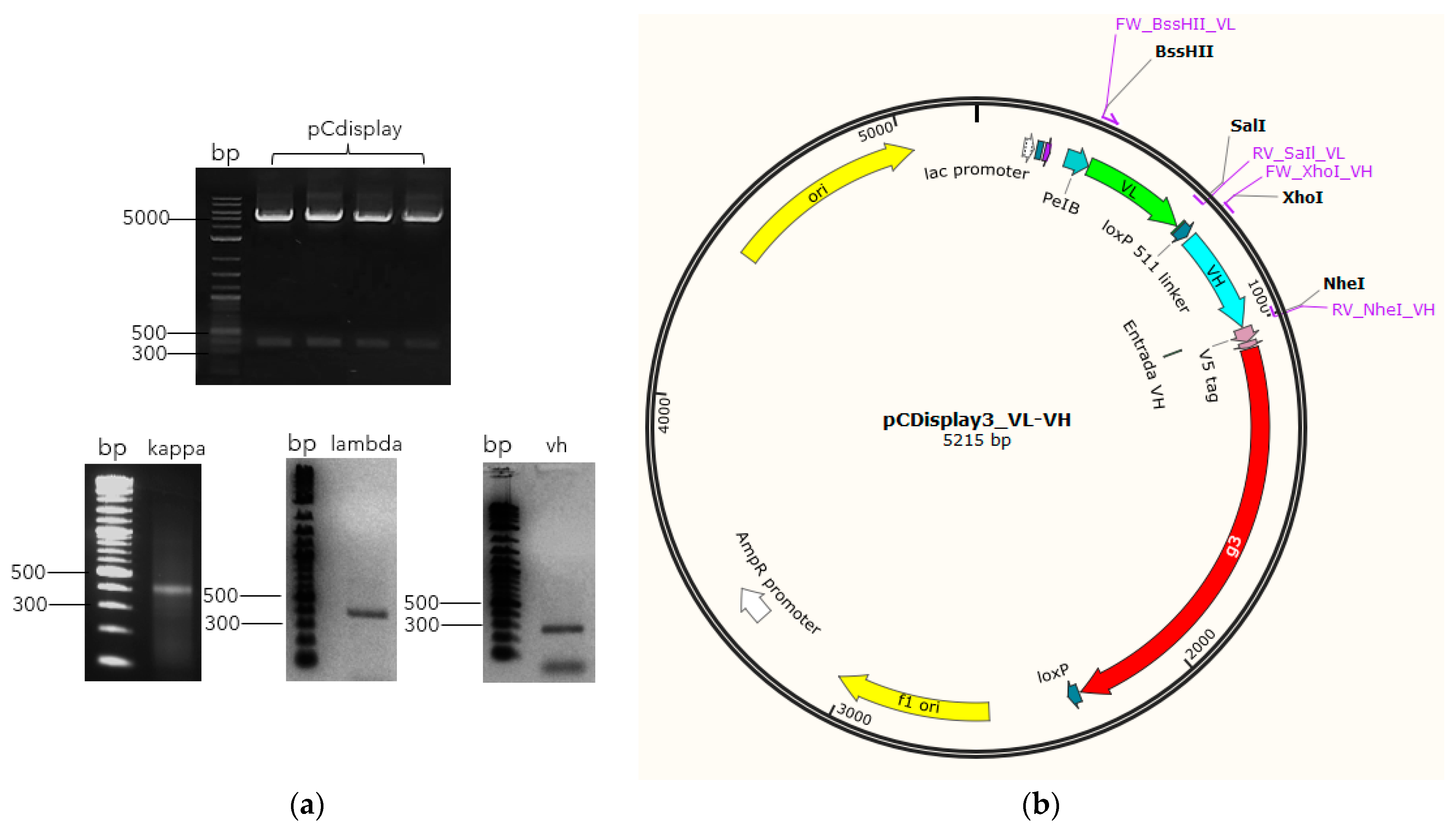
2.3. Cloning of VH and VL Segments into the Phagemid to Create the Library
2.4. Library Packaging and scFv—Phage Production
2.5. Recombinant Production of Claudin 18
2.6. Selection of scFv—Phages through Panning
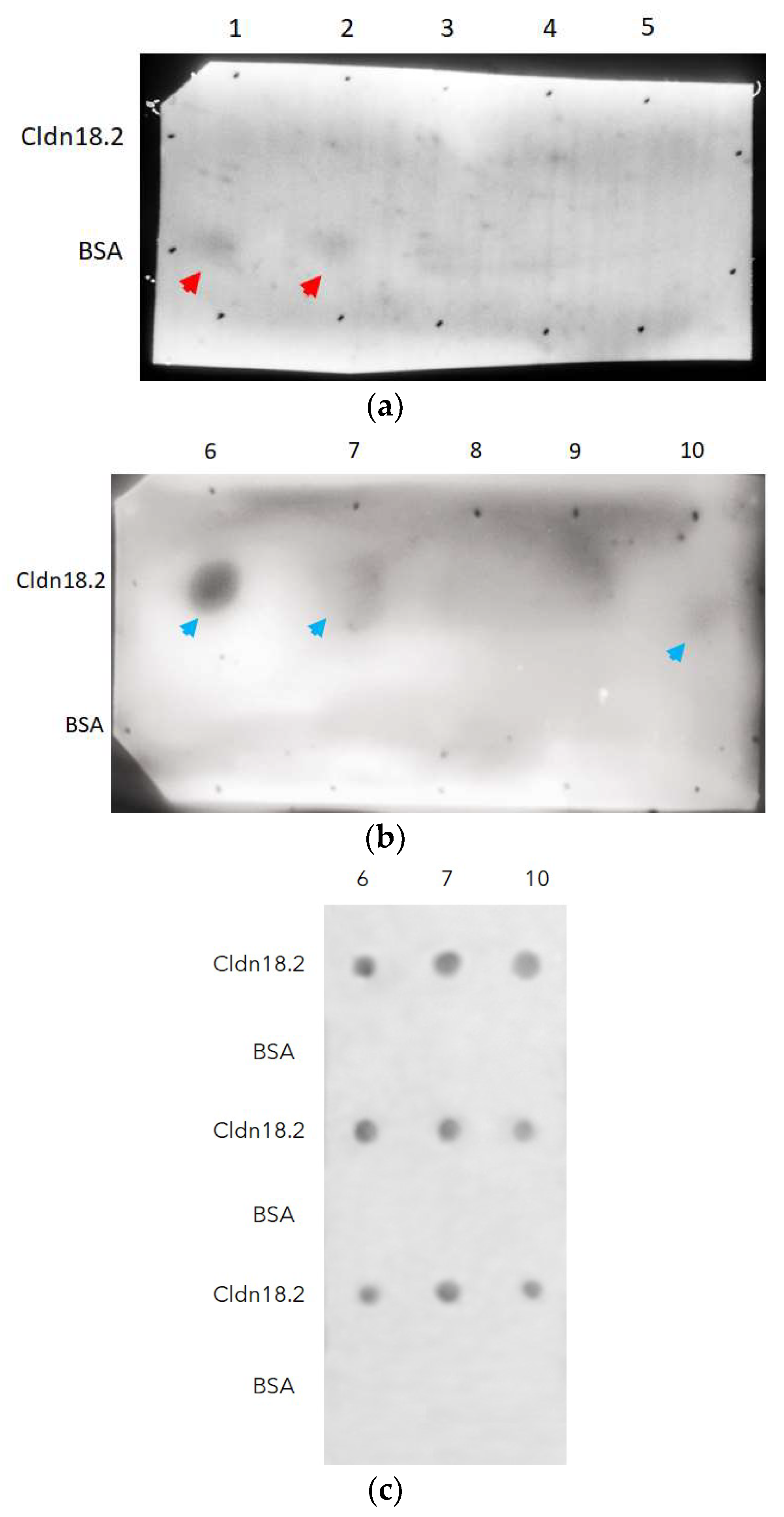
2.7. Phage Dot-Blotting Monoclonal Testing Antibody-Binding Specificity
3. Results
3.1. Amplification of VH and VL Genes
3.2. Immune Library Assembly and scFv-Phage Production
3.3. Recombinant Production of the Antigen cldn18.2
3.4. Selection of scFv-Phages against cldn18.2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roa, J.C.; García, P.; Kapoor, V.K.; Maithel, S.K.; Javle, M.; Koshiol, J. Gallbladder Cancer. Nat. Rev. Dis. Primers 2022, 8, 69. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Marcano-Bonilla, L.; Roberts, L.R. Gallbladder Cancer: Epidemiology and Genetic Risk Associations. Chin. Clin. Oncol. 2019, 8, 31. [Google Scholar] [CrossRef]
- Koshiol, J.; Wozniak, A.; Cook, P.; Adaniel, C.; Acevedo, J.; Azócar, L.; Hsing, A.W.; Roa, J.C.; Pasetti, M.F.; Miquel, J.F.; et al. Salmonella Enterica Serovar Typhi and Gallbladder Cancer: A Case–Control Study and Meta-Analysis. Cancer Med. 2016, 5, 3235–3310. [Google Scholar] [CrossRef]
- Koshiol, J.; Gao, Y.-T.; Dean, M.; Egner, P.; Nepal, C. Association of Aflatoxin and Gallbladder Cancer. Gastroenterology 2017, 153, 488–494. [Google Scholar] [CrossRef]
- Nemunaitis, J.M.; Brown-glabeman, U.; Soares, H.; Belmonte, J.; Liem, B.; Nir, I.; Phuoc, V.; Gullapalli, R.R. Gallbladder Cancer: Review of a Rare Orphan Gastrointestinal Cancer with a Focus on Populations of New Mexico. BMC Cancer 2018, 18, 665. [Google Scholar] [CrossRef]
- Hickman, L.; Contreras, C. Gallbladder Cancer: Diagnosis, Surgical Management, and Adjuvant Therapies. Surg. Clin. N. Am. 2019, 99, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Bizama, C.; García, P.; Espinoza, J.A.; Weber, H.; Leal, P.; Nervi, B.; Roa, J.C. Targeting Specific Molecular Pathways Holds Promise for Advanced Gallbladder Cancer Therapy. Cancer Treat. Rev. 2015, 41, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Tsao, L.-C.; Force, J.; Hartman, Z.C. Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res. 2021, 81, 4641–4651. [Google Scholar] [CrossRef] [PubMed]
- Effer, B.; Perez, I.; Ulloa, D.; Mayer, C.; Muñoz, F.; Bustos, D.; Rojas, C.; Manterola, C.; Vergara-Gómez, L.; Dappolonnio, C.; et al. Therapeutic Targets of Monoclonal Antibodies Used in the Treatment of Cancer: Current and Emerging. Biomedicines 2023, 11, 2086. [Google Scholar] [CrossRef]
- Zaroff, S.; Tan, G. Hybridoma Technology: The Preferred Method for Monoclonal Antibody Generation for in Vivo Applications. BioTechniques 2019, 67, 90–92. [Google Scholar] [CrossRef]
- Posner, J.; Barrington, T.; Brier, T.; Datta-Mannan, A. Monoclonal Antibodies: Past, Present and Future. In Concepts and Principles of Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 81–141. [Google Scholar]
- Ruschig, M.; Heine, P.A.; Fühner, V.; Zilkens, K.J.K.; Steinke, S.; Schubert, M.; Bertoglio, F.; Hust, M. Construction of Human Immune and Naive scFv Phage Display Libraries. In Phage Display Methods and Protocols; Hust, M., Lim, T.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 15–37. [Google Scholar]
- Giang, K.A.; Sidhu, S.S.; Nilvebrant, J. Construction of Synthetic Antibody Phage Display Libraries. In Phage Display Methods and Protocols; Hust, M., Lim, T.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 59–75. [Google Scholar]
- Nur, A.; Schubert, M.; Lai, J.Y.; Hust, M.; Choong, Y.S.; Isa, W.Y.H.W.; Lim, T.S. Antibody Phage Display. In Phage Display Methods and Protocols; Hust, M., Lim, T.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 3–12. [Google Scholar]
- Steinke, S.; Roth, K.D.R.; Ruschig, M.; Langreder, N.; Polten, S.; Schneider, K.-T.; Ballmann, R.; Russo, G.; Zilkens, K.J.K.; Schubert, M.; et al. Antibody Selection via Phage Display in Microtiter Plates. In Phage Display Methods and Protocols; Hust, M., Lim, T.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 247–260. [Google Scholar]
- Langreder, N.; Schäckermann, D.; Unkauf, T.; Schubert, M.; Frenzel, A.; Bertoglio, F.; Hust, M. Antibody Affinity and Stability Maturation by Error-Prone PCR. In Phage Display Methods and Protocols; Hust, M., Lim, T.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 395–410. [Google Scholar]
- Glaser, V.; Karsli-Ünal, Ü.; Hagedorn, M.; Pieper, T. Antibody Selection on Cells Targeting Membrane Proteins. In Phage Display Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Gray, A.C.; Sidhu, S.S.; Chandrasekera, P.C.; Hendriksen, C.F.M.; Borrebaeck, C.A.K. Animal-Friendly Affinity Reagents: Replacing the Needless in the Haystack. Trends Biotechnol. 2016, 34, 960–969. [Google Scholar] [CrossRef]
- Tomlinson, I.M.; Williams, S.C.; Corbett, S.J.; Cox, J.P.L.; Winter, G. MRC Centre for Protein Engineering, Cambridge, UK. Available online: https://www2.mrc-lmb.cam.ac.uk/vbase/alignments2.php#JLEX (accessed on 4 October 2023).
- Alfaleh, M.A.; Arora, N.; Yeh, M.; Bakker, C.J.D.; Howard, C.B.; Macpherson, P.; Allavena, R.E.; Chen, X.; Harkness, L.; Mahler, S.M.; et al. Canine CD117-Specific Antibodies with Diverse Binding Properties Isolated from a Phage Display Library Using Cell-Based Biopanning. Antibodies 2019, 8, 15. [Google Scholar] [CrossRef]
- Ramage, W.; Gaiotto, T.; Ball, C.; Risley, P.; Carnell, G.W.; Temperton, N.; Cheung, C.Y.; Engelhardt, O.G.; Hufton, S.E. Cross-Reactive and Lineage-Specific Single Domain Antibodies against Influenza B Hemagglutinin. Antibodies 2019, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, H.; Park, K.; Park, S.; Lim, J.; So, M.K.; Woo, H.; Ko, H.; Lee, J.; Lim, S.H.; et al. Selection and Characterization of Monoclonal Antibodies Targeting Middle East Respiratory Syndrome Coronavirus through a Human Synthetic Fab Phage Display Library Panning. Antibodies 2019, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, W.; Drabek, D.; Okba, N.M.A.; Haperen, R.V.; Osterhaus, A.D.M.E.; Kuppeveld, F.J.M.V.; Haagmans, B.L.; Grosveld, F.; Bosch, B. A Human Monoclonal Antibody Blocking SARS-CoV-2 Infection. Nat. Commun. 2020, 11, 2251. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, R.; Shen, L. Evaluation and Reflection on Claudin 18.2 Targeting Therapy in Advanced Gastric Cancer. Chin. J. Cancer Res. 2020, 32, 263–270. [Google Scholar] [CrossRef]
- Sweerus, K.; Lachowicz-Scroggins, M.; Gordon, E.; LaFemina, M.; Huang, X.; Parikh, M.; Kanegai, C.; Fahy, J.V.; Frank, J.A. Claudin-18 Deficiency Is Associated with Airway Epithelial Barrier Dysfunction and Asthma. J. Allergy Clin. Immunol. 2017, 139, 72–81.e1. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Kyuno, D.; Sawada, N. Targeting Claudin-4 in Human Pancreatic Cancer. Expert Opin. Ther. Targets 2012, 16, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Kotton, D.N. Claudin-18: Unexpected Regulator of Lung Alveolar Epithelial Cell Proliferation. J. Clin. Investig. 2018, 128, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.A.; Riquelme, I.; Sagredo, E.A.; Rosa, L.; García, P.; Bizama, C.; Apud-Bell, M.; Leal, P.; Weber, H.; Benavente, F.; et al. Mucin 5B, Carbonic Anhydrase 9 and Claudin 18 Are Potential Theranostic Markers of Gallbladder Carcinoma. Histopathology 2019, 74, 597–607. [Google Scholar] [CrossRef]
- Angerilli, V.; Ghelardi, F.; Nappo, F.; Grillo, F.; Parente, P.; Lonardi, S.; Luchini, C.; Pietrantonio, F.; Ugolini, C.; Vanoli, A. Claudin-18.2 Testing and Its Impact in the Therapeutic Management of Patients with Gastric and Gastroesophageal Adenocarcinomas: A Literature Review with Expert Opinion. Pathol. Res. Pract. 2024, 254, 155145. [Google Scholar] [CrossRef]
- Athauda, A.; Chau, I. Claudin 18.2—A FAST-Moving Target in Gastric Cancer? Ann. Oncol. 2021, 32, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Kugler, J.; Tomszak, F.; Frenzel, A.; Hust, M. Construction of Human Immune and Naive scFv Libraries. In Phage Display Methods and Protocols; Hust, M., Lim, T.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1701, pp. 3–24. ISBN 978-1-4939-7446-7. [Google Scholar]
- Sblattero, D.; Bradbury, A. Exploiting Recombination in Single Bacteria to Make Large Phage Antibody Libraries. Nat. Biotechnol. 2000, 18, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Cabarca, S.; Frazão de Souza, M.; Albert de Oliveira, A.; Vignoli Muniz, G.S.; Lamy, M.T.; Vinicius dos Reis, C.; Takarada, J.; Effer, B.; Souza, L.S.; Iriarte de la Torre, L.; et al. Structure of the Mycobacterium Tuberculosis cPknF and Conformational Changes Induced in Forkhead-Associated Regulatory Domains. Curr. Res. Struct. Biol. 2021, 3, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Barbas, C.; Burton, D.; Scott, J.; Silverman, G. Phage Display: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; ISBN 978-087969740-2. [Google Scholar]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.; Appel, R.; Bairoch, A. The Proteomics Protocols Handbook; Walker, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Noorsharmimi, O.; Lim, T.S. Construction of Naive and Immune Human Fab Phage-Display Library. Phage Disp. Methods Protoc. 2018, 1701, 25–44. [Google Scholar] [CrossRef]
- Lai, J.Y.; Lim, T.S. Infectious Disease Anti-Bodies for Biomedical Applications: A Mini Review of Immune Antibody Phage Library Repertoire. Int. J. Biol. Macromol. 2020, 163, 640–648. [Google Scholar] [CrossRef]
- Wu, B.-P.; Xiao, B.; Wan, T.-M.; Zhang, Y.-L.; Zhang, Z.-S.; Zhou, D.-Y.; Lai, Z.-S.; Gao, C.-F. Construction and Selection of the Natural Immune Fab Antibody Phage Display Library from Patients with Colorectal Cancer. World J. Gastroenterol. 2001, 7, 811. [Google Scholar] [CrossRef] [PubMed]
- Shui, X.; Huang, J.; Li, Y.-H.; Xie, P.-L.; Li, G.-C. Construction and Selection of Human Fab Antibody Phage Display Library of Liver Cancer. Hybridoma 2009, 28, 341–347. [Google Scholar] [CrossRef]
- Dong, Y.; Meng, F.; Wang, Z.; Yu, T.; Chen, A.; Xu, S.; Wang, J.; Yin, M.; Tang, L.; Hu, C.; et al. Construction and Application of a Human scFv Phage Display Library Based on Cre-LoxP Recombination for Anti-PCSK9 Antibody Selection. Int. J. Mol. Med. 2021, 47, 708–718. [Google Scholar] [CrossRef]
- Yang, Y.; Nian, S.; Li, L.; Wen, X.; Liu, Q.; Zhang, B.; Lan, Y.; Yuan, Q.; Ye, Y. Fully Human Recombinant Antibodies against EphA2 from a Multi-Tumor Patient Immune Library Suitable for Tumor-Targeted Therapy. Bioengineered 2021, 12, 10379–10400. [Google Scholar] [CrossRef]
- Hoet, R.M.; Cohen, E.H.; Kent, R.B.; Rookey, K.; Schoonbroodt, S.; Hogan, S.; Rem, L.; Frans, N.; Daukandt, M.; Pieters, H.; et al. Generation of High-Affinity Human Antibodies by Combining Donor-Derived and Synthetic Complementarity-Determining-Region Diversity. Nat. Biotechnol. 2005, 23, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Kügler, J.; Wilke, S.; Meier, D.; Tomszak, F.; Frenzel, A.; Schirrmann, T.; Dübel, S.; Garritsen, H.; Hock, B.; Toleikis, L.; et al. Generation and Analysis of the Improved Human HAL9/10 Antibody Phage Display Libraries. BMC Biotechnol. 2015, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, S.S.; Riazi-Rad, F.; Qamsari, E.S.; Bagheri, S.; Rahimi-Jamnani, F.; Sharifzadeh, Z. Development of a Human Phage Display-Derived Anti-PD-1 scFv Antibody: An Attractive Tool for Immune Checkpoint Therapy. BMC Biotechnol. 2022, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Shams, N.; Khoshtinat Nikkhoi, S.; Gu, Z.; Rahbarizadeh, F. Isolation and Characterization of Human Anti-CD20 Single-Chain Variable Fragment (scFv) from a Naive Human scFv Library. Med. Oncol. 2022, 39, 177. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight Junctions: From Simple Barriers to Multifunctional Molecular Gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.; Ragupathy, S.; Borchard, G. Target Specific Tight Junction Modulators. Adv. Drug Deliv. Rev. 2021, 171, 266–288. [Google Scholar] [CrossRef] [PubMed]
- Kyuno, D.; Takasawa, A.; Kikuchi, S.; Takemasa, I.; Osanai, M.; Kojima, T. Role of Tight Junctions in the Epithelial-to-Mesenchymal Transition of Cancer Cells. Biochim. Et Biophys. Acta (BBA) Biomembr. 2021, 1863, 183503. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; An, J.Y.; Lee, J.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Kim, K.-M.; Kang, W.K.; Kim, S.T. Claudin 18.2 Expression in Various Tumor Types and Its Role as a Potential Target in Advanced Gastric Cancer. Transl. Cancer Res. 2020, 9, 3367–3374. [Google Scholar] [CrossRef]
- Qi, C.; Xie, T.; Zhou, J.; Wang, X.; Gong, J.; Zhang, X.; Li, J.; Yuan, J.; Liu, C.; Shen, L. CT041 CAR T Cell Therapy for Claudin18.2-Positive Metastatic Pancreatic Cancer. J. Hematol. Oncol. 2023, 16, 102. [Google Scholar] [CrossRef]
- Keira, Y.; Takasawa, A.; Murata, M.; Nojima, M.; Takasawa, K.; Ogino, J.; Higashiura, Y.; Sasaki, A.; Kimura, Y.; Mizuguchi, T.; et al. An Immunohistochemical Marker Panel Including Claudin-18, Maspin, and P53 Improves Diagnostic Accuracy of Bile Duct Neoplasms in Surgical and Presurgical Biopsy Specimens. Virchows Arch. 2015, 466, 265–277. [Google Scholar] [CrossRef]
- Kyuno, D.; Takasawa, A.; Takasawa, K.; Ono, Y.; Aoyama, T.; Magara, K.; Nakamori, Y.; Takemasa, I.; Osanai, M. Claudin-18.2 as a Therapeutic Target in Cancers: Cumulative Findings from Basic Research and Clinical Trials. Tissue Barriers 2022, 10, 1967080. [Google Scholar] [CrossRef]
- Heine, P.A.; Ruschig, M.; Langreder, N.; Wenzel, E.; Schubert, M.; Bertoglio, F.; Hust, M. Antibody Selection in Solution Using Magnetic Beads. Methods Mol. Biol. 2023, 2702, 261–274. [Google Scholar] [CrossRef]
- Ch’ng, A.; Konthur, Z.; Lim, T.S. Magnetic Nanoparticle-Based Semi-Automated Panning for High-Throughput Antibody Selection. Methods Mol. Biol. 2023, 2702, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.S.; Ch’ng, A.; Song, B.; Lai, J.Y. Streptavidin-Coated Solid-Phase Extraction (SPE) Tips for Antibody Phage Display Biopanning. Methods Mol. Biol. 2023, 2702, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, J.; RajabiBazl, M.; Ebrahimizadeh, W.; Ahmadian, G.; Hosseini, H. Selection of Single Chain Antibody Fragments for Targeting Prostate Specific Membrane Antigen: A Compar-Ison between Cell-Based and Antigen-Based Approach. Protein Pept. Lett. 2016, 23, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.M. Antibody Therapeutics Approved or in Regulatory Review in the EU or US. 2024. Available online: www.antibodysociety.org (accessed on 3 January 2024).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Effer, B.; Ulloa, D.; Dappolonnio, C.; Muñoz, F.; Iturrieta-González, I.; Cotes, L.; Rojas, C.; Leal, P. Construction of a Human Immune Library from Gallbladder Cancer Patients for the Single-Chain Fragment Variable (scFv) Antibody Selection against Claudin 18.2 via Phage Display. Antibodies 2024, 13, 20. https://doi.org/10.3390/antib13010020
Effer B, Ulloa D, Dappolonnio C, Muñoz F, Iturrieta-González I, Cotes L, Rojas C, Leal P. Construction of a Human Immune Library from Gallbladder Cancer Patients for the Single-Chain Fragment Variable (scFv) Antibody Selection against Claudin 18.2 via Phage Display. Antibodies. 2024; 13(1):20. https://doi.org/10.3390/antib13010020
Chicago/Turabian StyleEffer, Brian, Daniel Ulloa, Camila Dappolonnio, Francisca Muñoz, Isabel Iturrieta-González, Loraine Cotes, Claudio Rojas, and Pamela Leal. 2024. "Construction of a Human Immune Library from Gallbladder Cancer Patients for the Single-Chain Fragment Variable (scFv) Antibody Selection against Claudin 18.2 via Phage Display" Antibodies 13, no. 1: 20. https://doi.org/10.3390/antib13010020
APA StyleEffer, B., Ulloa, D., Dappolonnio, C., Muñoz, F., Iturrieta-González, I., Cotes, L., Rojas, C., & Leal, P. (2024). Construction of a Human Immune Library from Gallbladder Cancer Patients for the Single-Chain Fragment Variable (scFv) Antibody Selection against Claudin 18.2 via Phage Display. Antibodies, 13(1), 20. https://doi.org/10.3390/antib13010020