A Narrative Review of the State of the Art of CCR4-Based Therapies in Cutaneous T-Cell Lymphomas: Focus on Mogamulizumab and Future Treatments
Abstract
:1. Introduction
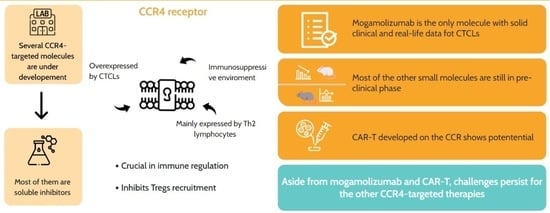
2. The CCR4 Receptor
CCR4 in Cutaneous Cell Lymphoma Development
3. The Developed and Under-Investigation Treatments to Address CCR4
| Drug Name | Type of Drug | Approved Status | Indication | References |
|---|---|---|---|---|
| Mogamulizumab | Monoclonal antibody | Approved for CTCLs | Cutaneous T-cell lymphoma (CTCL) | [59,60,61,62] |
| Compound 22 | Small-molecular antagonist | Pre-clinical investigation | Investigated for allergies, Th2-mediated infections, autoimmune diseases, and vaccinations | [57,63] |
| Compound 8c | Small-molecular antagonist | Pre-clinical investigation | Investigated for acute dermatitis treatment | [51] |
| RS-1748 | Small-molecular antagonist | Pre-clinical investigation | Investigated for airway inflammation | [52] |
| Zelnecirnon | Small-molecular antagonist | Clinical trial | Investigated for atopic dermatitis | [53] |
| GSK2239633 | Small-molecular antagonist | Clinical trial | Investigated for asthma indication | [64,65] |
| AZD-1678 | Small-molecular antagonist | Pre-clinical investigation | Investigated as CCR4 receptor antagonist candidate drugs | [55] |
| AZD-2098 | Small-molecular antagonist | Pre-clinical investigation | Investigated as CCR4 receptor antagonist candidate drugs | [55] |
| SP50 | Small-molecular antagonist | Pre-clinical investigation | Investigated for vaccinations | [49,56] |
| C021 | Small-molecular antagonist | Pre-clinical investigation | [57] | |
| K777 | Small-molecular antagonist | Pre-clinical investigation | Investigated as antiviral agent | [66,67] |
| CCR4-351 | Small-molecular antagonist | Pre-clinical investigation | Investigated for lymphoblastic and epithelial neoplasms with positivity for Epstein–Barr Virus | [68] |
| CCR4 antagonist 18a | Small-molecular antagonist | Pre-clinical investigation | Investigated as antiviral agent | [67] |
| C01 dihydrochloride | Small molecule | Pre-clinical investigation | Cutaneous T-cell lymphoma (CTCL) | [57] |
| CAR-T | Chimeric Antigen Receptor T-cells | Clinical trials for CTCLs | T-cell malignancies | [58,69] |
| Chloroquine/Hydroxychloroquine | Anti-malarial drug | Approved for autoimmune diseases, and investigational drug for CTCLs | Systemic lupus erythematosus, rheumatoid arthritis, porphyria cutanea tarda, q-fever, malaria, and inflammation | [70,71,72,73] |
3.1. Mogamolizumab
3.1.1. Real-Life Experience and Post Hoc Analysis
3.1.2. Report on Combination Treatment
3.1.3. Mogamulizumab-Associated Rash
3.1.4. Recent and Ongoing Clinical Trials
3.2. Zelnecirnon (RPT193)
3.3. K777
3.4. CCR4 Antagonist 18a
3.5. CCR4-351
3.6. AZD-1678 and AZD-2098
3.7. SP50 and Related Molecules
3.8. Compound 22
3.9. Compound 8c
3.10. RS-1748 and Related Compounds
3.11. GSK2239633
3.12. C01 Dihydrochloride
3.13. Hydroxychloroquine
3.14. CAR-T
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Duvic, M. Cutaneous T-Cell Lymphoma: Current and Emerging Therapies. Oncology 2023, 37, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Pileri, A.; Patrizi, A.; Agostinelli, C.; Neri, I.; Sabattini, E.; Bacci, F.; Piccaluga, P.P.; Pimpinelli, N.; Pileri, S.A. Primary cutaneous lymphomas: A reprisal. Semin. Diagn. Pathol. 2011, 28, 214–233. [Google Scholar] [CrossRef] [PubMed]
- Pileri, A.; Morsia, E.; Zengarini, C.; Torre, E.; Goteri, G.; Quaglino, P.; Pimpinelli, N.; Paulli, M.; Pileri, S.A.; Zinzani, P.L.; et al. Epidemiology of cutaneous T-cell lymphomas: State of the art and a focus on the Italian Marche region. Eur. J. Dermatol. 2023, 33, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Latzka, J. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2023. Eur. J. Cancer 2023, 195, 113343. [Google Scholar] [CrossRef] [PubMed]
- Cerroni, L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin. Cutan Med. Surg. 2018, 37, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Trautinger, F.; Eder, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Gniadecki, R.; Klemke, C.-D.; Ortiz-Romero, P.L.; Papadavid, E.; et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2017. Eur. J. Cancer 2017, 77, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Miyashiro, D.; Sanches, J.A. Mycosis fungoides and Sézary syndrome: Clinical presentation, diagnosis, staging, and therapeutic management. Front. Oncol. 2023, 13, 1141108. [Google Scholar] [CrossRef] [PubMed]
- Quadri, I.; Reneau, J.C.; Hanel, W.; Chung, C.G. Advancements in the treatment of mycosis fungoides and Sézary syndrome: Monoclonal antibodies, immunotherapies, and Janus kinase inhibitors. Front. Immunol. 2023, 14, 1291259. [Google Scholar] [CrossRef]
- Laghi, A.; Franceschini, C.; Mandel, V.D.; Teoli, M.; Musicco, F.; Sansone, M.; La Malfa, A.M.; Ardigò, M. Topical chlormethine gel in the treatment of mycosis fungoides: A single-center real-life experience and systematic review of the literature. Dermatol. Ther. 2022, 35, e15683. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Quaglino, P.; Pimpinelli, N.; Berti, E.; Baliva, G.; Rupoli, S.; Martelli, M.; Alaibac, M.; Borroni, G.; Chimenti, S.; et al. Prognostic factors in primary cutaneous B-cell lymphoma: The Italian Study Group for Cutaneous Lymphomas. J. Clin. Oncol. 2006, 24, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Bonthapally, V.; Huebner, D.; Lutes, R.; Chi, A.; Pileri, S. Panoptic clinical review of the current and future treatment of relapsed/refractory T-cell lymphomas: Peripheral T-cell lymphomas. Crit. Rev. Oncol. Hematol. 2016, 99, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Quaglino, P.; Violetti, S.A.; Cantonetti, M.; Goteri, G.; Onida, F.; Paulli, M.; Rupoli, S.; Barosi, G.; Pimpinelli, N. Critical concepts and management recommendations for cutaneous T-cell lymphoma: A consensus-based position paper from the Italian Group of Cutaneous Lymphoma. Hematol. Oncol. 2021, 39, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Maule, M.; Prince, H.M.; Porcu, P.; Horwitz, S.; Duvic, M.; Talpur, R.; Vermeer, M.; Bagot, M.; Guitart, J.; et al. Global patterns of care in advanced stage mycosis fungoides/Sezary syndrome: A multicenter retrospective follow-up study from the Cutaneous Lymphoma International Consortium. Ann. Oncol. 2019, 30, 494. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, H.; Miyagaki, T. Mycosis Fungoides and Sézary Syndrome: Updates and Review of Current Therapy. Curr. Treat. Options Oncol. 2021, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Sanches, J.A.; Cury-Martins, J.; Abreu, R.M.; Miyashiro, D.; Pereira, J. Mycosis fungoides and Sézary syndrome: Focus on the current treatment scenario. An. Bras. Dermatol. 2021, 96, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Stuver, R.; Geller, S. Advances in the treatment of mycoses fungoides and Sézary syndrome: A narrative update in skin-directed therapies and immune-based treatments. Front. Immunol. 2023, 14, 1284045. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, A.; Zengarini, C.; Agostinelli, C.; Sabattini, E.; Pileri, A. The Role of Cytokines in Cutaneous T-cell Lymphoma: Focus on the State of the Art and Possible Therapeutic Targets. Cells 2024, 13, 584. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Baliva, G.; Magagnoli, M.; Bendandi, M.; Modugno, G.; Gherlinzoni, F.; Orcioni, G.F.; Ascani, S.; Simoni, R.; Pileri, S.A.; et al. Gemcitabine Treatment in Pretreated Cutaneous T-Cell Lymphoma: Experience in 44 Patients. J. Clin. Oncol. 2000, 18, 2603–2606. [Google Scholar] [CrossRef]
- Marchi, E.; Alinari, L.; Tani, M.; Stefoni, V.; Pimpinelli, N.; Berti, E.; Pagano, L.; Bernengo, M.G.; Zaja, F.; Rupoli, S.; et al. Gemcitabine as frontline treatment for cutaneous T-cell lymphoma: Phase II study of 32 patients. Cancer 2005, 104, 2437–2441. [Google Scholar] [CrossRef]
- Welfringer-Morin, A.; Bataille, P.; Drummond, D.; Bellon, N.; Ingen-Housz-Oro, S.; Bonigen, J.; Schmartz, S.; Giraud-Kerleroux, L.; Moulin, F.; De Saint Blanquat, L.; et al. Comparison of idiopathic and drug-induced epidermal necrolysis in children. Br. J. Dermatol. 2023, 189, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Akpek, G.; Koh, H.K.; Bogen, S.; O’Hara, C.; Foss, F.M. Chemotherapy with etoposide, vincristine, doxorubicin, bolus cyclophosphamide, and oral prednisone in patients with refractory cutaneous T-cell lymphoma. Cancer 1999, 86, 1368–1376. [Google Scholar] [CrossRef]
- Molin, L.; Thomsen, K.; Volden, G.; Aronsson, A.; Hammar, H.; Hellbe, L.; Wantzin, G.L.; Roupe, G. Oral retinoids in mycosis fungoides and Sézary syndrome: A comparison of isotretinoin and etretinate. A study from the Scandinavian Mycosis Fungoides Group. Acta Derm. Venereol. 1987, 67, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.F.M.; Khot, A.; McCormack, C.; Lade, S.; Westerman, D.A.; Twigger, R.; Buelens, O.; Newland, K.; Tam, C.; Dickinson, M.; et al. Lack of durable disease control with chemotherapy for mycosis fungoides and Sézary syndrome: A comparative study of systemic therapy. Blood 2015, 125, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Seo, N.; Ohshima, A.; Itoh, T.; Itoh, N.; Horibe, T.; Yoshinari, Y.; Takigawa, M.; Hashizume, H. Chemokine receptor expression in cutaneous T cell and NK/T-cell lymphomas: Immunohistochemical staining and in vitro chemotactic assay. Am. J. Surg. Pathol. 2006, 30, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.; Kuttikrishnan, S.; Khan, A.Q.; Ahmad, F.; Alam, M.; Buddenkotte, J.; Ahmad, A.; Steinhoff, M.; Uddin, S. Molecular pathogenesis of Cutaneous T cell Lymphoma: Role of chemokines, cytokines, and dysregulated signaling pathways. Semin. Cancer Biol. 2022, 86, 382–399. [Google Scholar] [CrossRef] [PubMed]
- Maj, J.; Jankowska-Konsur, A.M.; Hałoń, A.; Woźniak, Z.; Plomer-Niezgoda, E.; Reich, A. Expression of CXCR4 and CXCL12 and their correlations to the cell proliferation and angiogenesis in mycosis fungoides. Postep. Dermatol. Alergol. 2015, 32, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T.; Sugaya, M.; Murakami, T.; Asano, Y.; Tada, Y.; Kadono, T.; Okochi, H.; Tamaki, K.; Sato, S. CCL11–CCR3 Interactions Promote Survival of Anaplastic Large Cell Lymphoma Cells via ERK1/2 Activation. Cancer Res. 2011, 71, 2056–2065. [Google Scholar] [CrossRef]
- Förster, R.; Schubel, A.; Breitfeld, D.; Kremmer, E.; Renner-Müller, I.; Wolf, E.; Lipp, M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999, 99, 23–33. [Google Scholar] [CrossRef]
- Narducci, M.G.; Scala, E.; Bresin, A.; Caprini, E.; Picchio, M.C.; Remotti, D.; Ragone, G.; Nasorri, F.; Frontani, M.; Arcelli, D.; et al. Skin homing of Sézary cells involves SDF-1-CXCR4 signaling and down-regulation of CD26/dipeptidylpeptidase IV. Blood 2006, 107, 1108–1115. [Google Scholar] [CrossRef]
- Notohamiprodjo, M.; Segerer, S.; Huss, R.; Hildebrandt, B.; Soler, D.; Djafarzadeh, R.; Buck, W.; Nelson, P.J.; von Luettichau, I. CCR10 is expressed in cutaneous T-cell lymphoma. Int. J. Cancer 2005, 115, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, J.; Zhang, H.; Ramakrishna, R.; Mintzlaff, D.; Mathes, D.W.; Pomfret, E.A.; Lucia, M.S.; Gao, D.; Haverkos, B.M.; et al. CCR4-IL2 bispecific immunotoxin is more effective than brentuximab for targeted therapy of cutaneous T-cell lymphoma in a mouse CTCL model. FEBS Open Bio 2023, 13, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Tuzova, M.; Richmond, J.; Wolpowitz, D.; Curiel-Lewandrowski, C.; Chaney, K.; Kupper, T.; Cruikshank, W. CCR4+ T cell recruitment to the skin in mycosis fungoides: Potential contributions by thymic stromal lymphopoietin and interleukin-16. Leuk. Lymphoma 2015, 56, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Kunicki, M.A.; Amaya Hernandez, L.C.; Davis, K.L.; Bacchetta, R.; Roncarolo, M.-G. Identity and Diversity of Human Peripheral Th and T Regulatory Cells Defined by Single-Cell Mass Cytometry. J. Immunol. 2018, 200, 336–346. [Google Scholar] [CrossRef]
- Santagata, S.; Ieranò, C.; Trotta, A.M.; Capiluongo, A.; Auletta, F.; Guardascione, G.; Scala, S. CXCR4 and CXCR7 Signaling Pathways: A Focus on the Cross-Talk Between Cancer Cells and Tumor Microenvironment. Front. Oncol. 2021, 11, 591386. [Google Scholar] [CrossRef] [PubMed]
- Yoshie, O.; Matsushima, K. CCR4 and its ligands: From bench to bedside. Int. Immunol. 2015, 27, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Rott, L.; Kunkel, E.J.; Genovese, M.C.; Andrew, D.P.; Wu, L.; Butcher, E.C. Rules of chemokine receptor association with T cell polarization in vivo. J. Clin. Investig. 2001, 108, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.M.; Stolberg, V.R.; Chiu, B.-C.; Lukacs, N.W.; Kunkel, S.L.; Chensue, S.W. CCR4 Participation in Th Type 1 (Mycobacterial) and Th Type 2 (Schistosomal) Anamnestic Pulmonary Granulomatous Responses. J. Immunol. 2006, 177, 4149–4158. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Nagakubo, D.; Komori, Y.; Fujisato, S.; Takeda, N.; Kitamatsu, M.; Nishiwaki, K.; Quan, Y.-S.; Kamiyama, F.; Oiso, N.; et al. CCR4 Is Critically Involved in Skin Allergic Inflammation of BALB/c Mice. J. Investig. Dermatol. 2018, 138, 1764–1773. [Google Scholar] [CrossRef]
- Araujo-Pires, A.C.; Vieira, A.E.; Francisconi, C.F.; Biguetti, C.C.; Glowacki, A.; Yoshizawa, S.; Campanelli, A.P.; Trombone, A.P.F.; Sfeir, C.S.; Little, S.R.; et al. IL-4/CCL22/CCR4 Axis Controls Regulatory T-Cell Migration That Suppresses Inflammatory Bone Loss in Murine Experimental Periodontitis. J. Bone Miner. Res. 2015, 30, 412–422. [Google Scholar] [CrossRef]
- Scheu, S.; Ali, S.; Ruland, C.; Arolt, V.; Alferink, J. The C-C Chemokines CCL17 and CCL22 and Their Receptor CCR4 in CNS Autoimmunity. Int. J. Mol. Sci. 2017, 18, 2306. [Google Scholar] [CrossRef] [PubMed]
- Mikhak, Z.; Strassner, J.P.; Luster, A.D. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J. Exp. Med. 2013, 210, 1855–1869. [Google Scholar] [CrossRef] [PubMed]
- Kempf, W.; Mitteldorf, C. Cutaneous T-cell lymphomas-An update 2021. Hematol. Oncol. 2021, 39 (Suppl. S1), 46–51. [Google Scholar] [CrossRef] [PubMed]
- Cowan, R.A.; Scarisbrick, J.J.; Zinzani, P.L.; Nicolay, J.P.; Sokol, L.; Pinter-Brown, L.; Quaglino, P.; Iversen, L.; Dummer, R.; Musiek, A.; et al. Efficacy and safety of mogamulizumab by patient baseline blood tumour burden: A post hoc analysis of the MAVORIC trial. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.-Y.; Chu, S.-F.; Chen, N.-H. The role of chemokines and chemokine receptors in multiple sclerosis. Int. Immunopharmacol. 2020, 83, 106314. [Google Scholar] [CrossRef] [PubMed]
- Geller, S.; Hollmann, T.J.; Horwitz, S.M.; Myskowski, P.L.; Pulitzer, M. C-C chemokine receptor 4 expression in CD8+ cutaneous T-cell lymphomas and lymphoproliferative disorders, and its implications for diagnosis and treatment. Histopathology 2020, 76, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Ishida, T.; Masaki, A.; Takeshita, M.; Iwasaki, H.; Yonekura, K.; Tashiro, Y.; Ito, A.; Kusumoto, S.; Iida, S.; et al. Clinicopathological significance of CD28 overexpression in adult T-cell leukemia/lymphoma. Cancer Sci. 2022, 113, 349–361. [Google Scholar] [CrossRef]
- Ishida, T.; Ueda, R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006, 97, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.N.; Bayry, J.; Tchilian, E.Z.; Vani, J.; Shaila, M.S.; Forbes, E.K.; Draper, S.J.; Beverley, P.C.L.; Tough, D.F.; Flower, D.R. Toward the Discovery of Vaccine Adjuvants: Coupling In Silico Screening and In Vitro Analysis of Antagonist Binding to Human and Mouse CCR4 Receptors. PLoS ONE 2009, 4, e8084. [Google Scholar] [CrossRef]
- Robles, O.; Jackson, J.J.; Marshall, L.; Talay, O.; Chian, D.; Cutler, G.; Diokno, R.; Hu, D.X.; Jacobson, S.; Karbarz, E.; et al. Novel Piperidinyl-Azetidines as Potent and Selective CCR4 Antagonists Elicit Antitumor Response as a Single Agent and in Combination with Checkpoint Inhibitors. J. Med. Chem. 2020, 63, 8584–8607. [Google Scholar] [CrossRef]
- Yokoyama, K.; Ishikawa, N.; Igarashi, S.; Kawano, N.; Masuda, N.; Hattori, K.; Miyazaki, T.; Ogino, S.; Orita, M.; Matsumoto, Y.; et al. Potent CCR4 antagonists: Synthesis, evaluation, and docking study of 2,4-diaminoquinazolines. Bioorg. Med. Chem. 2008, 16, 7968–7974. [Google Scholar] [CrossRef]
- Nakagami, Y.; Kawase, Y.; Yonekubo, K.; Nosaka, E.; Etori, M.; Takahashi, S.; Takagi, N.; Fukuda, T.; Kuribayashi, T.; Nara, F.; et al. RS-1748, a Novel CC Chemokine Receptor 4 Antagonist, Inhibits Ovalbumin-Induced Airway Inflammation in Guinea Pigs. Biol. Pharm. Bull. 2010, 33, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- RAPT Therapeutics, Inc. A Phase 2 Study to Evaluate the Efficacy and Safety of RPT193 as Monotherapy in Adults with Moderate-to-Severe Atopic Dermatitis; RAPT Therapeutics, Inc.: South San Francisco, CA, USA, 2023. Available online: https://clinicaltrials.gov/ (accessed on 1 January 2024).
- Procopiou, P.A.; Barrett, J.W.; Barton, N.P.; Begg, M.; Clapham, D.; Copley, R.C.B.; Ford, A.J.; Graves, R.H.; Hall, D.A.; Hancock, A.P.; et al. Synthesis and structure-activity relationships of indazole arylsulfonamides as allosteric CC-chemokine receptor 4 (CCR4) antagonists. J. Med. Chem. 2013, 56, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Kindon, N.; Andrews, G.; Baxter, A.; Cheshire, D.; Hemsley, P.; Johnson, T.; Liu, Y.-Z.; McGinnity, D.; McHale, M.; Mete, A.; et al. Discovery of AZD-2098 and AZD-1678, Two Potent and Bioavailable CCR4 Receptor Antagonists. ACS Med. Chem. Lett. 2017, 8, 981–986. [Google Scholar] [CrossRef]
- Bozza, S.; Iannitti, R.G.; Pariano, M.; Renga, G.; Costantini, C.; Romani, L.; Bayry, J. Small Molecule CCR4 Antagonists Protect Mice from Aspergillus Infection and Allergy. Biomolecules 2021, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Han, W.; Wang, X.; Velatooru, L.R.; Ni, X. CCR4 Antagonists in Cutaneous T-Cell Lymphoma (CTCL). Available online: https://openworks.mdanderson.org/cgi/viewcontent.cgi?article=1005&context=sumexp22 (accessed on 1 January 2024).
- Watanabe, K.; Gomez, A.M.; Kuramitsu, S.; Siurala, M.; Da, T.; Agarwal, S.; Song, D.; Scholler, J.; Rotolo, A.; Posey, A.D.; et al. Identifying highly active anti-CCR4 CAR T cells for the treatment of T-cell lymphoma. Blood Adv. 2023, 7, 3416–3430. [Google Scholar] [CrossRef]
- Roelens, M.; de Masson, A.; Andrillon, A.; Ram-Wolff, C.; Biard, L.; Boisson, M.; Mourah, S.; Battistella, M.; Toubert, A.; Bagot, M.; et al. Mogamulizumab induces long-term immune restoration and reshapes tumour heterogeneity in Sézary syndrome. Br. J. Dermatol. 2022, 186, 1010–1025. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Scarisbrick, J.J.; Dummer, R.; Whittaker, S.; Duvic, M.; Kim, Y.H.; Quaglino, P.; Zinzani, P.L.; Bechter, O.; Eradat, H.; et al. Randomized phase 3 ALCANZA study of brentuximab vedotin vs physician’s choice in cutaneous T-cell lymphoma: Final data. Blood Adv. 2021, 5, 5098–5106. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Beylot-Barry, M.; Booken, N.; Weishaupt, C.; Scarisbrick, J.; Wu, W.; Rosen, J.-P.; Medley, M.C. Impact of blood involvement on efficacy and time to response with mogamulizumab in mycosis fungoides and Sézary syndrome. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 311–316. [Google Scholar] [CrossRef]
- Zhou, Y.; Vedantham, P.; Lu, K.; Agudelo, J.; Carrion, R.; Nunneley, J.W.; Barnard, D.; Pöhlmann, S.; McKerrow, J.H.; Renslo, A.R.; et al. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral. Res. 2015, 116, 76–84. [Google Scholar] [CrossRef]
- Sato, T.; Iwase, M.; Miyama, M.; Komai, M.; Ohshima, E.; Asai, A.; Yano, H.; Miki, I. Internalization of CCR4 and Inhibition of Chemotaxis by K777, a Potent and Selective CCR4 Antagonist. Pharmacology 2013, 91, 305–313. [Google Scholar] [CrossRef]
- Ajram, L.; Begg, M.; Slack, R.; Cryan, J.; Hall, D.; Hodgson, S.; Ford, A.; Barnes, A.; Swieboda, D.; Mousnier, A.; et al. Internalization of the chemokine receptor CCR4 can be evoked by orthosteric and allosteric receptor antagonists. Eur. J. Pharmacol. 2014, 729, 75–85. [Google Scholar] [CrossRef]
- Hawkins, N.; Muszbek, N.; Evans, R.; Dequen-O’Byrne, P.; Jones, T.; McNamara, L. Adjusting for treatment crossover in the MAVORIC trial: Survival in advanced mycosis fungoides and Sézary syndrome. J. Comp. Eff. Res. 2022, 11, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Rixe, O.; Chiu, V.K.; Forde, P.M.; Dragovich, T.; Lou, Y.; Nayak-Kapoor, A.; Leidner, R.; Atkins, J.N.; Collaku, A.; et al. Mogamulizumab in Combination with Nivolumab in a Phase I/II Study of Patients with Locally Advanced or Metastatic Solid Tumors. Clin. Cancer Res. 2022, 28, 479–488. [Google Scholar] [CrossRef]
- Porcu, P.; Hudgens, S.; Horwitz, S.; Quaglino, P.; Cowan, R.; Geskin, L.; Beylot-Barry, M.; Floden, L.; Bagot, M.; Tsianakas, A.; et al. Quality of Life Effect of the Anti-CCR4 Monoclonal Antibody Mogamulizumab Versus Vorinostat in Patients with Cutaneous T-cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2021, 21, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Y.; Qi, H.; Xiao, J.; Gong, H.; Zhang, Y.; Xu, E.; Li, S.; Ma, D.; Wang, Y.; et al. A new antagonist for CCR4 attenuates allergic lung inflammation in a mouse model of asthma. Sci. Rep. 2017, 7, 15038. [Google Scholar] [CrossRef]
- Herschhorn, A.; Gu, C.; Espy, N.; Richard, J.; Finzi, A.; Sodroski, J.G. A broad HIV-1 inhibitor blocks envelope glycoprotein transitions critical for entry. Nat. Chem. Biol. 2014, 10, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.A.; Marubayashi, S.; Jorapur, A.; Jacobson, S.; Zibinsky, M.; Robles, O.; Hu, D.X.; Jackson, J.J.; Pookot, D.; Sanchez, J.; et al. Tumors establish resistance to immunotherapy by regulating Treg recruitment via CCR4. J. Immunother. Cancer 2020, 8, e000764. [Google Scholar] [CrossRef]
- Jorapur, A.; Marshall, L.A.; Jacobson, S.; Xu, M.; Marubayashi, S.; Zibinsky, M.; Hu, D.X.; Robles, O.; Jackson, J.J.; Baloche, V.; et al. EBV+ tumors exploit tumor cell-intrinsic and -extrinsic mechanisms to produce regulatory T cell-recruiting chemokines CCL17 and CCL22. PLoS Pathog. 2022, 18, e1010200. [Google Scholar] [CrossRef]
- Allen, S.; Newhouse, B.; Anderson, A.S.; Fauber, B.; Allen, A.; Chantry, D.; Eberhardt, C.; Odingo, J.; Burgess, L.E. Discovery and SAR of trisubstituted thiazolidinones as CCR4 antagonists. Bioorg. Med. Chem. Lett. 2004, 14, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Scarisbrick, J.J.; Prince, H.M.; Vermeer, M.H.; Quaglino, P.; Horwitz, S.; Porcu, P.; Stadler, R.; Wood, G.S.; Beylot-Barry, M.; Pham-Ledard, A.; et al. Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sézary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. J. Clin. Oncol. 2015, 33, 3766–3773. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, D.; Nishikawa, H.; Maeda, Y.; Nishioka, M.; Tanemura, A.; Katayama, I.; Ezoe, S.; Kanakura, Y.; Sato, E.; Fukumori, Y.; et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+ CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 17945–17950. [Google Scholar] [CrossRef] [PubMed]
- Beygi, S.; Duran, G.E.; Fernandez-Pol, S.; Rook, A.H.; Kim, Y.H.; Khodadoust, M.S. Resistance to mogamulizumab is associated with loss of CCR4 in cutaneous T-cell lymphoma. Blood 2022, 139, 3732–3736. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, K.; Amagai, R.; Kambayashi, Y.; Asano, Y.; Fujimura, T. Serum CCL22 Increased in Advanced Melanoma Patients with Liver Metastases: Report of 5 Cases. Case Rep. Oncol. 2022, 15, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Hisamoto, T.; Suga, H.; Kawana, Y.; Oka, T.; Miyagaki, T.; Sugaya, M.; Sato, S. A case of mycosis fungoides successfully treated with combination of bexarotene and mogamulizumab. Dermatol. Ther. 2021, 34, e14805. [Google Scholar] [CrossRef] [PubMed]
- Teoli, M.; Mandel, V.D.; Franceschini, C.; Saraceni, P.L.; Cicini, M.P.; Ardigò, M. Mogamulizumab and bexarotene are a promising association for the treatment of advanced cutaneous T-cell lymphomas: A case series. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8118–8128. [Google Scholar] [CrossRef]
- Fong, S.; Hong, E.K.; Khodadoust, M.S.; Li, S.; Hoppe, R.T.; Kim, Y.H.; Hiniker, S.M. Low-Dose Total Skin Electron Beam Therapy Combined With Mogamulizumab for Refractory Mycosis Fungoides and Sézary Syndrome. Adv. Radiat. Oncol. 2021, 6, 100629. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Muniz, C.A.; Sánchez-Velázquez, A.; Arroyo-Andrés, J.; Agud-de Dios, M.; Tarín-Vicente, E.J.; Falkenhain-López, D.; Ortiz-Romero, P.L. Mogamulizumab combined with extracorporeal photopheresis for the treatment of refractory mycosis fungoides and Sézary syndrome. Report of seven cases. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e102–e105. [Google Scholar] [CrossRef]
- Raval, N.S.; Snowden, C.K.; De Monnin, K.S.; Yokoyama, C.C.; Choi, J.; Mehta-Shah, N.; Rosman, I.S.; Pavlisin, J.; Musiek, A.C. Scarring alopecia developing after mogamulizumab-associated rash. Eur. J. Dermatol. 2021, 31, 841–843. [Google Scholar] [CrossRef]
- Musiek, A.C.M.; Rieger, K.E.; Bagot, M.; Choi, J.N.; Fisher, D.C.; Guitart, J.; Haun, P.L.; Horwitz, S.M.; Huen, A.O.-L.; Kwong, B.Y.; et al. Dermatologic Events Associated with the Anti-CCR4 Antibody Mogamulizumab: Characterization and Management. Dermatol. Ther. 2022, 12, 29–40. [Google Scholar] [CrossRef]
- Mitteldorf, C.; Langer, N.; Kempf, W.; Schön, M.P. Mogamulizumab-associated rash simulating lupus miliaris disseminatus faciei. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e479–e481. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, S.; Suh-Oh, H.-J.; Couselo-Rodríguez, C.; Soto-García, D.; Álvarez, C.; Flórez, Á. Mogamulizumab-associated rash: A challenging case report and literature review. Australas. J. Dermatol. 2023, 64, e191–e193. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, C.M.; Sharma, A.N.; Lee, B.A.; Pinter-Brown, L.C.; Smith, J.; Linden, K.; Mesinkovska, N.A. Alopecia areata-like presentations with mogamulizumab therapy. JAAD Case Rep. 2023, 41, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Breen, I.D.; Brumfiel, C.M.; Patel, M.H.; Rosenthal, A.C.; Rule, W.G.; DiCaudo, D.J.; Craig, F.E.; Pittelkow, M.R.; Mangold, A.R. Mogamulizumab-induced interface dermatitis drug rash treated successfully with methotrexate and extracorporeal photopheresis in a patient with Sézary syndrome. JAAD Case Rep. 2021, 9, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Caruso, L.; Castellino, A.; Dessì, D.; Flenghi, L.; Giordano, A.; Ibatici, A.; Massone, C.; Pileri, A.; Proietti, I.; Pupo, L.; et al. Italian Real-Life Experience on the Use of Mogamulizumab in Patients with Cutaneous T-Cell Lymphomas. Cancer Manag. Res. 2022, 14, 3205–3221. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Hirotsu, K.E.; Neal, T.M.; Raghavan, S.S.; Kwong, B.Y.; Khodadoust, M.S.; Brown, R.A.; Novoa, R.A.; Kim, Y.H.; Rieger, K.E. Histopathologic Characterization of Mogamulizumab-associated Rash. Am. J. Surg. Pathol. 2020, 44, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, K.E.; Neal, T.M.; Khodadoust, M.S.; Wang, J.Y.; Rieger, K.E.; Strelo, J.; Hong, E.; Kim, Y.H.; Kwong, B.Y. Clinical Characterization of Mogamulizumab-Associated Rash During Treatment of Mycosis Fungoides or Sézary Syndrome. JAMA Dermatol. 2021, 157, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Pileri, A.; Clarizio, G.; Zengarini, C.; Casadei, B.; Sabattini, E.; Agostinelli, C.; Zinzani, P.L. Mogamulizumab-associated rashes, their presentation and prognostic significance: A single-centre retrospective case series analysis. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e615–e617. [Google Scholar] [CrossRef]
- Barta, S.K.; Liu, N.; DerSarkissian, M.; Chang, R.; Ye, M.; Duh, M.S.; Surinach, A.; Fanale, M.; Yu, K.S. Real-World Treatment Patterns and Clinical Outcomes With Brentuximab Vedotin or Other Standard Therapies in Patients With Previously Treated Cutaneous T-Cell Lymphoma in the United States. Clin. Lymphoma Myeloma Leuk. 2023, 24, e21–e32. [Google Scholar] [CrossRef]
- Fujikawa, K.; Saito, T.; Kurose, K.; Kojima, T.; Funakoshi, T.; Sato, E.; Kakimi, K.; Iida, S.; Doki, Y.; Oka, M.; et al. Integrated analysis of phase 1a and 1b randomized controlled trials; Treg-targeted cancer immunotherapy with the humanized anti-CCR4 antibody, KW-0761, for advanced solid tumors. PLoS ONE 2023, 18, e0291772. [Google Scholar] [CrossRef] [PubMed]
- Izutsu, K.; Makita, S.; Nosaka, K.; Yoshimitsu, M.; Utsunomiya, A.; Kusumoto, S.; Morishima, S.; Tsukasaki, K.; Kawamata, T.; Ono, T.; et al. An open-label, single-arm phase 2 trial of valemetostat for relapsed or refractory adult T-cell leukemia/lymphoma. Blood 2023, 141, 1159–1168. [Google Scholar] [CrossRef]
- Saito, T.; Kurose, K.; Kojima, T.; Funakoshi, T.; Sato, E.; Nishikawa, H.; Nakajima, J.; Seto, Y.; Kakimi, K.; Iida, S.; et al. Phase Ib study on the humanized anti-CCR4 antibody, KW-0761, in advanced solid tumors. Nagoya J. Med. Sci. 2021, 83, 827–840. [Google Scholar] [CrossRef]
- Maeda, Y.; Wada, H.; Sugiyama, D.; Saito, T.; Irie, T.; Itahashi, K.; Minoura, K.; Suzuki, S.; Kojima, T.; Kakimi, K.; et al. Depletion of central memory CD8+ T cells might impede the antitumor therapeutic effect of Mogamulizumab. Nat. Commun. 2021, 12, 7280. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.; Zinzani, P.L.; Bagot, M.; Kim, Y.H.; Moskowitz, A.J.; Porcu, P.; Dwyer, K.; Sun, W.; Herr, F.M.; Scarisbrick, J. Lack of impact of type and extent of prior therapy on outcomes of mogamulizumab therapy in patients with cutaneous T cell lymphoma in the MAVORIC trial. Leuk. Lymphoma 2021, 62, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.P.; Miljkovic, M.D.; Fleisher, T.A.; Pittaluga, S.; Hsu-Albert, J.; Bryant, B.R.; Petrus, M.N.; Perera, L.P.; Müller, J.R.; Shih, J.H.; et al. Short-course IL-15 given as a continuous infusion led to a massive expansion of effective NK cells: Implications for combination therapy with antitumor antibodies. J. Immunother. Cancer 2021, 9, e002193. [Google Scholar] [CrossRef]
- Rolf, D.; Elsayad, K.; Eich, H.T. Acute and sub-acute toxicity profile of ultra-hypofractionated low-dose total skin electron beam with two 4 Gy fractions for cutaneous T cell lymphoma. J. Cancer Res. Clin. Oncol. 2021, 147, 1757–1761. [Google Scholar] [CrossRef]
- Zamarin, D.; Hamid, O.; Nayak-Kapoor, A.; Sahebjam, S.; Sznol, M.; Collaku, A.; Fox, F.E.; Marshall, M.A.; Hong, D.S. Mogamulizumab in Combination with Durvalumab or Tremelimumab in Patients with Advanced Solid Tumors: A Phase I Study. Clin. Cancer Res. 2020, 26, 4531–4541. [Google Scholar] [CrossRef]
- Hirosawa, M.; Yamaguchi, T.; Tanaka, A.; Kominato, Y.; Higashi, T.; Morimoto, H.; Tsukada, J. Reduced-intensity haploidentical peripheral blood stem cell transplantation using low-dose thymoglobulin for aggressive adult T cell leukemia/lymphoma patients in non-complete remission. Ann. Hematol. 2020, 99, 599–607. [Google Scholar] [CrossRef]
- Mukai, M.; Mould, D.; Maeda, H.; Narushima, K.; Greene, D. Exposure-Response Analysis for Mogamulizumab in Adults With Cutaneous T-Cell Lymphoma. J. Clin. Pharmacol. 2020, 60, 50–57. [Google Scholar] [CrossRef]
- Matsuo, K.; Hatanaka, S.; Kimura, Y.; Hara, Y.; Nishiwaki, K.; Quan, Y.S.; Kamiyama, F.; Oiso, N.; Kawada, A.; Kabashima, K.; et al. A CCR4 antagonist ameliorates atopic dermatitis-like skin lesions induced by dibutyl phthalate and a hydrogel patch containing ovalbumin. Biomed. Pharmacother. 2019, 109, 1437–1444. [Google Scholar] [CrossRef]
- Bissonnette, R.; DuBois, J.; Facheris, P.; Del Duca, E.; Kim, M.; Correa Da Rosa, J.; Trujillo, D.L.; Bose, S.; Pagan, A.D.; Wustrow, D.; et al. Clinical and molecular effects of oral CCR4 antagonist RPT193 in atopic dermatitis: A Phase 1 study. Allergy 2023, 79, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Ndao, M.; Nath-Chowdhury, M.; Sajid, M.; Marcus, V.; Mashiyama, S.T.; Sakanari, J.; Chow, E.; Mackey, Z.; Land, K.M.; Jacobson, M.P.; et al. A cysteine protease inhibitor rescues mice from a lethal Cryptosporidium parvum infection. Antimicrob. Agents Chemother. 2013, 57, 6063–6073. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.C.; Beck, K.R.; Holloway, C.B.; Hemings, R.A.; Dix, T.A.; Norris, R.A. The C-C Chemokine Receptor Type 4 Is an Immunomodulatory Target of Hydroxychloroquine. Front. Pharmacol. 2020, 11, 1253. [Google Scholar] [CrossRef] [PubMed]
- Shukla, L.; Ajram, L.A.; Begg, M.; Evans, B.; Graves, R.H.; Hodgson, S.T.; Lynn, S.M.; Miah, A.H.; Percy, J.M.; Procopiou, P.A.; et al. 2,8-Diazaspiro[4.5]decan-8-yl)pyrimidin-4-amine potent CCR4 antagonists capable of inducing receptor endocytosis. Eur. J. Med. Chem. 2016, 115, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Raymondi Silva, J.; Iftinca, M.; Fernandes Gomes, F.I.; Segal, J.P.; Smith, O.M.A.; Bannerman, C.A.; Silva Mendes, A.; Defaye, M.; Robinson, M.E.C.; Gilron, I.; et al. Skin-resident dendritic cells mediate postoperative pain via CCR4 on sensory neurons. Proc. Natl. Acad. Sci. USA 2022, 119, e2118238119. [Google Scholar] [CrossRef] [PubMed]
- Solari, R.; Pease, J.E. Targeting chemokine receptors in disease—A case study of CCR4. Eur. J. Pharmacol. 2015, 763, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, J.; Pawlik, K.; Ciapała, K.; Ciechanowska, A.; Mika, J. CC Chemokine Receptor 4 (CCR4) as a Possible New Target for Therapy. Int. J. Mol. Sci. 2022, 23, 15638. [Google Scholar] [CrossRef] [PubMed]
- Purandare, A.V.; Wan, H.; Somerville, J.E.; Burke, C.; Vaccaro, W.; Yang, X.; McIntyre, K.W.; Poss, M.A. Core exploration in optimization of chemokine receptor CCR4 antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 679–682. [Google Scholar] [CrossRef]
- Yamamoto, S.; Matsuo, K.; Nagakubo, D.; Higashiyama, S.; Nishiwaki, K.; Oiso, N.; Kawada, A.; Yoshie, O.; Nakayama, T. A CCR4 antagonist enhances DC activation and homing to the regional lymph node and shows potent vaccine adjuvant activity through the inhibition of regulatory T-cell recruitment. J. Pharmacol. Sci. 2018, 136, 165–171. [Google Scholar] [CrossRef]
- Cahn, A.; Hodgson, S.; Wilson, R.; Robertson, J.; Watson, J.; Beerahee, M.; Hughes, S.C.; Young, G.; Graves, R.; Hall, D.; et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of GSK2239633, a CC-chemokine receptor 4 antagonist, in healthy male subjects: Results from an open-label and from a randomised study. BMC Pharmacol. Toxicol. 2013, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Beaumont, K.; Maurer, T.S.; Di, L. Relevance of Half-Life in Drug Design. J. Med. Chem. 2018, 61, 4273–4282. [Google Scholar] [CrossRef]
- Jin, C.; Gao, B.-B.; Zhou, W.-J.; Zhao, B.-J.; Fang, X.; Yang, C.-L.; Wang, X.-H.; Xia, Q.; Liu, T.-T. Hydroxychloroquine attenuates autoimmune hepatitis by suppressing the interaction of GRK2 with PI3K in T lymphocytes. Front. Pharmacol. 2022, 13, 972397. [Google Scholar] [CrossRef] [PubMed]
- Gadhe, C.G.; Kim, M. Insights into the binding modes of CC chemokine receptor 4 (CCR4) inhibitors: A combined approach involving homology modelling, docking, and molecular dynamics simulation studies. Mol. BioSyst. 2015, 11, 618–634. [Google Scholar] [CrossRef]
- Fauzi, Y.R.; Nakahata, S.; Chilmi, S.; Ichikawa, T.; Nueangphuet, P.; Yamaguchi, R.; Nakamura, T.; Shimoda, K.; Morishita, K. Antitumor effects of chloroquine/hydroxychloroquine mediated by inhibition of the NF-κB signaling pathway through abrogation of autophagic p47 degradation in adult T-cell leukemia/lymphoma cells. PLoS ONE 2021, 16, e0256320. [Google Scholar] [CrossRef]
- Wang, C.; Reusser, N.; Shelton, M.; Reed, J.; Doan, H.; Torres-Cabala, C.A.; Dabaja, B.; Duvic, M. An unusual case of cytotoxic peripheral T-cell lymphoma. JAAD Case Rep. 2015, 1, 257–260. [Google Scholar] [CrossRef]
- Heyl, J.; Mehregan, D.; Kado, J.; Campbell, M. A case of idiopathic follicular mucinosis treated with bexarotene gel. Int. J. Dermatol. 2014, 53, 838–841. [Google Scholar] [CrossRef]
- Schneider, S.W.; Metze, D.; Bonsmann, G. Treatment of so-called idiopathic follicular mucinosis with hydroxychloroquine. Br. J. Dermatol. 2010, 163, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Westin, J. CAR T-Cells. Adv. Exp. Med. Biol. 2020, 1244, 215–233. [Google Scholar] [CrossRef]
- Perera, L.P.; Zhang, M.; Nakagawa, M.; Petrus, M.N.; Maeda, M.; Kadin, M.E.; Waldmann, T.A.; Perera, P.-Y. Chimeric antigen receptor modified T cells that target chemokine receptor CCR4 as a therapeutic modality for T-cell malignancies. Am. J. Hematol. 2017, 92, 892–901. [Google Scholar] [CrossRef]

| Title | Author | Date | Research Field | Results | References |
|---|---|---|---|---|---|
| Real-World Treatment Patterns and Clinical Outcomes with Brentuximab Vedotin or Other Standard Therapies in Patients with Previously Treated Cutaneous T-Cell Lymphoma in the United States | Stefan K Barta et al. | 2023 | Real-world data; brentuximab–vedotin vs. other treatments (including mogamolizumab) in CTCLs | Favourable outcomes with BV vs. OST in patients with CTCL previously treated with ≥1 systemic therapy | [92] |
| Integrated analysis of phase 1a and 1b randomised controlled trials; Treg-targeted cancer immunotherapy with the humanised anti-CCR4 antibody, KW-0761, for advanced solid tumours | Kaoru Fujikawa et al. | 2023 | Phase 1a and 1b trials to examine the safety and efficacy of mogamolizumab in augmenting immune therapy response in solid cancers | Durable clinical response in some patients | [93] |
| An open-label, single-arm phase 2 trial of valemetostat for relapsed or refractory adult T-cell leukaemia/lymphoma | Koji Izutsu et al. | 2023 | Phase 2 trial enrolled patients with R/R aggressive ATL treated with valemetostat, with some of them pretreated with mogamolizumab | Patients pretreated with mogamulizumab had an ORR of 45.8% (four complete and seven partial remissions) | [94] |
| Impact of blood involvement on efficacy and time to response with mogamulizumab in mycosis fungoides and Sézary syndrome | Marie Beylot-Barry et al. | 2023 | Post hoc analyses were carried out using data from MAVORIC | Compared with vorinostat, superior results were seen for ORR, PFS, and TTNT in mogamulizumab-treated patients with MF | [62] |
| Adjusting for treatment crossover in the MAVORIC trial: survival in advanced mycosis fungoides and Sézary syndrome | Neil Hawkins et al. | 2022 | Post hoc analyses were carried out using data from MAVORIC | OS of mogamulizumab relative to vorinostat may be underestimated in MAVORIC due to the presence of crossover | [66] |
| Phase Ib study on the humanised anti-CCR4 antibody, KW-0761, in advanced solid tumours | Takuro Saito et al. | 2021 | Phase 1b trial to assess the efficacy of mogamulizumab as an immunotherapeutic drug in solid tumours | Mogamulizumab resulted in the depletion of Tregs in peripheral blood and potential immune responses | [95] |
| Depletion of central memory CD8+ T-cells might impede the antitumor therapeutic effect of Mogamulizumab | Yuka Maeda et al. | 2021 | Phase 1b study, the cohort analysis of patients with advanced CCR4-negative solid cancer | Mogamulizumab’s current doses may deplete effector components in immune therapy | [96] |
| Mogamulizumab in Combination with Nivolumab in Phase I/II Study of Patients with Locally Advanced or Metastatic Solid Tumors | David S Hong et al. | 2022 | Phase I/II study, multicentric, to assess mogamulizumab with nivolumab in solid tumours | Combination therapy did not result in enhanced efficacy | [67] |
| Lack of impact of type and extent of prior therapy on outcomes of mogamulizumab treatment in patients with cutaneous T-cell lymphoma in the MAVORIC trial | Steven Horwitz et al. | 2021 | Post hoc analyses were carried out using data from MAVORIC | ORR and DOR remained consistent regardless of the type of immediately prior therapy. Additionally, the immunomodulatory activity of the last prior therapy and time from prior treatment generally did not affect the ORR or PFS | [97] |
| Short-course IL-15 given as a continuous infusion led to a massive expansion of effective NK cells: implications for combination therapy with antitumor antibodies | Sigrid P Dubois et al. | 2021 | Phase 1 study analysis | Mogamulizumab could benefit from NK expansion induced by IL-15 administration | [98] |
| Acute and sub-acute toxicity profile of ultra-hypofractionated low-dose total skin electron beam with two 4 Gy fractions for cutaneous T-cell lymphoma. | Daniel Rolf et al. | 2021 | Prospective study | Ultra-hypofractionated low-dose TSEBT followed by systemic therapy, including mogamulizumab, seems to be a feasible alternative to the conventional fractionated TSEBT | [99] |
| Quality of Life Effect of the Anti-CCR4 Monoclonal Antibody Mogamulizumab Versus Vorinostat in Patients with Cutaneous T-cell Lymphoma. | Pierluigi Porcu et al. | 2020 | Multicenter phase 3 trial | The symptoms, functions, and overall QoLs of patients with MF/SS favoured mogamulizumab over vorinostat across all time points | [68] |
| Mogamulizumab in Combination with Durvalumab or Tremelimumab in Patients with Advanced Solid Tumors: A Phase I Study. | Dmitriy Zamarin et al. | 2021 | Multicenter, phase I, dose-escalation study | There is no clear correlation of clinical response with peripheral or intratumoral reduction in CCR4+ eTregs or with the baseline degree of CCR4+ expression | [100] |
| Reduced-intensity haploidentical peripheral blood stem cell transplantation using low-dose thymoglobulin for aggressive adult T-cell leukaemia/lymphoma patients in non-complete remission | Makoto Hirosawa et al. | 2020 | Prospective study | CR was achieved in all the patients after transplantation, including one with pretransplant mogamulizumab therapy; however, T-cell receptor repertoire diversities were low even 1 year after transplantation in next-generation sequencing | [101] |
| Exposure-Response Analysis for Mogamulizumab in Adults with Cutaneous T-Cell Lymphoma | Mayumi Mukai et al. | 2020 | Registrational clinical trial | No variable was found to impact efficacy or safety, indicating that there is no need to modify the dose on the basis of this parameter | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengarini, C.; Guglielmo, A.; Mussi, M.; Motta, G.; Agostinelli, C.; Sabattini, E.; Piraccini, B.M.; Pileri, A. A Narrative Review of the State of the Art of CCR4-Based Therapies in Cutaneous T-Cell Lymphomas: Focus on Mogamulizumab and Future Treatments. Antibodies 2024, 13, 32. https://doi.org/10.3390/antib13020032
Zengarini C, Guglielmo A, Mussi M, Motta G, Agostinelli C, Sabattini E, Piraccini BM, Pileri A. A Narrative Review of the State of the Art of CCR4-Based Therapies in Cutaneous T-Cell Lymphomas: Focus on Mogamulizumab and Future Treatments. Antibodies. 2024; 13(2):32. https://doi.org/10.3390/antib13020032
Chicago/Turabian StyleZengarini, Corrado, Alba Guglielmo, Martina Mussi, Giovanna Motta, Claudio Agostinelli, Elena Sabattini, Bianca Maria Piraccini, and Alessandro Pileri. 2024. "A Narrative Review of the State of the Art of CCR4-Based Therapies in Cutaneous T-Cell Lymphomas: Focus on Mogamulizumab and Future Treatments" Antibodies 13, no. 2: 32. https://doi.org/10.3390/antib13020032