Shark Variable New Antigen Receptor (VNAR) Single Domain Antibody Fragments: Stability and Diagnostic Applications
Abstract
:1. Introduction
2. Results and Discussion
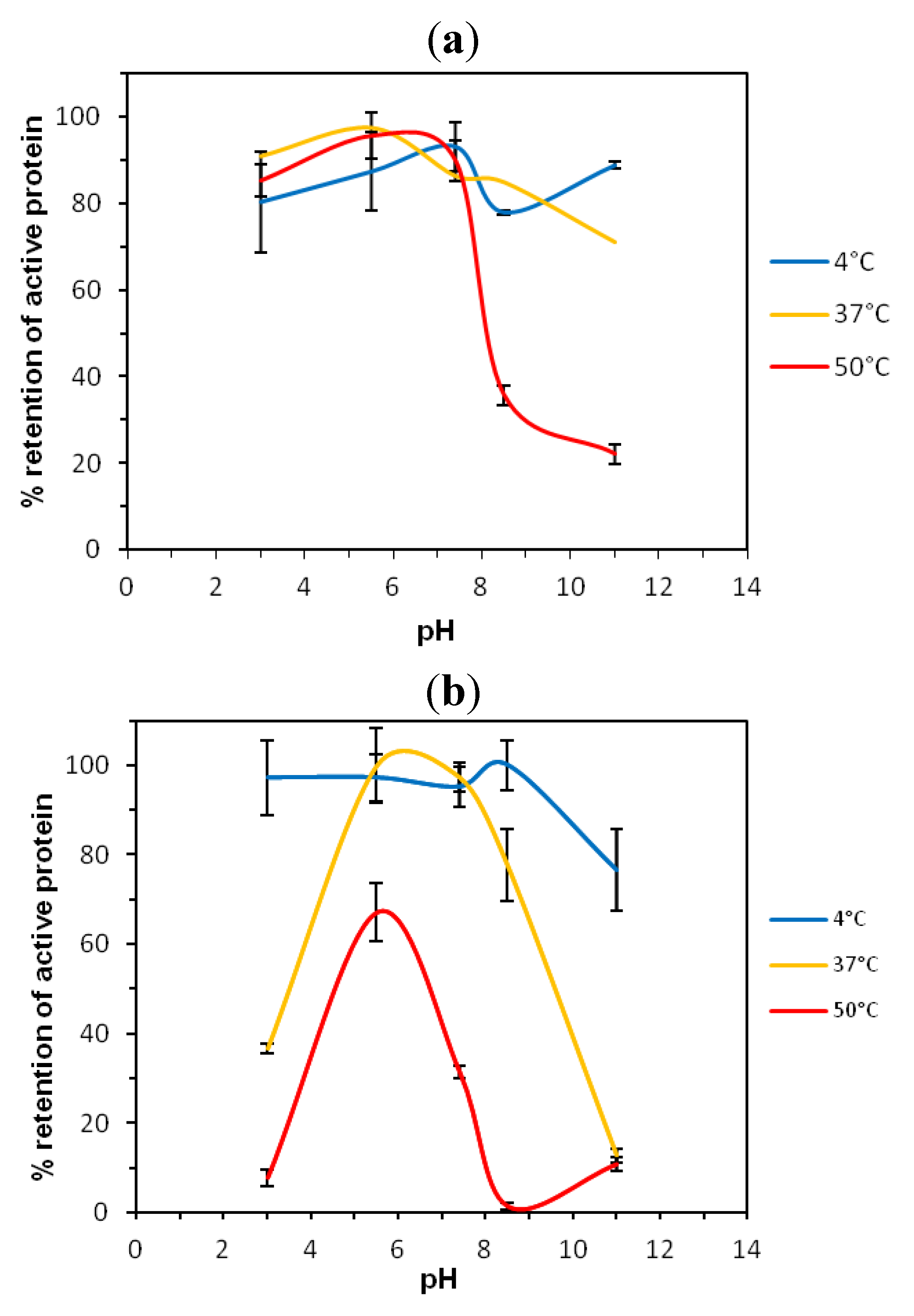
2.1. Thermostability of VNARs at Extreme pH
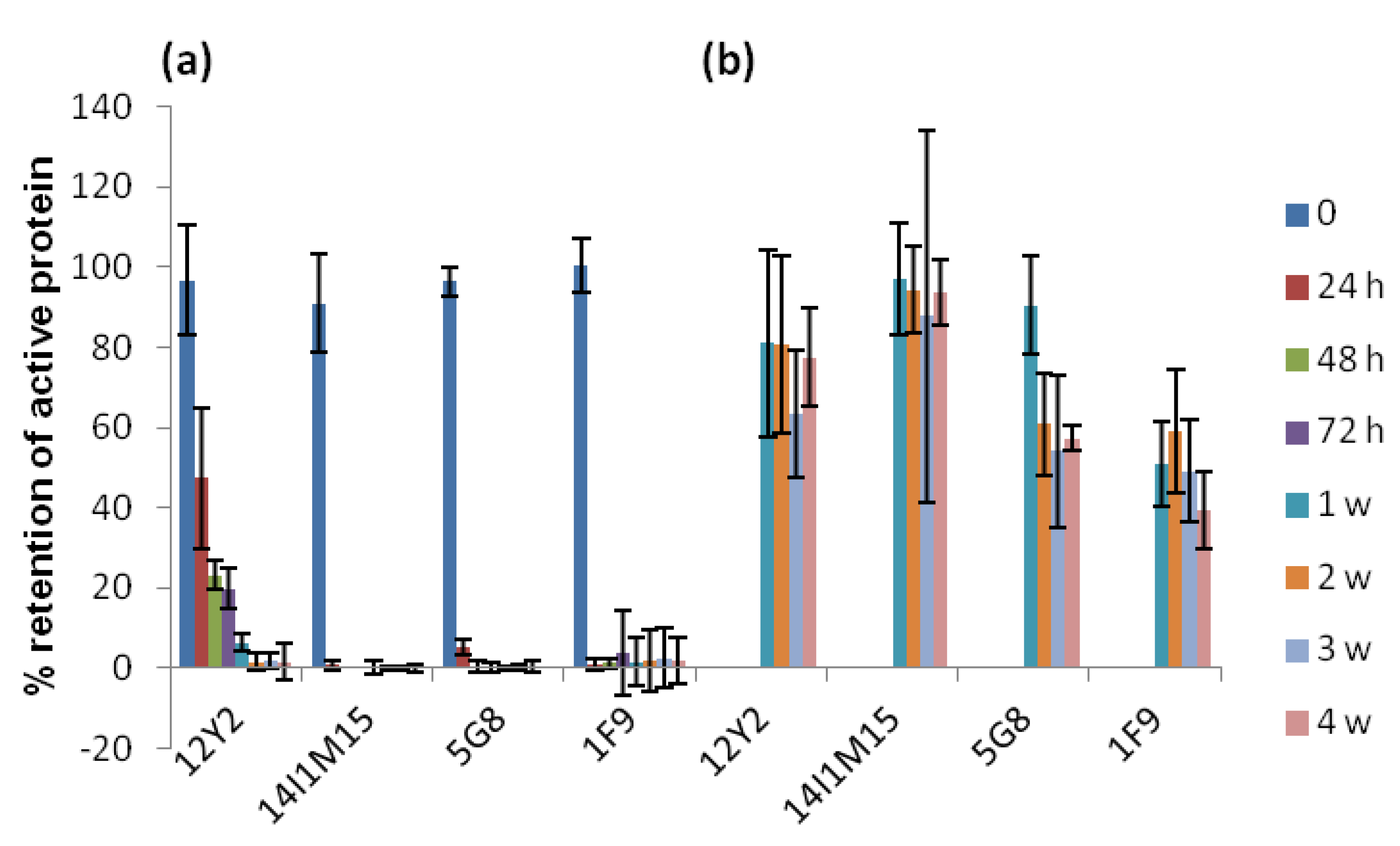
2.2. Thermostability of VNARs and Mabs in Liquid and Lyophilized Formats
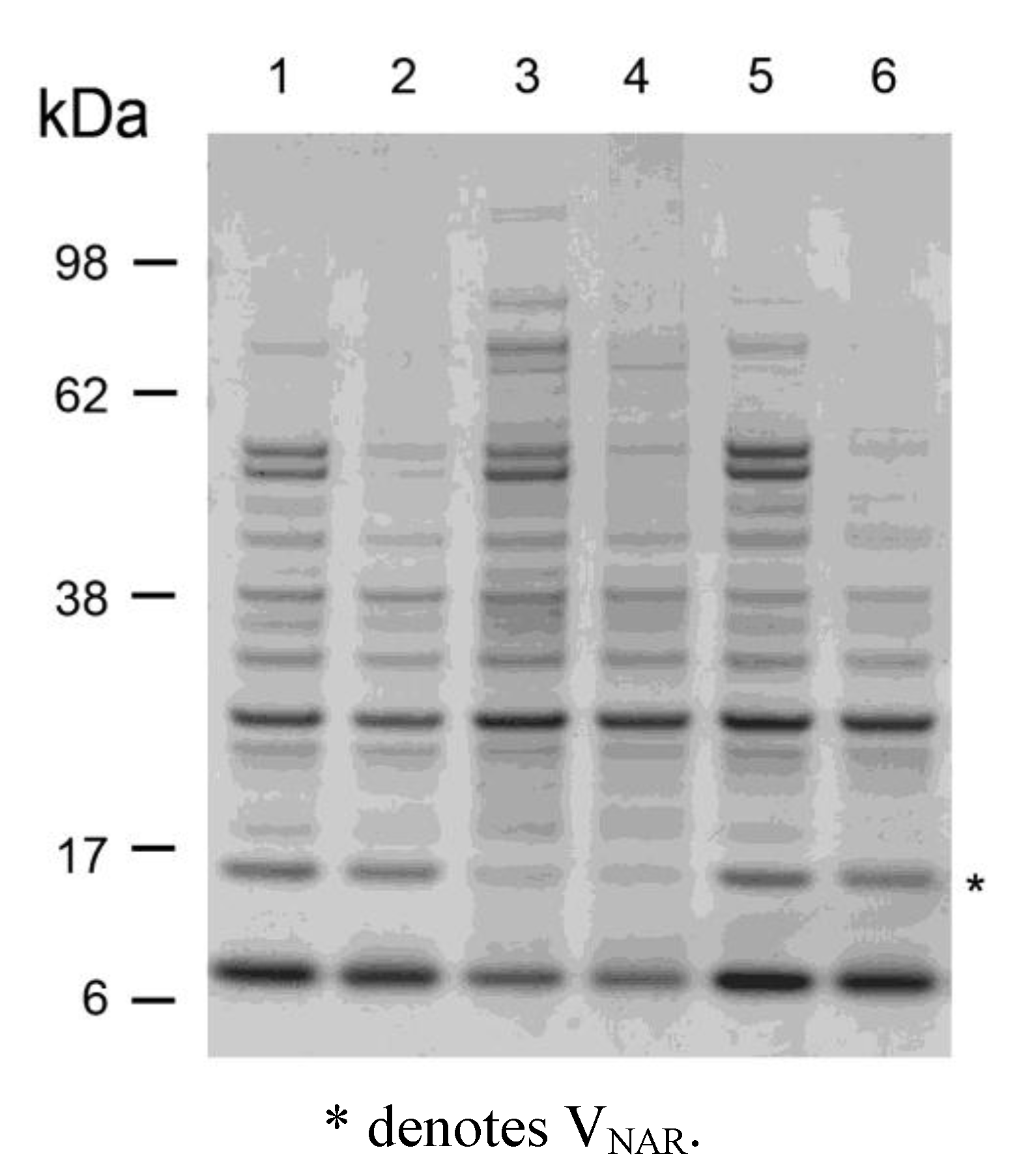
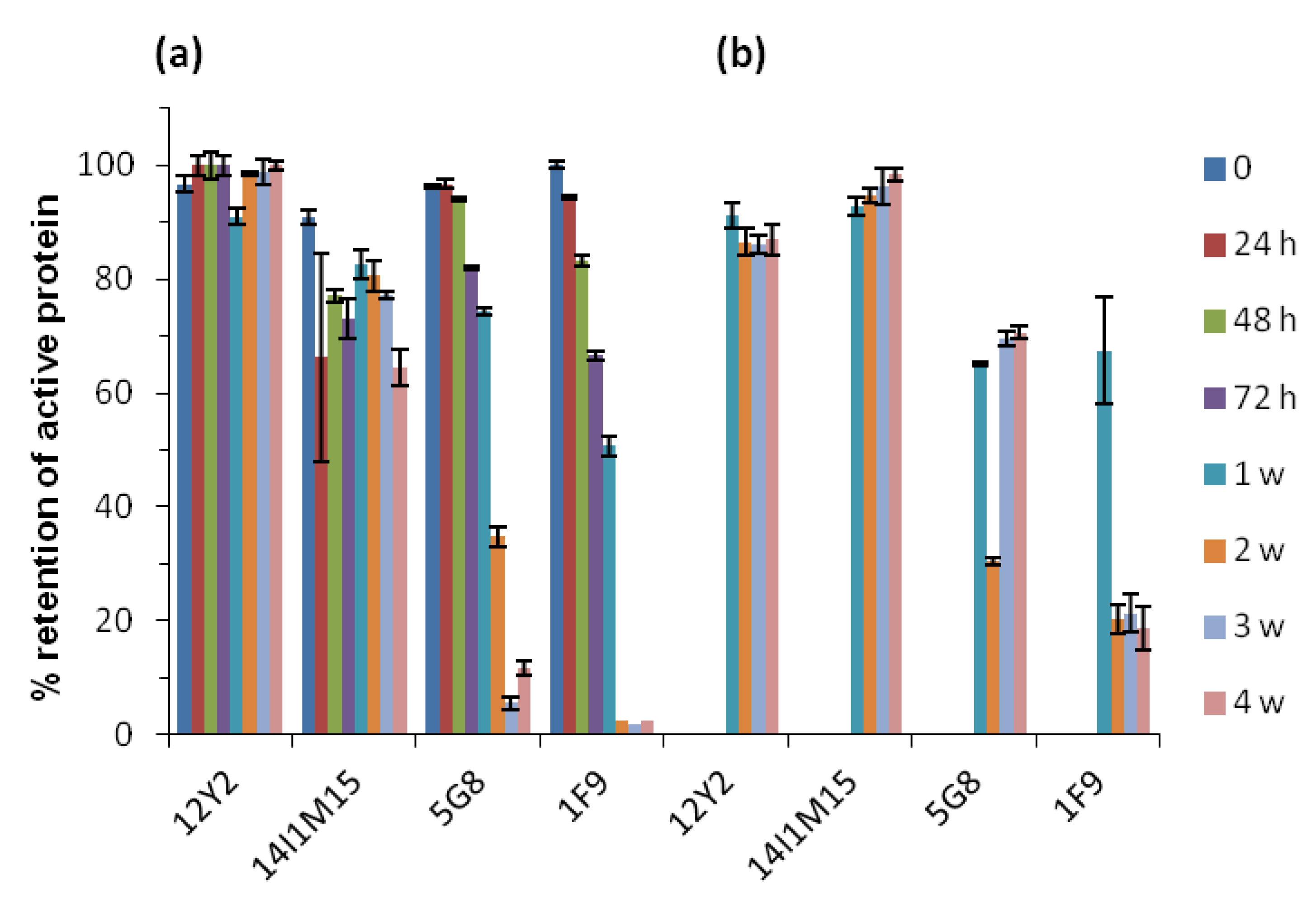
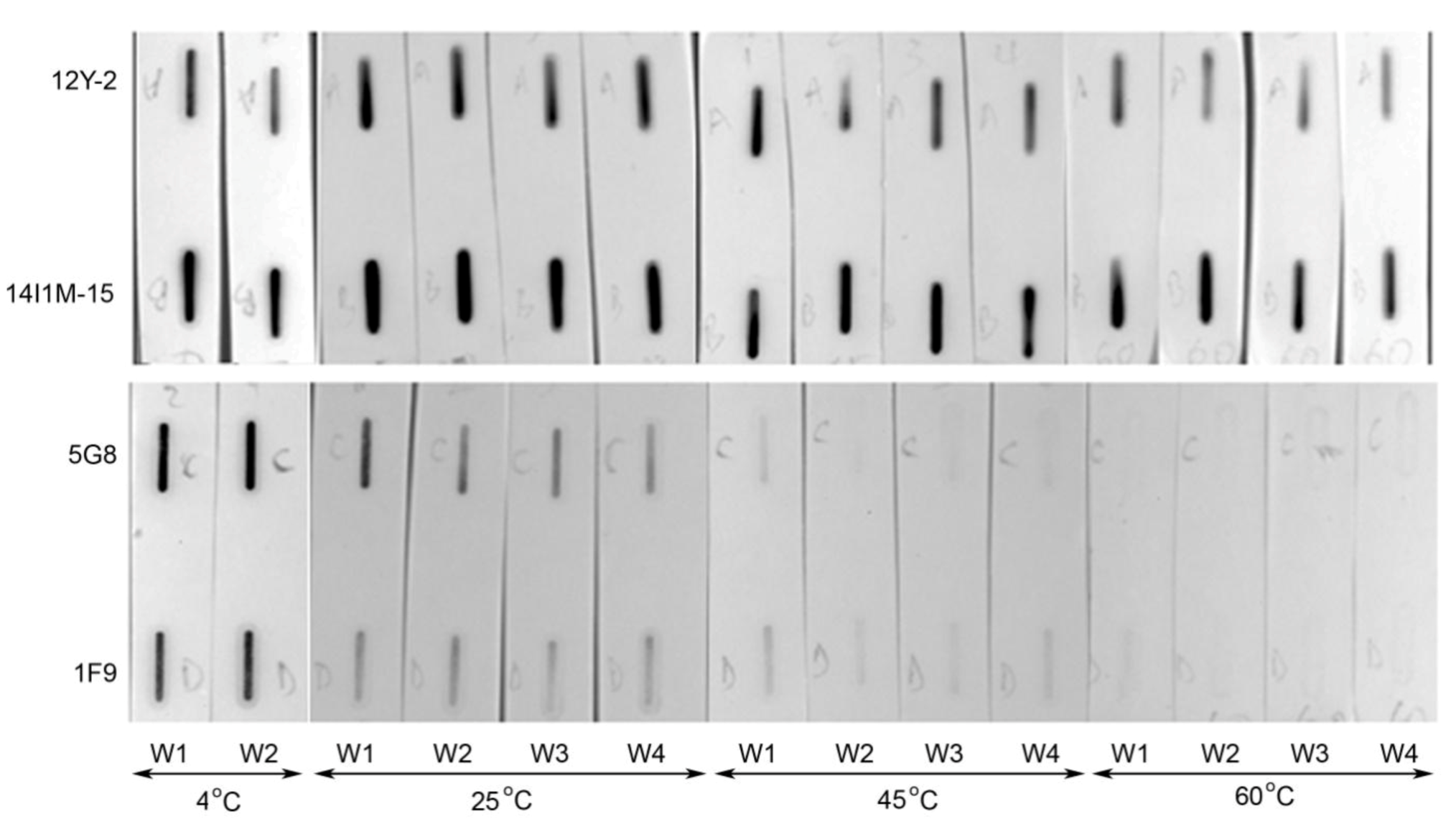
2.3. Thermostability of VNARs in Biological Samples

2.4. Analysis of Secondary Structure by Circular Dichroism Spectroscopy

3. Experimental Section
3.1. Nucleic Acid Isolation and Cloning
3.2. Soluble Expression of VNAR Constructs from Expression Vector pGC
3.3. Thermal and pH Stability of VNARs and mAbs
3.3.1. pH Stability of VNAR 4A-1
3.3.2. Heating VNARs in Periplasmic Extracts at 100 °C
3.3.3. Heating VNARs and mAbs at 45 °C and 60 °C for up to 4 Weeks
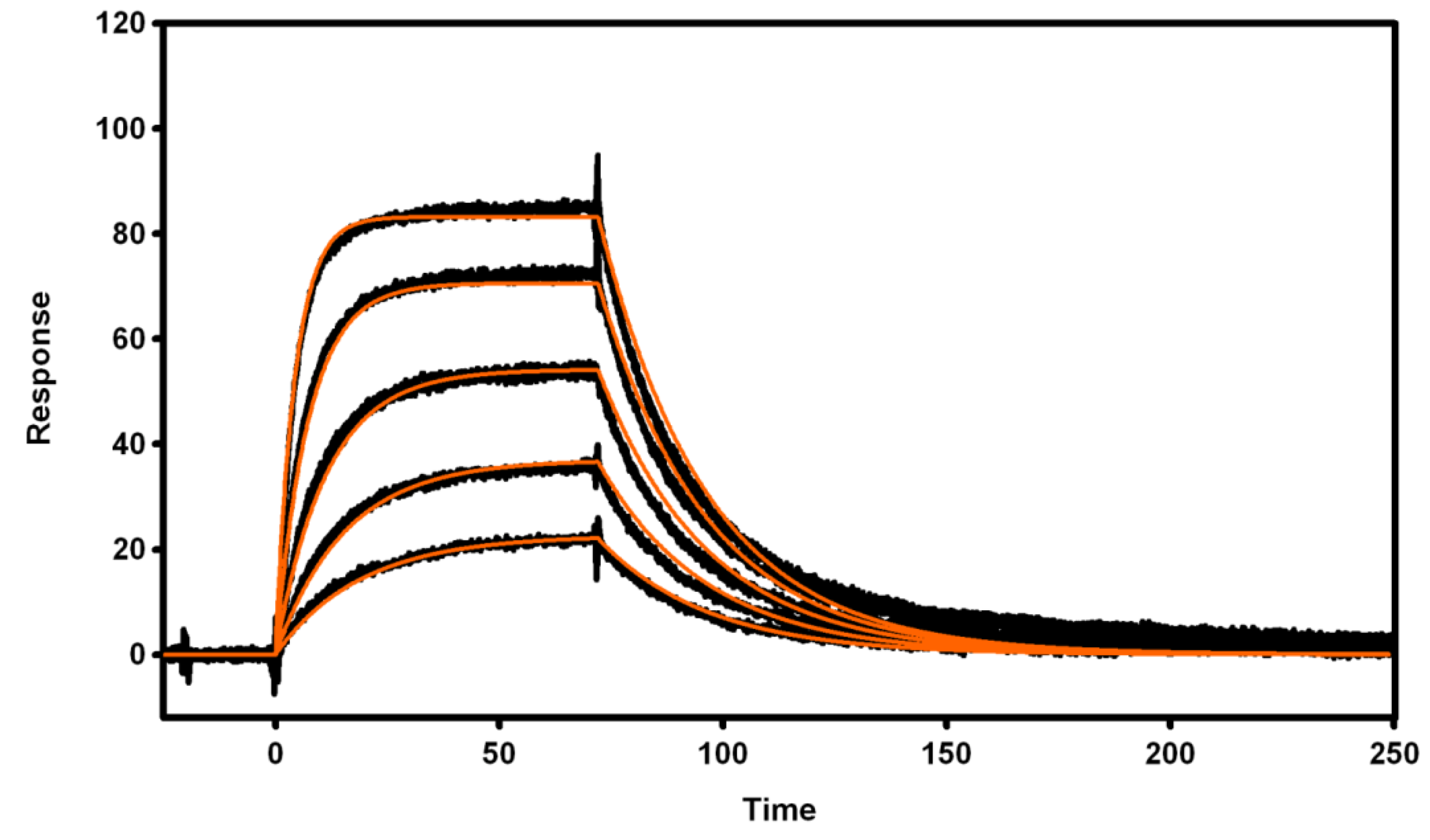
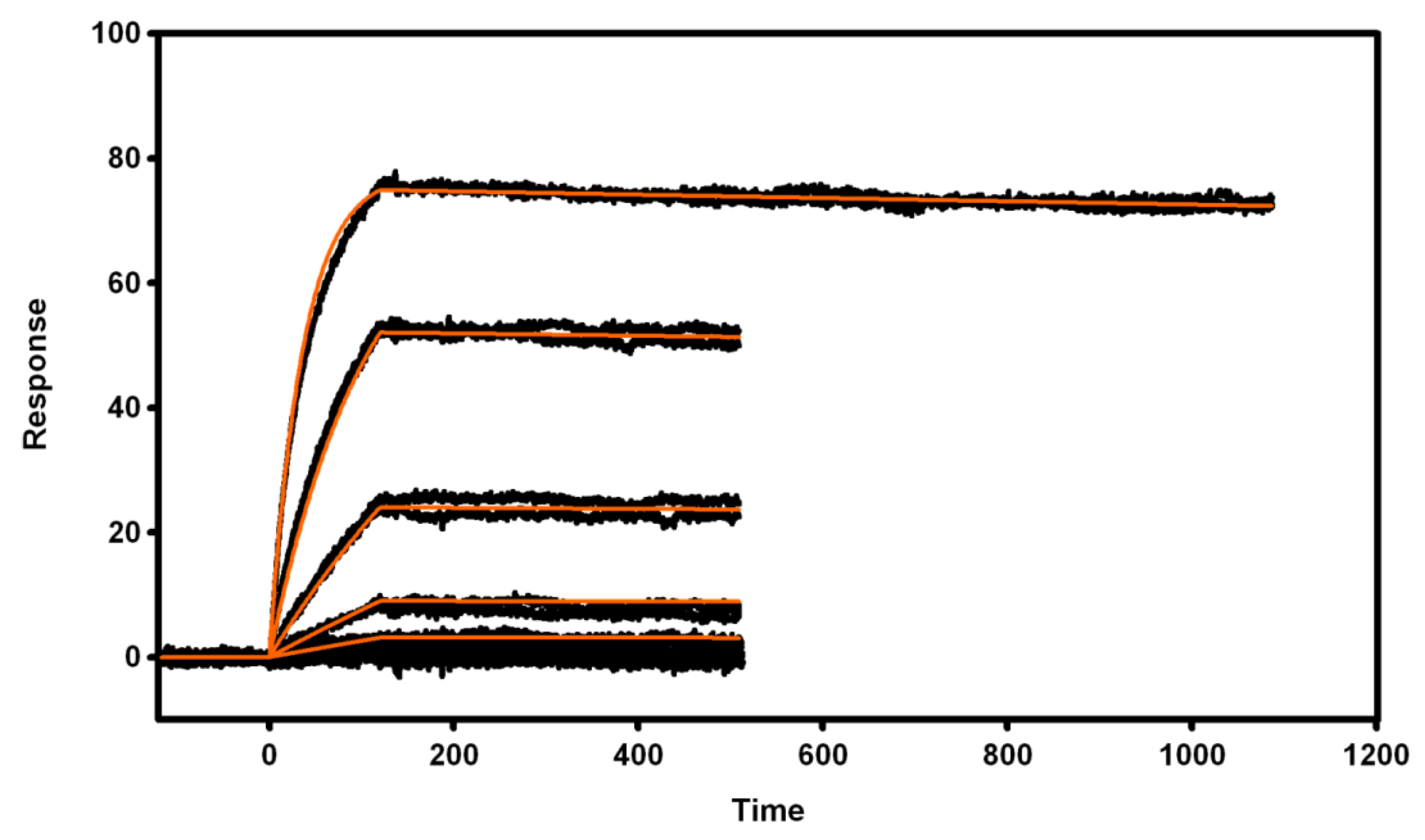
3.4. Biosensor Analysis of VNAR Proteins
3.4.1. Immobilization of Recombinant Proteins
3.4.2. Active Concentration Determinations
3.5. Nitrocellulose Dot Blot and Western Analysis
3.6. Exposure of VNAR 14M-15 to Murine stomach and Intestinal Scrapings
3.7. Circular Dichroism Spectroscopy
4. Conclusions
Acknowledgments
References and Notes
- Murray, C.K.; Gasser, R.A., Jr.; Magill, A.J.; Miller, R.S. Update on rapid diagnostic testing for malaria. Clin. Microbiol. Rev. 2008, 21, 97–110. [Google Scholar] [CrossRef]
- Roux, K.H.; Greenberg, A.S.; Greene, L.; Strelets, L.; Avila, D.; McKinney, E.C.; Flajnik, M.F. Structural analysis of the nurse shark (new) antigen receptor (nar): Molecular convergence of nar and unusual mammalian immunoglobulins. Proc. Natl. Acad. Sci. USA 1998, 95, 11804–11809. [Google Scholar]
- Streltsov, V.A.; Varghese, J.N.; Carmichael, J.A.; Irving, R.A.; Hudson, P.J.; Nuttall, S.D. Structural evidence for evolution of shark ig new antigen receptor variable domain antibodies from a cell-surface receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 12444–12449. [Google Scholar]
- Stanfield, R.L.; Dooley, H.; Flajnik, M.F.; Wilson, I.A. Crystal structure of a shark single-domain antibody V region in complex with lysozyme. Science 2004, 305, 1770–1773. [Google Scholar] [CrossRef]
- Nuttall, S.D.; Humberstone, K.S.; Krishnan, U.V.; Carmichael, J.A.; Doughty, L.; Hattarki, M.; Coley, A.M.; Casey, J.L.; Anders, R.F.; Foley, M.; et al. Selection and affinity maturation of IgNAR variable domains targeting Plasmodium falciparum AMA-1. Proteins 2004, 55, 187–197. [Google Scholar] [CrossRef]
- Kopsidas, G.; Roberts, A.S.; Coia, G.; Streltsov, V.A.; Nuttall, S.D. In vitro improvement of a shark IgNAR antibody by Qβ replicase mutation and ribosome display mimics in vivo affinity maturation. Immunol. Lett. 2006, 107, 163–168. [Google Scholar] [CrossRef]
- Simmons, D.P.; Abregu, F.A.; Krishnan, U.V.; Proll, D.F.; Streltsov, V.A.; Doughty, L.; Hattarki, M.K.; Nuttall, S.D. Dimerisation strategies for shark IgNAR single domain antibody fragments. J. Immunol. Methods 2006, 315, 171–184. [Google Scholar] [CrossRef]
- Henderson, K.A.; Streltsov, V.A.; Coley, A.M.; Dolezal, O.; Hudson, P.J.; Batchelor, A.H.; Gupta, A.; Bai, T.; Murphy, V.J.; Anders, R.F.; et al. Structure of an IgNAR-AMA1 complex: Targeting a conserved hydrophobic cleft broadens malarial strain recognition. Structure 2007, 15, 1452–1466. [Google Scholar] [CrossRef]
- Simmons, D.P.; Streltsov, V.A.; Dolezal, O.; Hudson, P.J.; Coley, A.M.; Foley, M.; Proll, D.F.; Nuttall, S.D. Shark IgNAR antibody mimotopes target a murine immunoglobulin through extended CDR3 loop structures. Proteins 2008, 71, 119–130. [Google Scholar] [CrossRef]
- Coley, A.M.; Campanale, N.V.; Casey, J.L.; Hodder, A.N.; Crewther, P.E.; Anders, R.F.; Tilley, L.M.; Foley, M. Rapid and precise epitope mapping of monoclonal antibodies against Plasmodium falciparum AMA-1 by combined phage display of fragments and random peptides. Protein Eng. 2001, 14, 691–698. [Google Scholar] [CrossRef]
- Bell, D.; Wongsrichanalai, C.; Barnwell, J.W. Ensuring quality and access for malaria diagnosis: How can it be achieved? Nat. Rev. Microbiol. 2006, 4, S7–S20. [Google Scholar]
- Chiodini, P.L.; Bowers, K.; Jorgensen, P.; Barnwell, J.W.; Grady, K.K.; Luchavez, J.; Moody, A.H.; Cenizal, A.; Bell, D. The heat stability of Plasmodium lactate dehydrogenase-based and histidine-rich protein 2-based malaria rapid diagnostic tests. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 331–337. [Google Scholar] [CrossRef]
- Ashley, E.A.; Touabi, M.; Ahrer, M.; Hutagalung, R.; Htun, K.; Luchavez, J.; Dureza, C.; Proux, S.; Leimanis, M.; Lwin, M.M.; et al. Evaluation of three parasite lactate dehydrogenase-based rapid diagnostic tests for the diagnosis of falciparum and vivax malaria. Malar. J. 2009, 8, 241. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Dudgeon, K.; Rouet, R.; Kokmeijer, I.; Schofield, P.; Stolp, J.; Langley, D.; Stock, D.; Christ, D. General strategy for the generation of human antibody variable domains with increased aggregation resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 10879–10884. [Google Scholar]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Hussack, G.; Arbabi-Ghahroudi, M.; van Faassen, H.; Songer, J.G.; Ng, K.K.; MacKenzie, R.; Tanha, J. Neutralization of Clostridium difficile toxin a with single-domain antibodies targeting the cell receptor binding domain. J. Biol. Chem. 2011, 286, 8961–8976. [Google Scholar]
- Urdea, M.; Penny, L.A.; Olmsted, S.S.; Giovanni, M.Y.; Kaspar, P.; Shepherd, A.; Wilson, P.; Dahl, C.A.; Buchsbaum, S.; Moeller, G.; et al. Requirements for high impact diagnostics in the developing world. Nature 2006, 444, 73–79. [Google Scholar]
- Romer, T.; Leonhardt, H.; Rothbauer, U. Engineering antibodies and proteins for molecular in vivo imaging. Curr. Opin. Biotechnol. 2011, 22, 882–887. [Google Scholar] [CrossRef]
- Adair, J.R.; Howard, P.W.; Hartley, J.A.; Williams, D.G.; Chester, K.A. Antibody-drug conjugates—A perfect synergy. Expert Opin. Biol. Ther. 2012, 12, 1191–1206. [Google Scholar] [CrossRef]
- Olichon, A.; Schweizer, D.; Muyldermans, S.; de Marco, A. Heating as a rapid purification method for recovering correctly-folded thermotolerant VH and VHH domains. BMC Biotechnol. 2007, 7, 7. [Google Scholar]
- Muller, R.M.; Saunders, K.; Grace, C.; Jin, M.; Piche-Nicholas, N.; Steven, J.; O'Dwyer, R.; Wu, L.; Khetemenee, L.; Vugmeyster, Y.; et al. Improving the pharmacokinetic properties of biologics by fusion to an anti-HSA shark VNAR domain. mAbs 2012, 4, 673–685. [Google Scholar] [CrossRef]
- Fennell, B.J.; Darmanin-Sheehan, A.; Hufton, S.E.; Calabro, V.; Wu, L.; Muller, M.R.; Cao, W.; Gill, D.; Cunningham, O.; Finlay, W.J. Dissection of the IgNAR V domain: Molecular scanning and orthologue database mining define novel ignar hallmarks and affinity maturation mechanisms. J. Mol. Biol. 2010, 400, 155–170. [Google Scholar] [CrossRef]
- Nuttall, S.D.; Krishnan, U.V.; Doughty, L.; Pearson, K.; Ryan, M.T.; Hoogenraad, N.J.; Hattarki, M.; Carmichael, J.A.; Irving, R.A.; Hudson, P.J. Isolation and characterization of an IgNAR variable domain specific for the human mitochondrial translocase receptor tom70. Eur. J. Biochem. 2003, 270, 3543–3554. [Google Scholar] [CrossRef]
- Hodder, A.N.; Crewther, P.E.; Anders, R.F. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 2001, 69, 3286–3294. [Google Scholar] [CrossRef]
- Coia, G.; Ayres, A.; Lilley, G.G.; Hudson, P.J.; Irving, R.A. Use of mutator cells as a means for increasing production levels of a recombinant antibody directed against Hepatitis B. Gene 1997, 201, 203–209. [Google Scholar] [CrossRef]
- Nuttall, S.D.; Krishnan, U.V.; Doughty, L.; Nathanielsz, A.; Ally, N.; Pike, R.N.; Hudson, P.J.; Kortt, A.A.; Irving, R.A. A naturally occurring NAR variable domain binds the Kgp protease from Porphyromonas gingivalis. FEBS Lett. 2002, 516, 80–86. [Google Scholar] [CrossRef]
- Nuttall, S.D.; Rousch, M.J.; Irving, R.A.; Hufton, S.E.; Hoogenboom, H.R.; Hudson, P.J. Design and expression of soluble CTLA-4 variable domain as a scaffold for the display of functional polypeptides. Proteins 1999, 36, 217–227. [Google Scholar] [CrossRef]
- Minsky, A.; Summers, R.G.; Knowles, J.R. Secretion of beta-lactamase into the periplasm of Escherichia coli: Evidence for a distinct release step associated with a conformational change. Proc. Natl. Acad. Sci. USA 1986, 83, 4180–4184. [Google Scholar] [CrossRef]
- Gupta, A.; Bai, T.; Murphy, V.; Strike, P.; Anders, R.F.; Batchelor, A.H. Refolding, purification, and crystallization of apical membrane antigen 1 from Plasmodium falciparum. Protein Expr. Purif. 2005, 41, 186–198. [Google Scholar] [CrossRef]
- Gapper, L.W.; Copestake, D.E.; Otter, D.E.; Indyk, H.E. Analysis of bovine immunoglobulin g in milk, colostrum and dietary supplements: A review. Anal. Bioanal. Chem. 2007, 389, 93–109. [Google Scholar] [CrossRef]
- Every, A.L.; Ng, G.Z.; Skene, C.D.; Harbour, S.N.; Walduck, A.K.; McGuckin, M.A.; Sutton, P. Localized suppression of inflammation at sites of helicobacter pylori colonization. Infect. Immun. 2011, 79, 4186–4192. [Google Scholar] [CrossRef]
- Chionh, Y.T.; Walduck, A.K.; Mitchell, H.M.; Sutton, P. A comparison of glycan expression and adhesion of mouse-adapted strains and clinical isolates of Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2009, 57, 25–31. [Google Scholar] [CrossRef]
- Atkinson, S.C.; Dogovski, C.; Downton, M.T.; Pearce, F.G.; Reboul, C.F.; Buckle, A.M.; Gerrard, J.A.; Dobson, R.C.; Wagner, J.; Perugini, M.A. Crystal, solution and in silico structural studies of dihydrodipicolinate synthase from the common grapevine. PLoS One 2012, 7, e38318. [Google Scholar]
Appendix
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Griffiths, K.; Dolezal, O.; Parisi, K.; Angerosa, J.; Dogovski, C.; Barraclough, M.; Sanalla, A.; Casey, J.L.; González, I.; Perugini, M.A.; et al. Shark Variable New Antigen Receptor (VNAR) Single Domain Antibody Fragments: Stability and Diagnostic Applications. Antibodies 2013, 2, 66-81. https://doi.org/10.3390/antib2010066
Griffiths K, Dolezal O, Parisi K, Angerosa J, Dogovski C, Barraclough M, Sanalla A, Casey JL, González I, Perugini MA, et al. Shark Variable New Antigen Receptor (VNAR) Single Domain Antibody Fragments: Stability and Diagnostic Applications. Antibodies. 2013; 2(1):66-81. https://doi.org/10.3390/antib2010066
Chicago/Turabian StyleGriffiths, Katherine, Olan Dolezal, Kathy Parisi, Julie Angerosa, Con Dogovski, Miles Barraclough, Abdulmonem Sanalla, Joanne L. Casey, Iveth González, Matthew A. Perugini, and et al. 2013. "Shark Variable New Antigen Receptor (VNAR) Single Domain Antibody Fragments: Stability and Diagnostic Applications" Antibodies 2, no. 1: 66-81. https://doi.org/10.3390/antib2010066



