Investigations of Laser Produced Plasmas Generated by Laser Ablation on Geomaterials. Experimental and Theoretical Aspects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Details
2.2. Characterization Methods
3. Results and Discussions
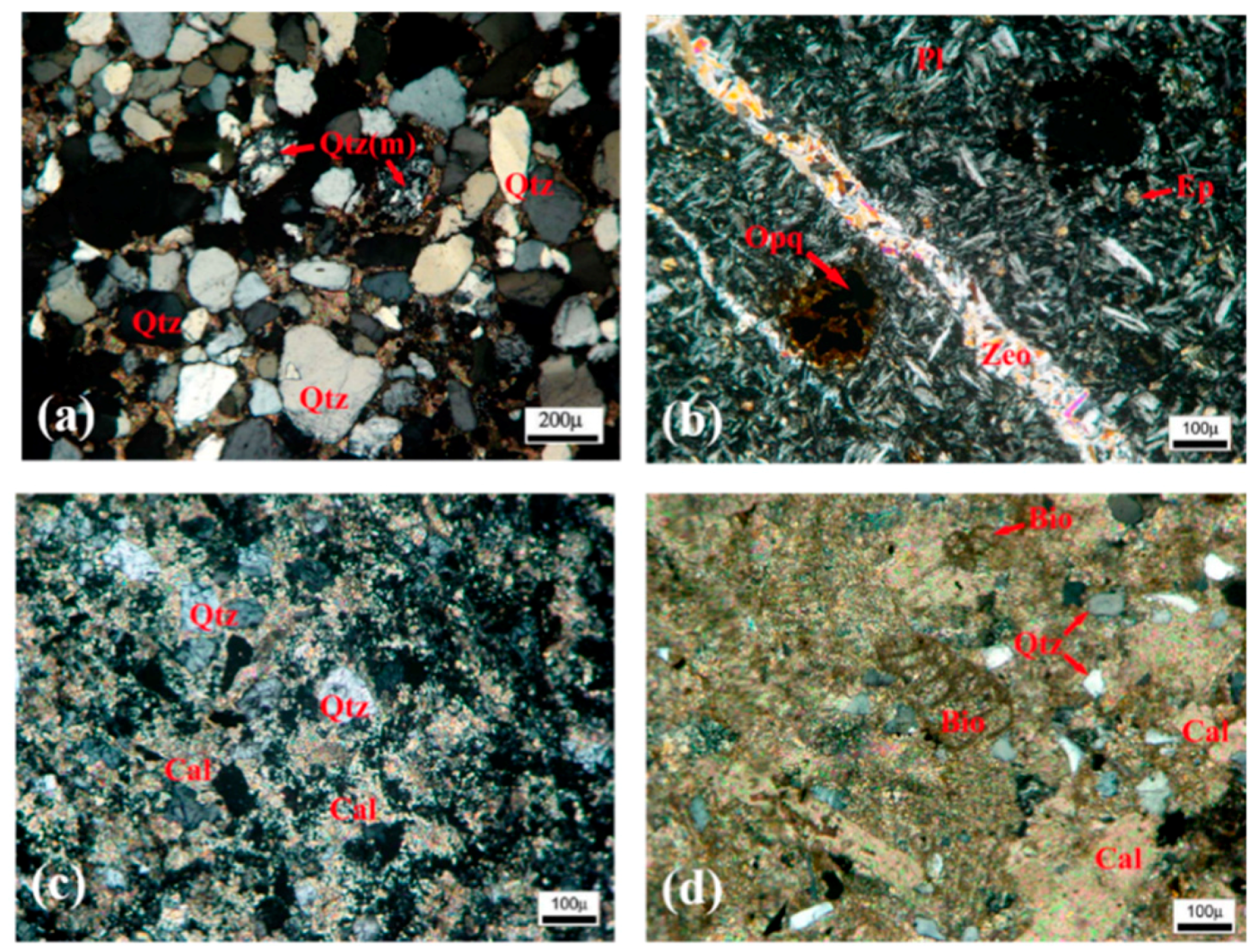
3.1. Structural Investigations
3.2. Optical Investigations of Laser Produced Plasmas
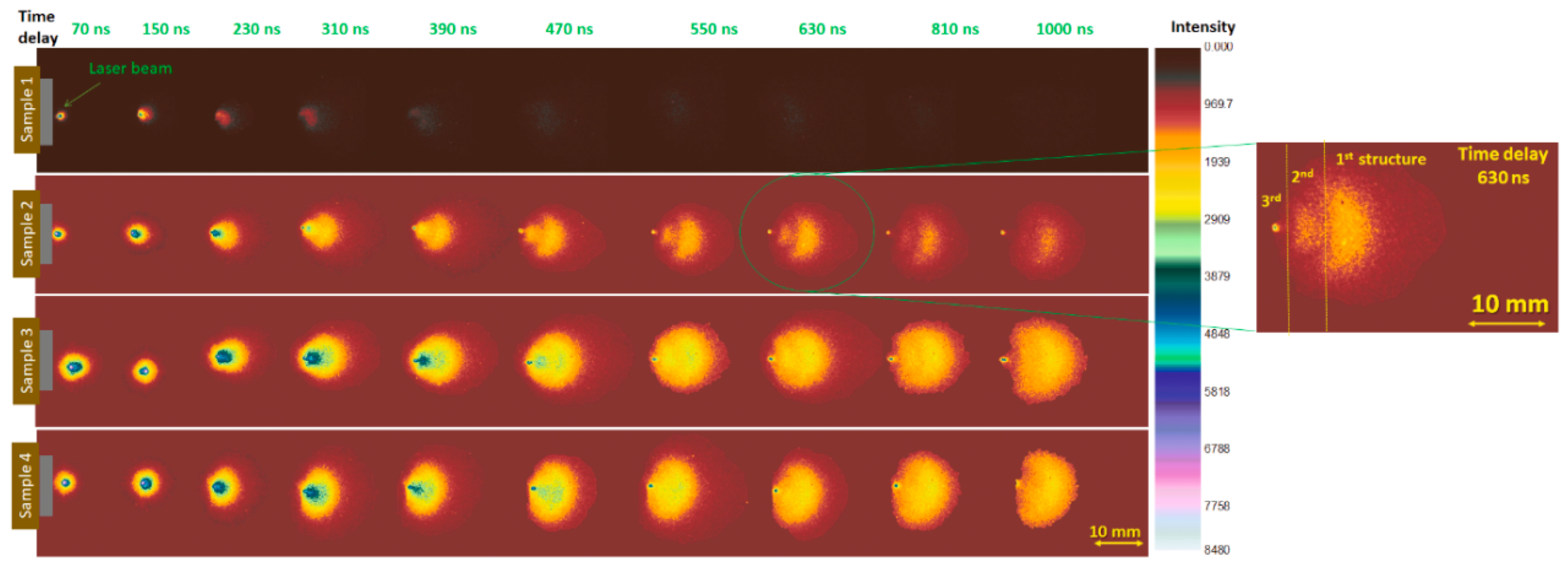
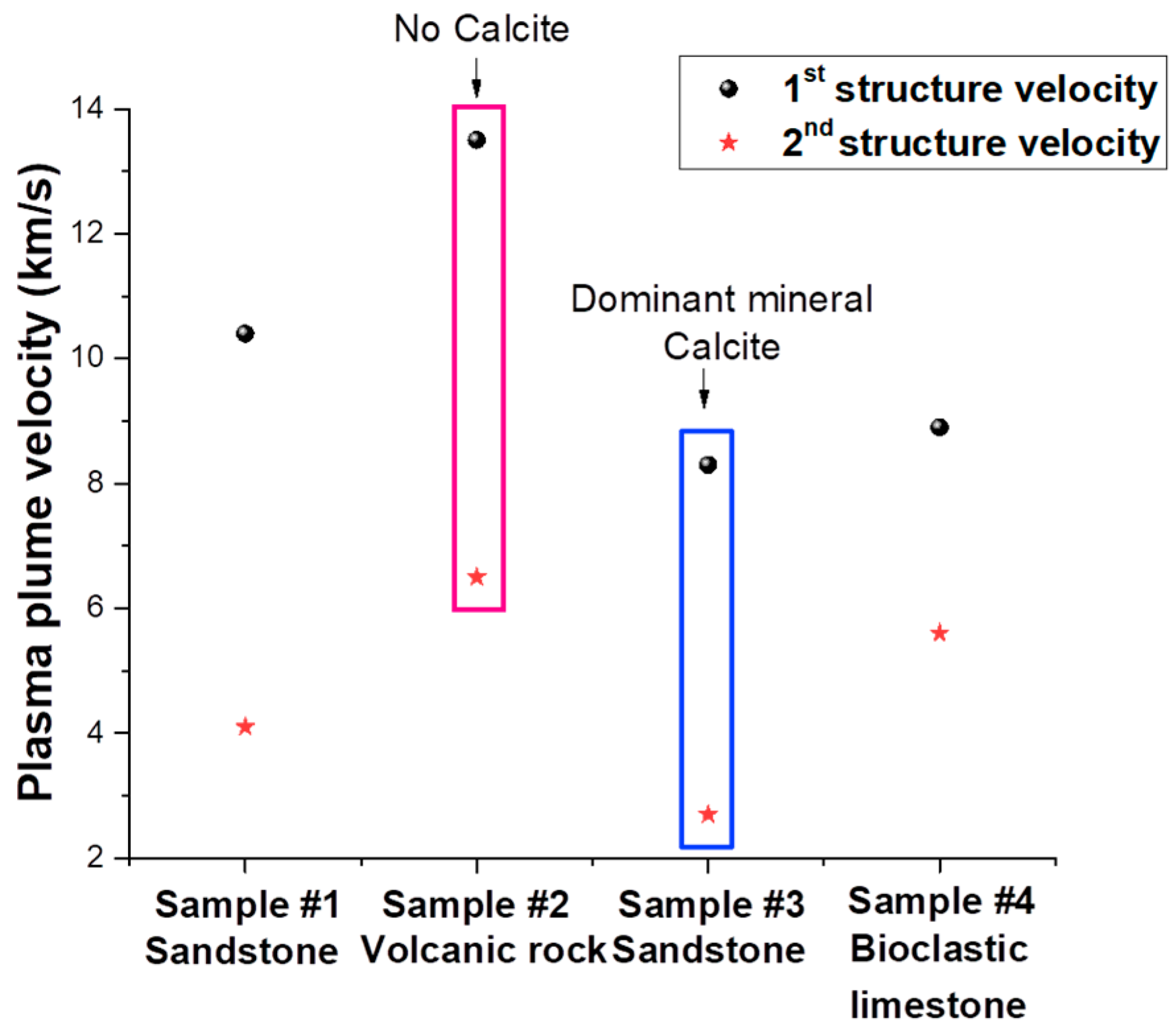
3.2.1. ICCD Fast Camera Imaging
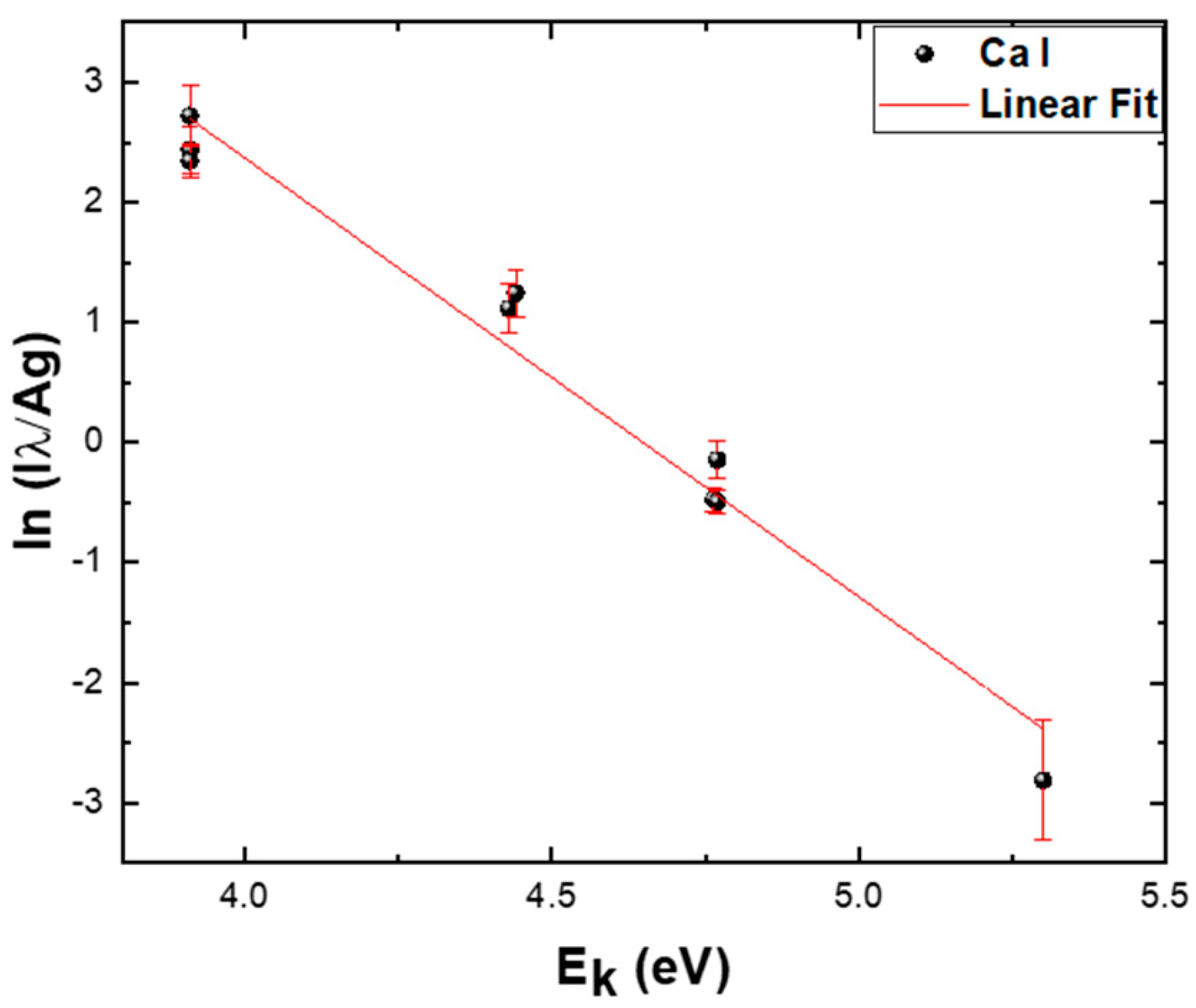
3.2.2. Optical Emission Spectroscopy
4. Mathematical Model
4.1. Fractal Analysis
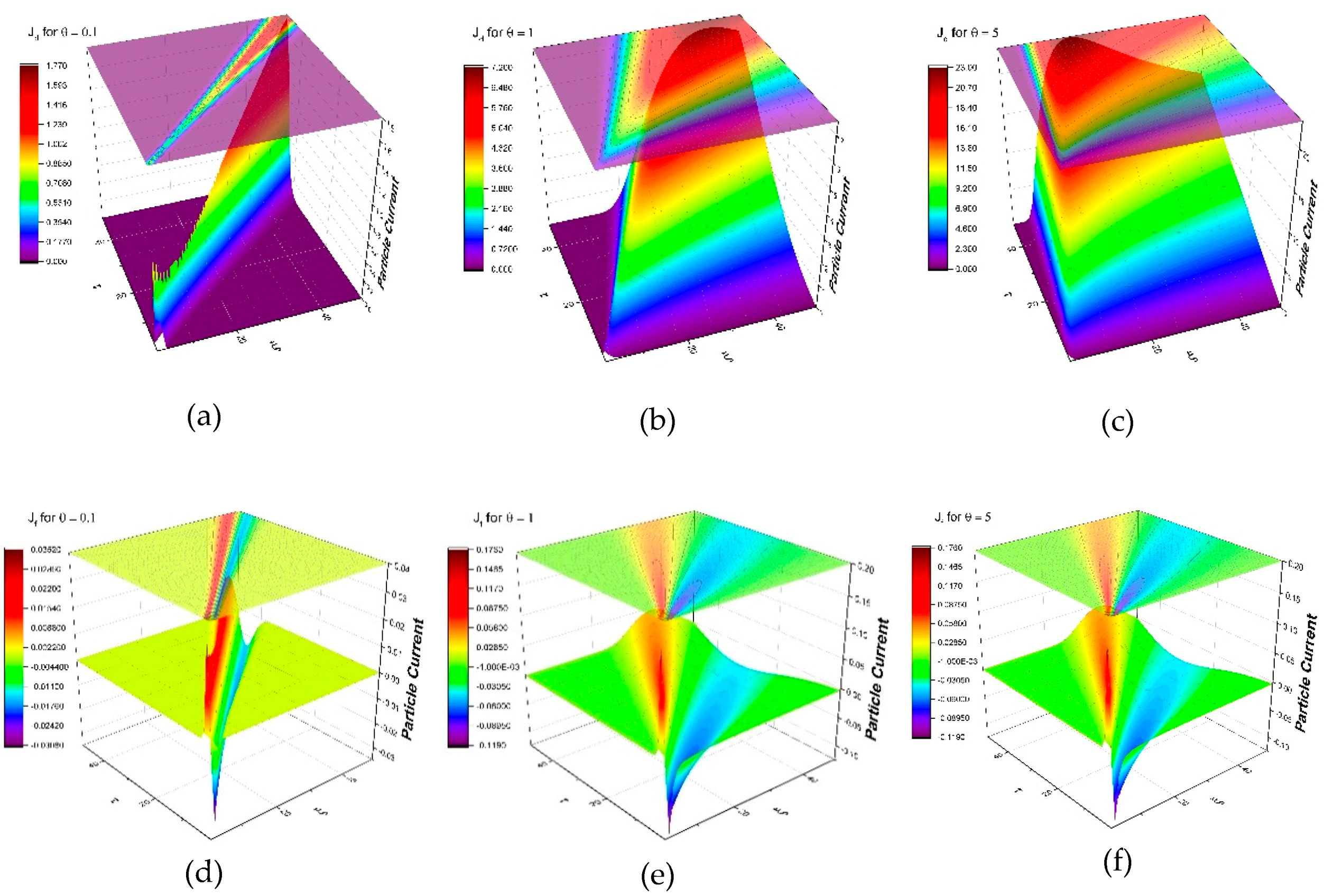
4.2. Group Invariance Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davari, S.A.; Hu, S.; Mukherjee, D. Calibration-free quantitative analysis of elemental ratios in intermetallic nanoalloys and nanocomposites using Laser Induced Breakdown Spectroscopy (LIBS). Talanta 2017, 164, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.W.; Omenetto, N. Laser-Induced Breakdown Spectroscopy (LIBS), Part I: Review of Basic Diagnostics and Plasma–Particle Interactions: Still-Challenging Issues Within the Analytical Plasma Community. Appl. Spectrosc. 2010, 64, 335–366. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, J.; Shi, Q.; He, Y.; Niu, G.; Duan, Y. Multi-elemental surface mapping and analysis of carbonaceous shale by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B-At. Spectrosc. 2016, 115, 31–39. [Google Scholar] [CrossRef]
- Xie, S.; Xu, T.; Han, X.; Lin, Q.; Duan, Y. Accuracy improvement of quantitative LIBS analysis using wavelet threshold de-noising. J. Anal. At. Spectrom. 2017, 32, 629–637. [Google Scholar] [CrossRef]
- El Haddad, J.; Canioni, L.; Bousquet, B. Good practices in LIBS analysis: Review and advices. Spectrochim. Acta-Part B At. Spectrosc. 2014, 101, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Legnaioli, S.; Lorenzetti, G.; Pardini, L.; Cavalcanti, G.H.; Palleschi, V. Applications of LIBS to the Analysis of Metals. In Laser-Induced Breakdown Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Eppler, A.S.; Cremers, D.A.; Hickmott, D.D.; Ferris, M.J.; Koskelo, A.C. Matrix effects in the detection of Pb and Ba in soils using laser-induced breakdown spectroscopy. Appl. Spectrosc. 1996, 50, 1175–1181. [Google Scholar] [CrossRef]
- Sanghapi, H.K.; Jain, J.; Bol’Shakov, A.; Lopano, C.; McIntyre, D.; Russo, R. Determination of elemental composition of shale rocks by laser induced breakdown spectroscopy. Spectrochim. Acta-Part B At. Spectrosc. 2016, 122, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Popov, A.M.; Zaytsev, S.M.; Seliverstova, I.V.; Zakuskin, A.S.; Labutin, T.A. Matrix effects on laser-induced plasma parameters for soils and ores. Spectrochim. Acta-Part B At. Spectrosc. 2018, 148, 205–210. [Google Scholar] [CrossRef]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Dyar, M.D.; Carmosino, M.L.; Breves, E.A.; Ozanne, M.V.; Clegg, S.M.; Wiens, R.C. Comparison of partial least squares and lasso regression techniques as applied to laser-induced breakdown spectroscopy of geological samples. Spectrochim. Acta-Part B At. Spectrosc. 2012, 70, 51–67. [Google Scholar] [CrossRef]
- Harmon, R.S.; Russo, R.E.; Hark, R.R. Applications of laser-induced breakdown spectroscopy for geochemical and environmental analysis: A comprehensive review. Spectrochim. Acta-Part B At. Spectrosc. 2013, 87, 11–26. [Google Scholar] [CrossRef]
- Tognoni, E.; Cristoforetti, G.; Legnaioli, S.; Palleschi, V.; Salvetti, A.; Müller, M.; Gornushkin, I. A numerical study of expected accuracy and precision in calibration-free laser-induced breakdown spectroscopy in the assumption of ideal analytical plasma. Spectrochim. Acta-Part B At. Spectrosc. 2007, 62, 1287–1302. [Google Scholar] [CrossRef]
- Bulajic, D.; Corsi, M.; Cristoforetti, G.; Legnaioli, S.; Palleschi, V.; Salvetti, A.; Tognoni, E. A procedure for correcting self-absorption in calibration free-laser induced breakdown spectroscopy. Spectrochim. Acta-Part B At. Spectrosc. 2002, 57, 339–353. [Google Scholar] [CrossRef]
- González, R.; Lucena, P.; Tobaria, L.M.; Laserna, J.J. Standoff LIBS detection of explosive residues behind a barrier. J. Anal. At. Spectrom. 2009, 24, 1123–1126. [Google Scholar] [CrossRef]
- Waltham, T. The sandstone fantasy of Petra. Geol. Today 1994, 10, 105–111. [Google Scholar] [CrossRef]
- Makhlouf, I.M.; Abed, A.M. Depositional facies and environments in the Umm Ishrin Sandstone Formation, Dead Sea area, Jordan. Sediment. Geol. 1991, 71, 177–187. [Google Scholar] [CrossRef]
- Migoń, P.; Goudie, A. Sandstone geomorphology of south-west Jordan, Middle East. Quaest. Geogr. 2014, 33, 123–130. [Google Scholar] [CrossRef]
- Amireh, B.S. Mineral composition of the Cambrian-Cretaceous Nubian Series of Jordan: Provenance, tectonic setting and climatological implications. Sediment. Geol. 1991, 71, 99–119. [Google Scholar] [CrossRef]
- Leitch, H.C.B. Contributions to the Geology of Bowen Island. Ph.D. Thesis, University of British Columbia, Kelowna, BC, Canada, 1947. [Google Scholar]
- Boyle, D.R.; Turner, R.J.W.; Hall, G.E.M. Anomalous arsenic concentrations in groundwaters of an island community, Bowen Island. British Columbia. Environ. Geochem. Health 1998, 20, 199–212. [Google Scholar] [CrossRef]
- Friedman, R.M.; Monger, J.W.H.; Tipper, H.W. Age of the Bowen Island Group, southwestern Coast Mountains, British Columbia. Can. J. Earth Sci. 1990, 27, 1456–1461. [Google Scholar] [CrossRef]
- Valero Garcés, B.L.; Aguilar, J.G. Shallow carbonate lacustrine facies models in the Permian of the aragon-bearn basin (Western Spanish-French Pyrenees). Carbonates Evaporites 1992, 7, 94–107. [Google Scholar] [CrossRef]
- Sibuet, J.C.; Srivastava, S.P.; Spakman, W. Pyrenean orogeny and plate kinematics. J. Geophys. Res. 2004, 109, B08104. [Google Scholar] [CrossRef]
- Dunham, R.J. Classification of Carbonate Rocks According to Depositional Textures. In Classification of Carbonate Rocks—A Symposium; Ham, W.E., Ed.; American Association of Petroleum Geologists: Vancuver, BC, Canada, 1962; pp. 108–121. [Google Scholar]
- Roigé, M.; Gómez-Gras, D.; Remacha, E.; Daza, R.; Boya, S. Tectonic control on sediment sources in the Jaca basin (Middle and Upper Eocene of the South-Central Pyrenees). C. R. Geosci. 2016, 348, 236–245. [Google Scholar] [CrossRef]
- Rodríguez, L.; Cuevas, J.; Tubía, J.M. Structural Evolution of the Sierras Interiores (Aragón and Tena Valleys, South Pyrenean Zone): Tectonic Implications. J. Geol. 2014, 122, 99–111. [Google Scholar] [CrossRef]
- Irimiciuc, S.; Bulai, G.; Agop, M.; Gurlui, S. Influence of laser-produced plasma parameters on the deposition process: In situ space- and time-resolved optical emission spectroscopy and fractal modeling approach. Appl. Phys. A Mater. Sci. Process. 2018, 124, 615. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Mihaila, I.; Agop, M. Experimental and theoretical aspects of a laser produced plasma. Phys. Plasmas 2014, 21, 93509. [Google Scholar] [CrossRef]
- Irimiciuc, S.; Boidin, R.; Bulai, G.; Gurlui, S.; Nemec, P.; Nazabal, V.; Focsa, C. Laser ablation of (GeSe2)100−x (Sb2Se3)x chalcogenide glasses: Influence of the target composition on the plasma plume dynamics. Appl. Surf. Sci. 2017, 418, 594–600. [Google Scholar] [CrossRef]
- Puretzky, A.A.; Geohegan, D.B.; Haufler, R.E.; Hettich, R.L.; Zheng, X.Y.; Compton, R.N. Laser ablation of graphite in different buffer gases. AIP Conf. Proc. 1993, 288, 365–374. [Google Scholar] [Green Version]
- Amoruso, S.; Armenante, M.; Bruzzese, R.; Spinelli, N.; Velotta, R.; Wang, X. Emission of prompt electrons during excimer laser ablation of aluminum targets. Appl. Phys. Lett. 1999, 75, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Amoruso, S.; Unitá, I.; Fisiche, S.; Federico, N.; Angelo, M.S.; Cintia, V.; Napoli, I.; Toftmann, B.; Schou, J. Thermalization of a UV laser ablation plume in a background gas: From a directed to a diffusionlike flow. Phys. Rev. E 2004, 69, 56403. [Google Scholar] [CrossRef]
- Harilal, S.S.; Bindhu, C.V.; Tillack, M.S.; Najmabadi, F.; Gaeris, A.C. Plume splitting and sharpening in laser-produced aluminium plasma. J. Phys. D Appl. Phys. 2002, 35, 2935–2938. [Google Scholar] [CrossRef] [Green Version]
- Harilal, S.S.; Bindhu, C.V.; Tillack, M.S.; Najmabadi, F.; Gaeris, A.C. Internal structure and expansion dynamics of laser ablation plumes into ambient gases. J. Appl. Phys. 2003, 93, 2380–2388. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Bogaerts, A. Laser ablation of Cu and plume expansion into 1 atm ambient gas. J. Appl. Phys. 2005, 97, 063305. [Google Scholar] [CrossRef]
- O’Mahony, D.; Lunney, J.; Dumont, T.; Canulescu, S.; Lippert, T.; Wokaun, A. Laser-produced plasma ion characteristics in laser ablation of lithium manganate. Appl. Surf. Sci. 2007, 254, 811–815. [Google Scholar] [CrossRef]
- Ershov-Pavlov, E.A.; Katsalap, K.Y.; Stepanov, K.L.; Stankevich, Y.A. Time-space distribution of laser-induced plasma parameters and its influence on emission spectra of the laser plumes. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 1024–1037. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J. NIST ASD Team, NIST Atomic Spectra Database Lines Form, NIST At. Spectra Database (Ver. 5.2). 2014. Available online: http://physics.nist.gov/asd (accessed on 9 October 2018).
- Rao, K.H.; Smijesh, N.; Nivas, J.J.; Philip, R. Ultrafast laser produced zinc plasma: Stark broadening of emission lines in nitrogen ambient. Phys. Plasmas 2016, 23, 43503. [Google Scholar] [CrossRef]
- Aguilera, J.A.; Aragón, C. Multi-element Saha-Boltzmann and Boltzmann plots in laser-induced plasmas. Spectrochim. Acta-Part B At. Spectrosc. 2007, 62, 378–385. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Gurlui, S.; Agop, M. Particle distribution in transient plasmas generated by ns-laser ablation on ternary metallic alloys. Appl. Phys. B 2019, 125, 190. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Bulai, G.; Gurlui, S.; Agop, M. On the separation of particle flow during pulse laser deposition of heterogeneous materials—A multi-fractal approach. Powder Technol. 2018, 339, 273–280. [Google Scholar] [CrossRef]
- Lee, Y.I.; Sawan, S.P.; Thiem, T.L.; Teng, Y.Y.; Sneddon, J. Interaction of a laser beam with metals. Part II: Space-resolved studies of laser-ablated plasma emission. Appl. Spec. 1992, 46, 436–441. [Google Scholar] [CrossRef]
- Milan, M.; Laserna, J.J. Diagnostics of silicon plasmas produced by visible nanosecond laser ablation. Spectrochim. Acta-Part B At. Spectrosc. 2001, 56, 275–288. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Gurlui, S.; Nica, P.; Focsa, C.; Agop, M. A compact non-differential approach for modeling laser ablation plasma dynamics. J. Appl. Phys. 2017, 12, 083301. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Agop, M.; Nica, P.; Gurlui, S.; Mihaileanu, D.; Toma, S.; Focsa, C. Dispersive effects in laser ablation plasmas. Jpn. J. Appl. Phys. 2014, 53, 116202. [Google Scholar] [CrossRef]
- Merches, I.; Agop, M. Differentiability and Fractality in Dynamics of Physical Systems; World Scientific: Singapore, 2015. [Google Scholar]
- Mandelbrot, B. The Fractal Geometry of Nature; WH Freeman Publisher: New York, NY, USA, 1993. [Google Scholar]
- Nottale, L. Scale Relativity and Fractal Space-Time: An Approach to Unifying Relativity and Quantum Mechanics; Imperial College Press: London, UK, 2011. [Google Scholar]
- Agop, M.; Merches, I. Operational Procedures Describing Physical Systems; CRC Press, Taylor and Francis Group: London, UK, 2019. [Google Scholar]
- Mihaileanu, M. Differential, Projective and Analytical Geometry; Didactical and Pedagogical Publishing House: Bucuresti, Romania, 1972. [Google Scholar]
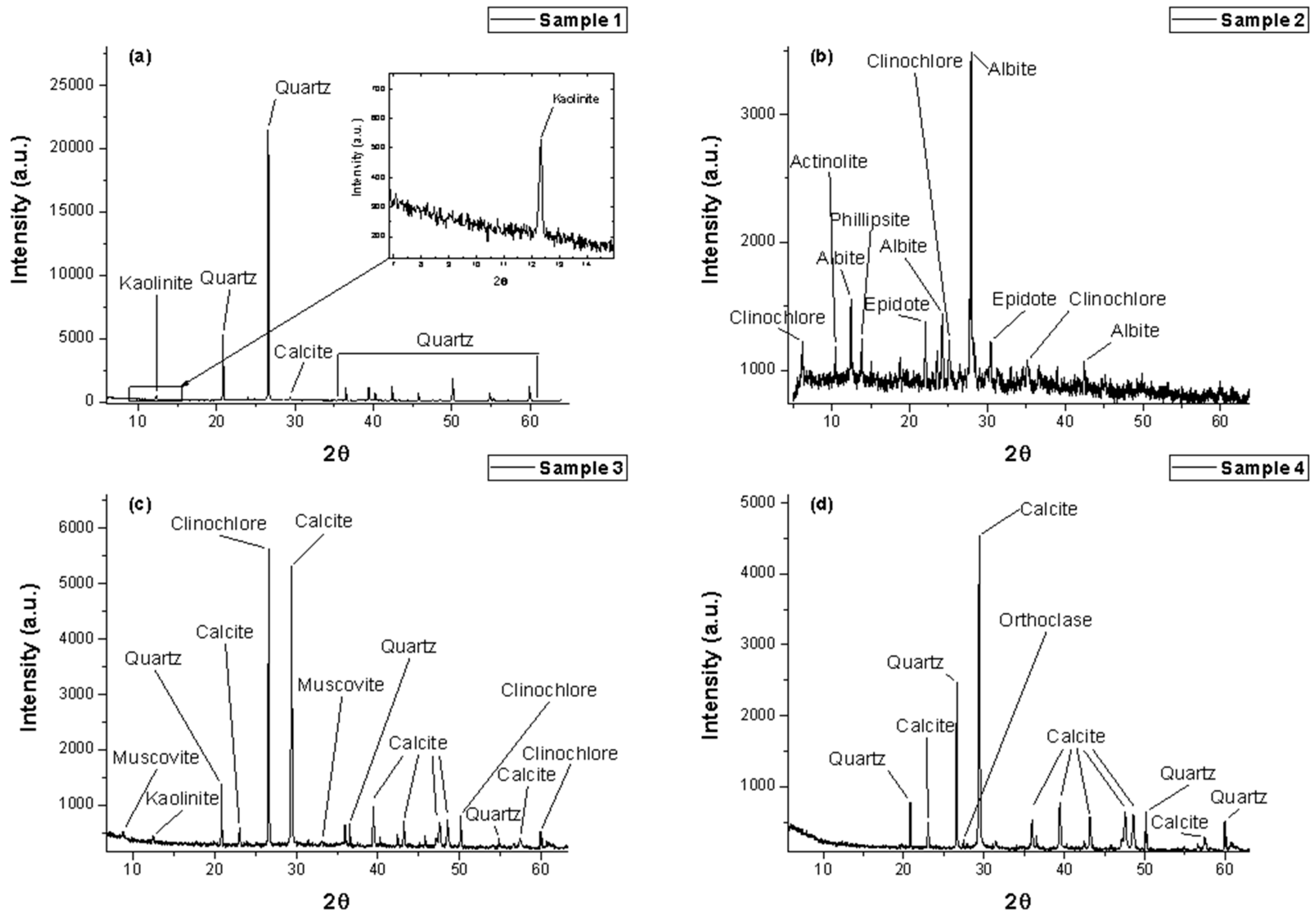



| Rocks | Confirmed Minerals from XRD Database |
|---|---|
| Sample #1 | PDF 01-070-7244 SiO2 Quartz PDF 00-058-2001 (Al2Si2)5(OH)4 Kaolinite 1A PDF 00-005-0586 CaCO3 Calcite |
| Sample #2 | PDF 00-073-2147 Ca2Fe0.33Al2.67Si3O12OH Ca Fe (Epidote) PDF 00-046-1427 (K,Na)2(Si,Al)8O16*4H2O (Philipsite) PDF 00-019-0749 Mg5Al(Si3Al)O10(OH)8 (Clinochlore) PDF 00-001-0739 NaAlSi3O8 (albite) PDF 00-080-0521 Ca2(Mg,Fe)5Si8O22(OH) (Actinolite) |
| Sample #3 | PDF 01-070-7344 SiO2 Quartz PDF 00-024-0027 CaCO3 Calcite PDF 01-078-2110 Al4(OH)8(Si4O10) Kaolinite PDF 01-070-1869 K0.77Al1.93(Al0.5Si3.5)O10(OH)2 Muscovite-2M2 PDF 00-007-0078 (Mg,Fe,Al)6(Si,Al)4O10(OH)8 Clinochlore |
| Sample #4 | PDF 01-083-1762 Ca(CO3) Calcite PDF 01-070-7344 SiO2 Quartz PDF 01-083-1324 K0.59Ba0.19Na0.33(Al0.18Si2.82O8) Orthoclase |
| Sample #1 (Petra) | Sample #2 (Bowen Island) | Sample #3 (Pico Anayet) | Sample #4 (Pico Collarada) | ||||
|---|---|---|---|---|---|---|---|
| Element | At % | Element | At % | Element | At % | Element | At % |
| Si | 19.14 ± 0.25 | Si | 21.97 ± 0.25 | Ca | 31.85 ± 0.2 | Ca | 24.62 ± 0.3 |
| Ca | 5.78 ± 0.1 | Fe | 6.50 ± 0.13 | Fe | 1.33 ± 0.1 | Si | 17.98 ± 0.2 |
| C | 1.74 ± 0.1 | Al | 9.31 ± 0.16 | C | 0.27 ± 0.05 | Al | 2.84 ± 0.1 |
| Al | 2.14 ± 0.12 | Mg | 5.03 ± 0.11 | Si | 1.00 ± 0.08 | Mg | 0.93 ± 0.08 |
| Ti | 0.23 ± 0.05 | Ba | 0.68 ± 0.1 | Al | 0.6 ± 0.05 | K | 4.39 ± 0.1 |
| Fe | 0.68 ± 0.05 | Ca | 2.04 ± 0.11 | Mn | 0.67 ± 0.06 | Fe | 0.81 ± 0.06 |
| Mg | 0.53 ± 0.05 | K | 1.63 ± 0.08 | K | 1.47 ± 0.08 | C | 0.34 ± 0.05 |
| O | 69.71 ± 1.1 | Na | 0.73 ± 0.07 | O | 62.77 ± 1.2 | O | 48.06 ± 1.0 |
| Cl | 0.58 ± 0.05 | ||||||
| P | 1.05 ± 0.07 | ||||||
| C | 1.13 ± 0.05 | ||||||
| O | 49.27 ± 1.0 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enescu, F.; Irimiciuc, S.A.; Cimpoesu, N.; Bedelean, H.; Bulai, G.; Gurlui, S.; Agop, M. Investigations of Laser Produced Plasmas Generated by Laser Ablation on Geomaterials. Experimental and Theoretical Aspects. Symmetry 2019, 11, 1391. https://doi.org/10.3390/sym11111391
Enescu F, Irimiciuc SA, Cimpoesu N, Bedelean H, Bulai G, Gurlui S, Agop M. Investigations of Laser Produced Plasmas Generated by Laser Ablation on Geomaterials. Experimental and Theoretical Aspects. Symmetry. 2019; 11(11):1391. https://doi.org/10.3390/sym11111391
Chicago/Turabian StyleEnescu, Florin, Stefan Andrei Irimiciuc, Nicanor Cimpoesu, Horea Bedelean, Georgiana Bulai, Silviu Gurlui, and Maricel Agop. 2019. "Investigations of Laser Produced Plasmas Generated by Laser Ablation on Geomaterials. Experimental and Theoretical Aspects" Symmetry 11, no. 11: 1391. https://doi.org/10.3390/sym11111391






