Mechanical Vibration for the Control of Membrane Fouling in Direct Contact Membrane Distillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Fabrication and Characterization
2.2. Module Fabrication
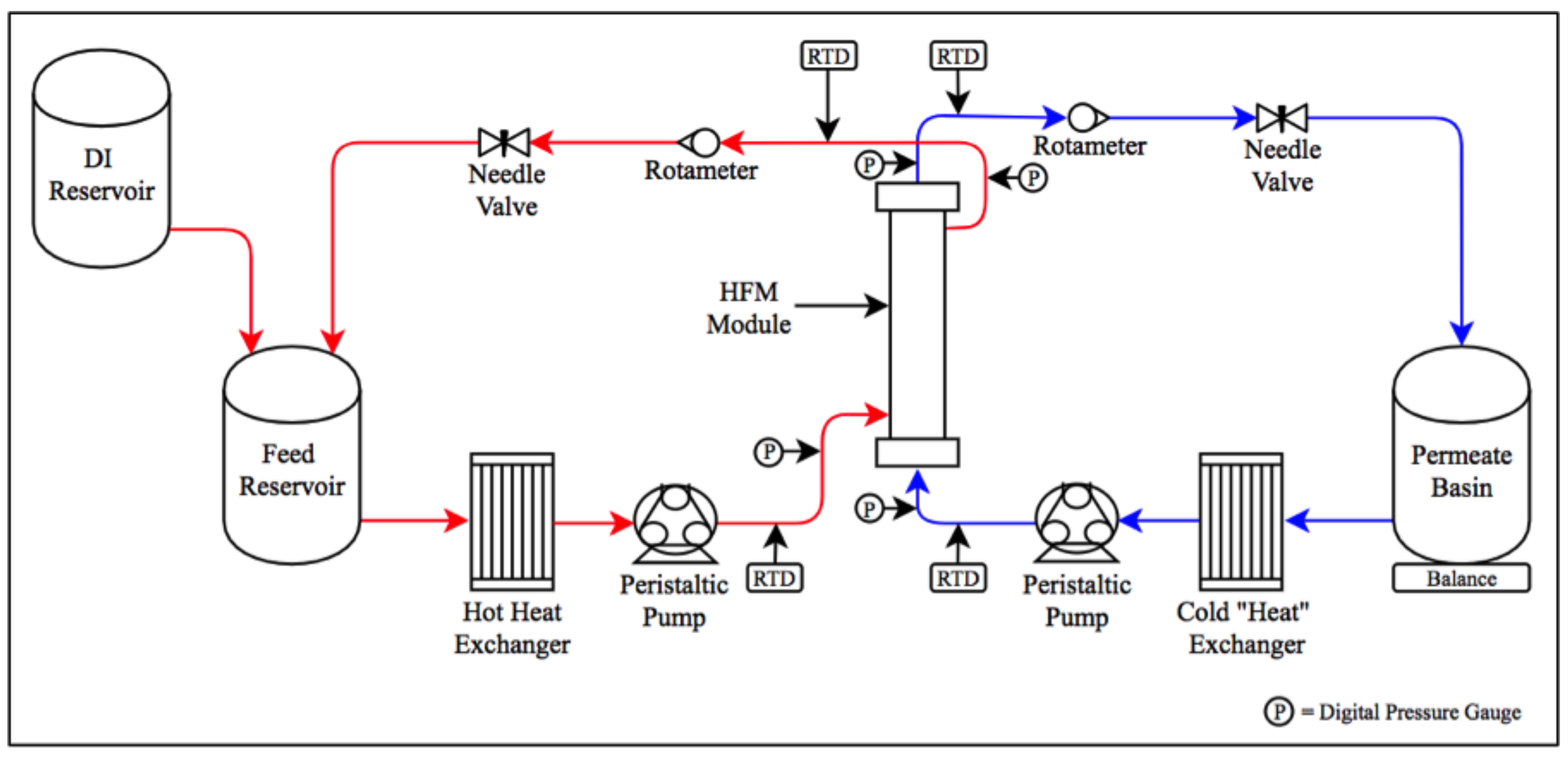
2.3. DCMD Experimental Setup
2.4. Operational Constraints
2.5. Simulated FGD Wastewater
2.6. Fouling Experiments
Vibrational Force Application
3. Results and Discussion
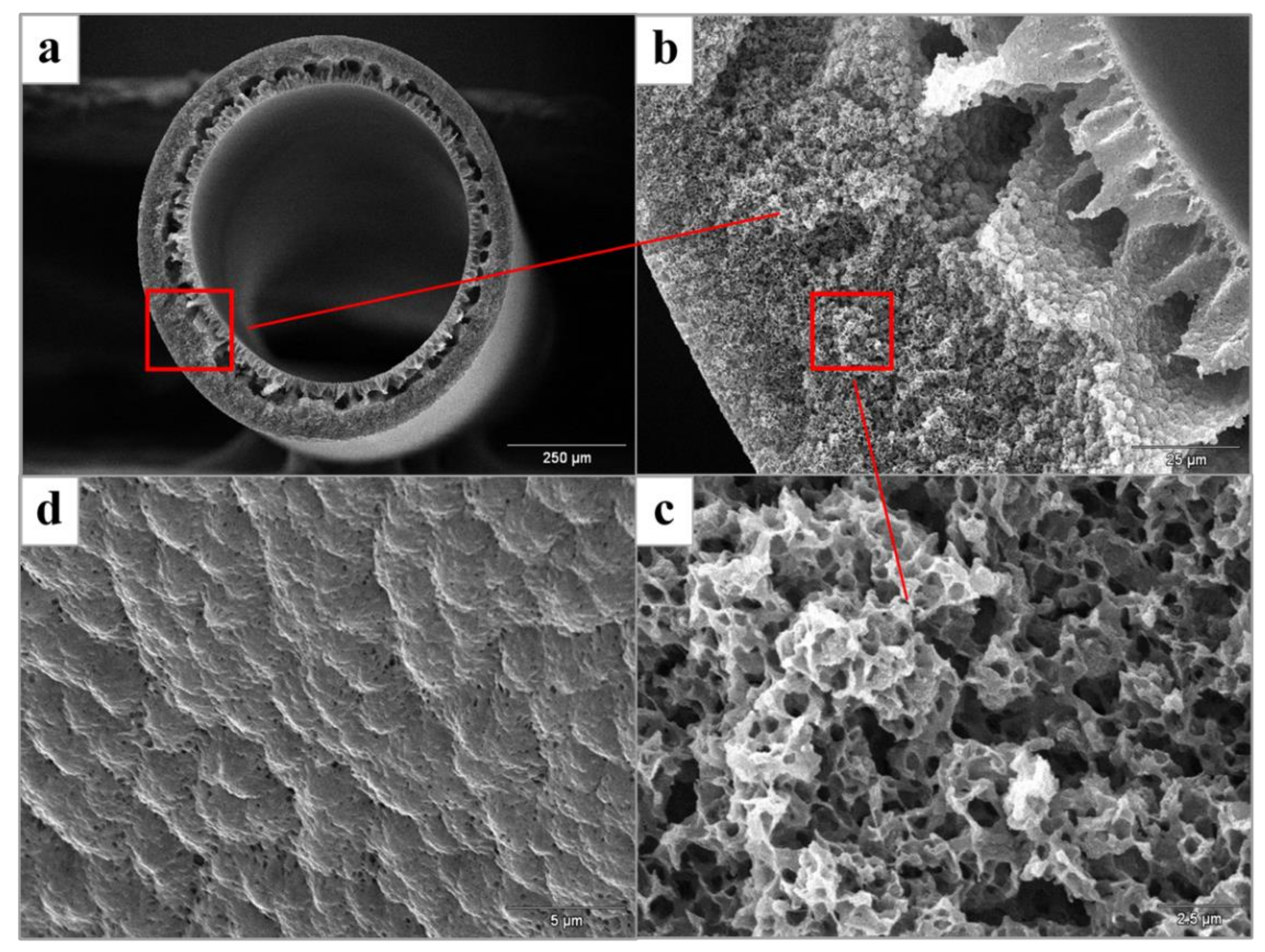
3.1. Membrane Characterization
3.2. Membrane Performance
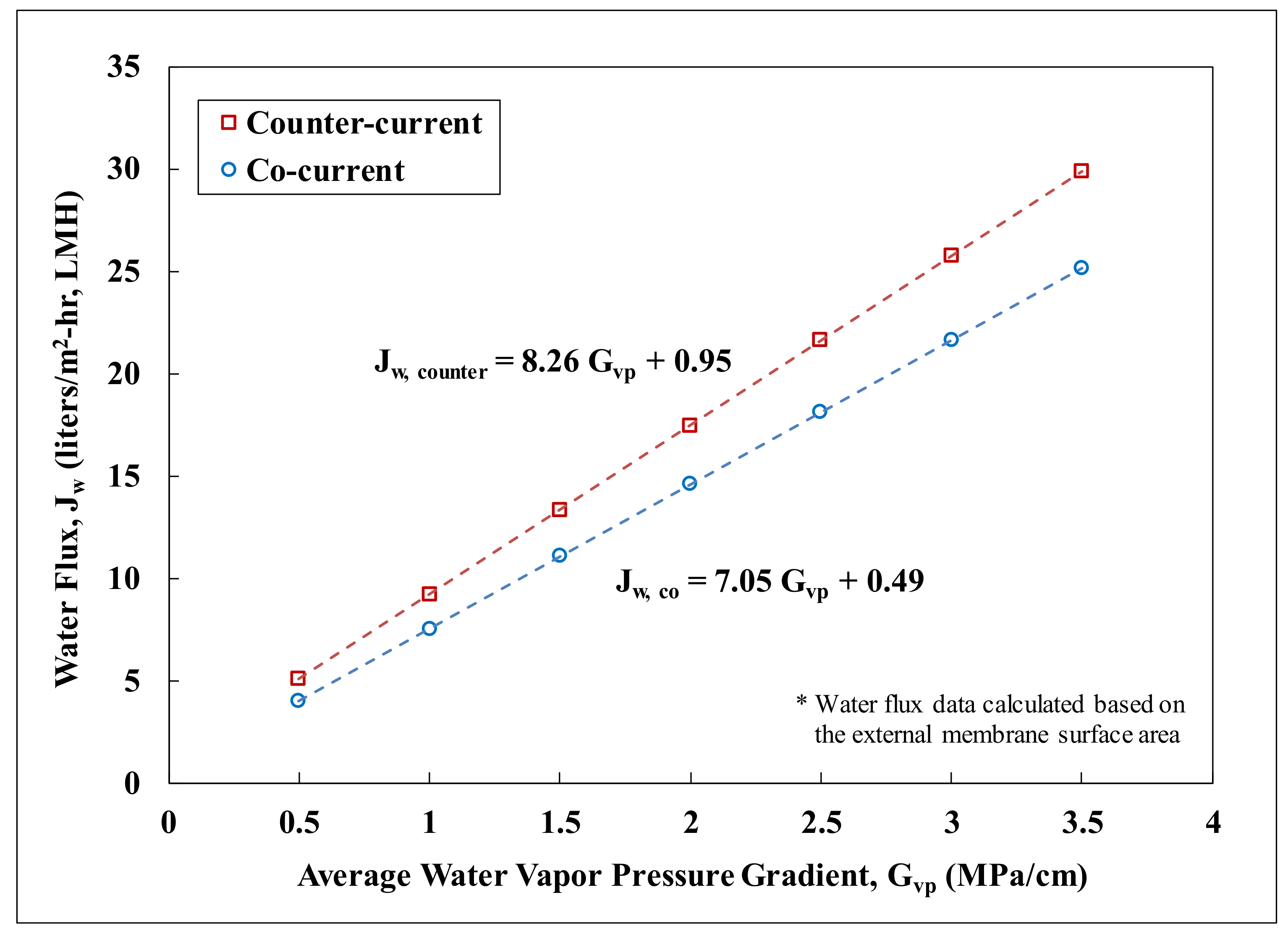
3.2.1. Effects of Flow Configurations
3.2.2. Liquid Entry Pressure (LEP)
3.2.3. Mass Transfer Limit (MTL)
3.3. Membrane Fouling
3.3.1. Precipitation Potential
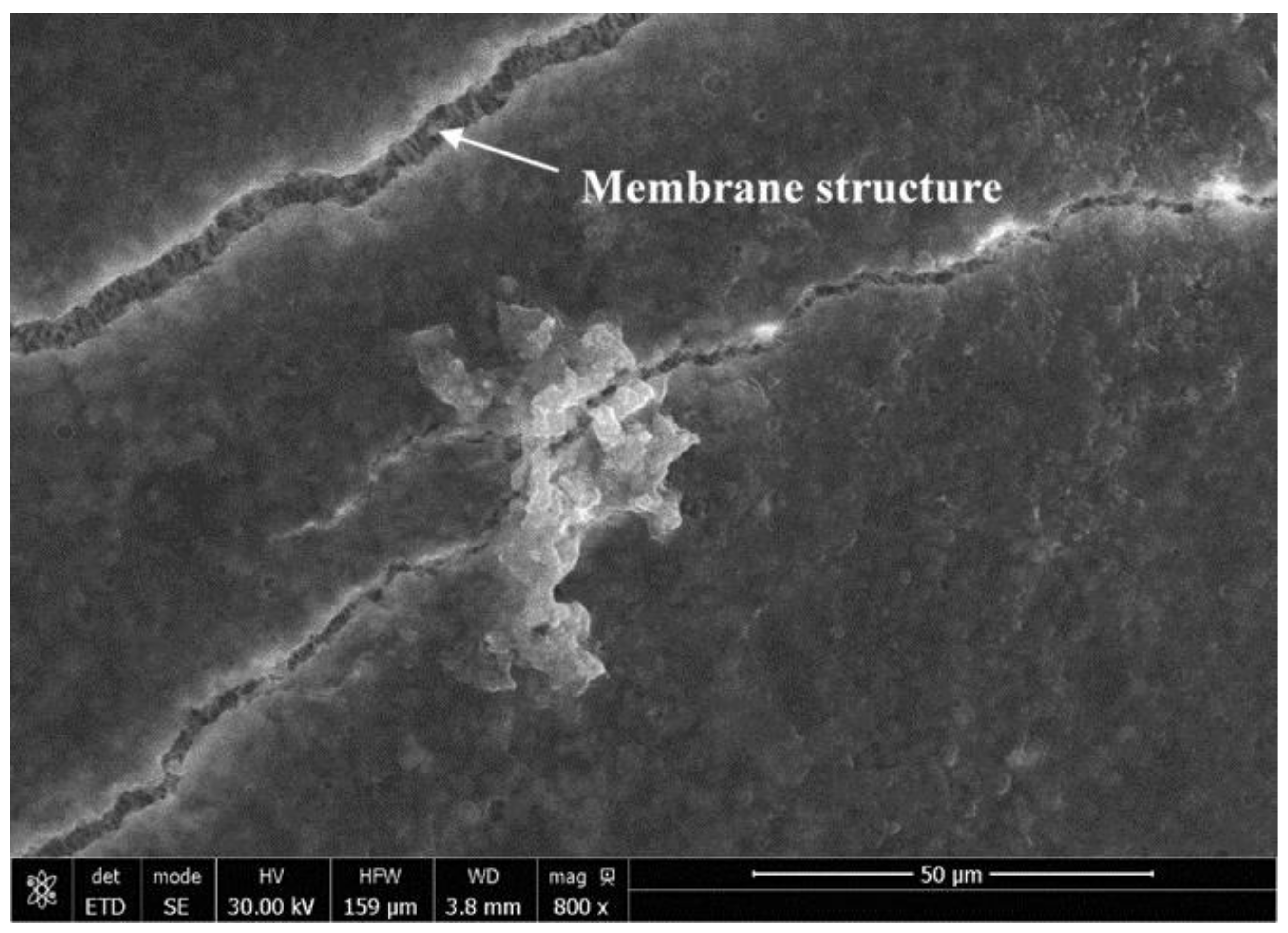
3.3.2. Effects of Mechanical Vibration on Precipitate Fouling
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gleick, P.H. Roadmap for sustainable water resources in southwestern North America. Proc. Natl. Acad. Sci. USA 2012, 107, 21300–21305. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, A.N.; Snyder, S.A. Wastewater treatment and reuse: Past, present, and future. Water 2015, 7, 4887–4895. [Google Scholar] [CrossRef]
- Diehl, T.H.; Harris, M.A.; Murphy, J.C.; Hutson, S.S.; Ladd, D.E. Methods for Estimating Water Consumption for Thermoelectric Power Plants in the United States; Scientific Investigations Report 2013–5188; U.S. Geological Survey, U.S. Department of the Interior: Washington DC, USA, 2013.
- Tow, E.W.; Warsinger, D.M.; Trueworthy, A.M.; Swaminathan, J.; Thiel, G.P.; Zubair, S.M.; Myerson, A.S.; Lienhard, J.H. Comparison of fouling propensity between reverse osmosis, forward osmosis, and membrane distillation. J. Membr. Sci. 2018, 556, 352–364. [Google Scholar] [CrossRef] [Green Version]
- Susanto, H. Towards practical implementations of membrane distillation. Chem. Eng. Process. 2011, 50, 139–150. [Google Scholar] [CrossRef]
- Simone, S.; Figoli, A.; Criscuoli, A.; Carnevale, M.C.; Rosselli, A.; Drioli, E. Preparation of hollow fibre membranes from PVDF/PVP blends and their application in VMD. J. Membr. Sci. 2010, 364, 219–232. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Huang, F.Y.C.; Laumbach, L.; Reprogle, R.; Medin, C. Geothermal Membrane Distillation in Industrial Greenhouse Applications: Membrane Fabrication and Characterization. Environ. Eng. Sci. 2018, 35, 815–828. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T. Molecular elucidation of morphology and mechanical properties of PVDF hollow fiber membranes from aspects of phase inversion, crystallization and rheology. J. Membr. Sci. 2009, 340, 192–205. [Google Scholar] [CrossRef]
- Teoh, M.M.; Chung, T.; Yeo, Y.S. Dual-layer PVDF/PTFE composite hollow fibers with a thin macrovoid-free selective layer for water production via membrane distillation. Chem. Eng. J. 2011, 171, 684–691. [Google Scholar] [CrossRef]
- Li, D.F.; Chung, T.S.; Wang, R.; Liu, Y. Fabrication of fluoropolyimide/polyethersulfone (PES) dual-layer asymmetric hollow fiber membranes for gas separation. J. Membr. Sci. 2002, 198, 211–223. [Google Scholar] [CrossRef]
- Li, D.F.; Chung, T.S.; Wang, R. Morphological aspects and structure control of dual-layer asymmetric hollow fiber membranes formed by a simultaneous co-extrusion approach. J. Membr. Sci. 2004, 243, 155–175. [Google Scholar] [CrossRef]
- Wang, P.; Teoh, M.M.; Chung, T. Morphological architecture of dual-layer hollow fiber for membrane distillation with higher desalination performance. Water Res. 2011, 45, 5489–5500. [Google Scholar] [CrossRef]
- Gryta, M.; Barancewicz, M. Influence of morphology of PVDF capillary membranes on the performance of direct contact membrane distillation. J. Membr. Sci. 2010, 358, 158–167. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane Distillation. J. Memb Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Li, J.; Guan, Y.; Cheng, F.; Liu, Y. Treatment of high salinity brines by direct contact membrane distillation: Effect of membrane characteristics and salinity. Chemosphere 2015, 140, 143–149. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.S.; Lee, S.; Kim, S.H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef] [Green Version]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard V, J.H. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef] [Green Version]
- Fard, A.K.; Rhadfi, T.; Khraisheh, M.; Atieh, M.A.; Khraisheh, M.; Hilal, N. Reducing flux decline and fouling of direct contact membrane distillation by utilizing thermal brine from MSF desalination plant. Desalination 2016, 379, 172–181. [Google Scholar] [CrossRef]
- Gryta, M. Fouling in direct contact membrane distillation process. J. Membr. Sci. 2008, 325, 383–394. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Ge, J.; Peng, Y.; Li, Z.; Chen, P.; Wang, S. Membrane fouling and wetting in a DCMD process for RO brine concentration. Desalination 2014, 344, 97–107. [Google Scholar] [CrossRef]
- Peng, Y.; Ge, J.; Li, Z.; Wang, S. Effects of anti-scaling and cleaning chemicals on membrane scale in direct contact membrane distillation process for RO brine concentrate. Sep. Purif. Technol. 2015, 154, 22–26. [Google Scholar] [CrossRef]
- Hou, D.; Wang, Z.; Li, G.; Fan, H.; Wang, J.; Huang, H. Ultrasonic assisted direct contact membrane distillation hybrid process for membrane scaling mitigation. Desalination 2015, 375, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Jaffrin, M.Y. Dynamic shear-enhanced membrane filtration: A review of rotating disks, rotating membranes and vibrating systems. J. Membr. Sci. 2008, 324, 7–25. [Google Scholar] [CrossRef]
- Li, T.; Law, A.W.K.; Cetin, M.; Fane, A.G. Fouling control of submerged hollow fibre membranes by vibrations. J. Membr. Sci. 2013, 427, 230–239. [Google Scholar] [CrossRef]
- Huang, F. Full-Scale Testing of the Global Filter™ System. Final Report for Global Filter, Inc.; Department of Civil and Environmental Engineering, New Mexico Tech: Socorro, NM, USA, 2007. [Google Scholar]
- Zhu, C.; Liu, G. Modeling of ultrasonic enhancement on membrane distillation. J. Membr. Sci. 2000, 176, 31–41. [Google Scholar] [CrossRef]
- Hsu, S.T.; Cheng, K.T.; Chiou, J.S. Seawater desalination by direct contact membrane distillation. Desalination 2002, 143, 279–287. [Google Scholar] [CrossRef]
- Basile, A.; Figoli, A.; Khayet, M. Pervaporation, Vapour Permeation, and Membrane Distillation Principles and Applications; Elsevier Ltd.: Oxford, UK, 2015; pp. 147–377. [Google Scholar]
- Wei, Q.; (Trevi Systems, Petaluma, CA, USA). Personal communication, 2018.
- Sinha, A. Vibration of Mechanical Systems; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Hibbeler, R.C. Engineering Mechanics: Statics, 11th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Su, M.; Teoh, M.M.; Wang, K.Y.; Su, J.; Chung, T.S. Effect of inner-layer thermal conductivity on flux enhancement of dual-layer hollow fiber membranes in direct contact membrane distillation. J. Membr. Sci. 2010, 364, 278–289. [Google Scholar] [CrossRef]
- Parkhust, D.L.; Appelo, C.A. User’s Guide to PHREEQC (Version 2)—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; Water-Resources Investigations Report; U.S. Geological Survey, U.S. Department of the Interior: Denver, CO, USA, 1999.
- Snoeyink, V.L.; Jenkins, D. Water Chemistry; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Brady, P.V. Surface complexation and mineral growth: Sepiolite. In Proceedings of the 7th International Symposium on Water-Rock Interaction, Park City, UT, USA, 13–18 July 1992; Kharaka, Y.K., Maest, A.S., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1992. [Google Scholar]
- Azimi, G.; Papangelakis, V.G.; Dutrizac, J.E. Modelling of calcium sulphate solubility in concentrated multi-component sulphate solutions. Fluid Phase Equilibria 2007, 260, 300–315. [Google Scholar] [CrossRef]
- Alexander, G.B.; Heston, W.M.; Iler, R.K. The solubility of amorphous silica in water. J. Phys. Chem. 1954, 58, 453–455. [Google Scholar] [CrossRef]
- Dabb, L.M. Calcium Carbonate Dissolution and Precipitation in Water: Factors Affecting the Carbonate Saturometer Method. M.S. Thesis, Utah State University, Logan, UT, USA, 1971. [Google Scholar]
- Medin, C. Prevention and Mitigation of Hollow Fiber Membrane Fouling in Direct Contact Membrane Distillation Applications. M.S. Thesis, New Mexico Institute of Mining and Technology, Socorro, NM, USA, 2018. [Google Scholar]
- Paiz, J. Module Packing Density and Performance Optimization for Hollow Fiber Direct Contact Membrane Distillation. M.S. Thesis, New Mexico Institute of Mining and Technology, Socorro, NM, USA, 2018. [Google Scholar]




| Spinning Parameters | Values |
|---|---|
| Dope solution | |
| PVDF/EG/NMP, wt% | 12/12/76 |
| Bore solution | |
| IPA/DI, wt% | 60/40 |
| Coagulation bath | |
| IPA/DI, wt% | 60/40 |
| Flow rate, ml/min | |
| dope solution | 1 |
| bore solution | 1 |
| Air-gap distance, cm (inch) | 15.24 (6) |
| Take-up velocity, m/min | 1.2 |
| Coagulation temperature, °C | 20 ± 1 |
| Parameter | FGD Wastewater | Synthetic FGD Water |
|---|---|---|
| Ca2+ | 679.7 | 679.7 |
| Mg2+ | 527 | 527 |
| K+ | 88.5 | 88.5 |
| Na+ | 6195.4 | 6195.4 |
| NH4+ | 76.8 | 76.8 |
| Fe2+ | 0.12 | na ** |
| Cu2+ | 0.05 | na ** |
| F- | 17.7 | 17.7 |
| Cl- | 9823 | 9530 |
| SO42- | 3704.3 | 3704.3 |
| NO3- | 139 | 139 |
| Si (as SiO2) | 19.85 | 19.85 |
| Alkalinity (as CaCO3) | 297.4 | 297.4 |
| pH | 8.34 | 8.34 |
| TDS | 22230 | 21140 |
| Temperature (°C) | 50-70 | 70 |
| Membrane Characteristic | PVDF |
|---|---|
| Outer diameter (µm) * | 779 ± 18 |
| Wall thickness (µm) * | 112 ± 13 |
| Pore size (µm) | 0.319/0.333/0.422 ** |
| Porosity | 0.79 ± 0.05 |
| Failure stress (MPa) | 6 |
| Young’s modulus (MPa) | 35 |
| Potential Precipitate | SI of the Feed b | Retrograde Soluble c |
|---|---|---|
| Calcite, CaCO3 | 1.77 | yes |
| Sepiolite, Mg4Si6O15(OH)2⋅6H2O | 8.44 | yes |
| Silica (amorphous), SiO2 | −1.32 | no |
| Gypsum, CaSO4 | −0.22 | yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.Y.C.; Medin, C.; Arning, A. Mechanical Vibration for the Control of Membrane Fouling in Direct Contact Membrane Distillation. Symmetry 2019, 11, 126. https://doi.org/10.3390/sym11020126
Huang FYC, Medin C, Arning A. Mechanical Vibration for the Control of Membrane Fouling in Direct Contact Membrane Distillation. Symmetry. 2019; 11(2):126. https://doi.org/10.3390/sym11020126
Chicago/Turabian StyleHuang, Frank Y.C., Carolyn Medin, and Allie Arning. 2019. "Mechanical Vibration for the Control of Membrane Fouling in Direct Contact Membrane Distillation" Symmetry 11, no. 2: 126. https://doi.org/10.3390/sym11020126




