The Role of Strontium Enriched Hydroxyapatite and Tricalcium Phosphate Biomaterials in Osteoporotic Bone Regeneration
Abstract
:1. Introduction
2. Results
2.1. Trabecular Bone Area
2.2. Immunohistochemical Analysis
2.2.1. Group A
2.2.2. Group B
2.2.3. Group C
2.2.4. Healthy Bone Compared to Operated Bone Samples
2.2.5. Comparison Between Operated Groups
2.2.6. Comparison Between Intact Leg Bone Samples of the Operated Groups
2.3. Correlation Between the Analyzed Factors
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Biomaterial Preparation
4.3. Design of the Experiment.
4.3.1. Animal Model
4.3.2. Induction of Osteoporosis
4.3.3. Biomaterial Implantation
4.3.4. Harvest of Bone Samples
4.4. Routine Morphology and Immunohistochemistry
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Metcalfe, D. The pathophysiology of osteoporotic hip fracture. Mcgill. J. Med. 2008, 11, 51–57. [Google Scholar]
- Xu, D.F.; Bi, F.G.; Ma, C.Y.; Wen, Z.F.; Cai, X.Z. A systematic review of undisplaced femoral neck fracture treatments for patients over 65 years of age, with a focus on union rates and avascular necrosis. J. Ortho Surg. Res. 2017, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Offermanns, V.; Andersen, O.Z.; Riede, G.; Sillassen, M.; Jeppesen, C.S.; Almtoft, K.P.; Talasz, H.; Öhman-Mägi, C.; Lethaus, B.; Tolba, R.; et al. Effect of strontium surface-functionalized implants on early and late osseointegration: A histological, spectrometric and tomographic evaluation. Acta Biomater. 2018, 69, 385–394. [Google Scholar] [CrossRef] [PubMed]
- El-Rashidy, A.A.; Roether, J.A.; Harjaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Herford, S.A.; Cicciu, M.; Eftimie, L.F.; Miller, M.; Signorino, F.; Fama, F.; Cervino, G.; Giudice, G.L.; Bramanti, E.; Lauritano, F.; et al. rhBMP-2 applied as support of distraction osteogenesis: A split-mouth histological study over nonhuman primates mandibles. Int. J. Clin. Exp. Med. 2016, 9, 17187–17194. [Google Scholar]
- Poli, P.P.; Beretta, M.; Cicciù, M.; Maiorana, C. Alveolar ridge augmentation with titanium mesh. A retrospective clinical study. Open Dent. J. 2014, 8, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Pao, J.I.; Chen, C.S.; Chen, Y.C.; Chang, C.C.; Hung, F.M.; Chang, C.H. Evaluation of New Biphasic Calcium Phosphate Bone Substitute: Rabbit Femur Defect Model and Preliminary Clinical Results. J. Med. Biol. Eng. 2017, 37, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Gu, J.; Song, R.; Wang, D.; Sun, Z.; Sui, C.; Zhang, C.; Liu, X.; Bian, J.; Liu, Z. Osteoprotegerin inhibit osteoclast differentiation and bone resorption by enhancing autophagy via AMPK/mTOR/p70S6K signaling pathway in vitro. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wan, P.; Ge, Y.; Fan, X.; Tan, L.; Li, J.; Yang, K. Tailoring the degradation and biological response of a magnesium-strontium alloy for potential bone substitute application. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Neves, N.; Linhares, D.; Costa, G.; Ribeiro, C.C.; Barbosa, M.A. In vivo and clinical application of strontium-enriched biomaterials for bone regeneration: A systematic review. Bone Joint Res. 2017, 6, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioactive Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.X.; Xu, C.; Wang, F.Q.; Feng, Y.F.; Zhao, X.; Yan, Y.B.; Lei, W. Temporal changes of microarchitectural and mechanical parameters of cancellous bone in the osteoporotic rabbit. Biomed. Res. Int. 2015, 263434. [Google Scholar] [CrossRef] [PubMed]
- Rathore, B.; Singh, M.; Kumar, V.; Misra, A. Osteocalcin: An emerging biomarker for bone turnover. Int. J. Res. Med. Sci. 2016, 4, 3670–3674. [Google Scholar] [CrossRef]
- Abu-Amer, Y. NF-κB signaling and bone resorption. Osteop. Int. 2013, 24, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzidis, D.; Gilmore, T.D. Transcription factor cross-talk: The estrogen receptor and NF-kappaB. Trends Endocrinol. Metab. 2005, 16, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.K.; Gautieri, A.; Chang, S.W.; Buehler, M.J. Molecular mechanics of mineralized collagen fibrils in bone. Nat. Commun. 2013, 1724. [Google Scholar] [CrossRef] [PubMed]
- Poon, B.; Kha, T.; Tran, S.; Dass, C.R. Bone morphogenetic protein-2 and bone therapy: Successes and pitfalls. J. Pharm. Pharmacol. 2016, 68, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Paiva, K.B.S.; Granjeiro, J.M. Bone tissue remodeling and development: Focus on matrix metalloproteinase functions. Arch. Biochem. Biophys. 2014, 1, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.P.H.; Xu, J.; Xue, M.; Jackson, C.J. Matrix metalloproteinases in bone development and pathology: Current knowledge and potential clinical utility. Metall. Med. 2016, 3, 93–102. [Google Scholar] [CrossRef]
- Paiva, K.B.S.; Granjeiro, J.M. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Prog. Mol. Biol. Transl. Sci. 2017, 148, 203–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Fujikado, N.; Manaka, H.; Yasuda, H.; Iwakura, Y. IL-1 plays an important role in the bone metabolism under physiological conditions. Int. Immunol. 2010, 22, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, B.; Yan, F.; Guo, J.; Zhu, X.; Ma, S.; Yang, W. Interleukin-10 inhibits bone resorption: A potential therapeutic strategy in periodontitis and other bone loss diseases. BioMed Res. Int. 2014, 284836. [Google Scholar] [CrossRef] [PubMed]
- Zarins, J.; Pilmane, M.; Sidhoma, E.; Salma, I.; Locs, J. Immunohistochemical evaluation after Sr-enriched biphasic ceramic implantation in rabbits femoral neck: Comparison of seven different bone conditions. J. Mater. Sci. Mater. Med. 2018, 29, 119. [Google Scholar] [CrossRef] [PubMed]
- Zarins, J.; Pilmane, M.; Sidhoma, E.; Salma, I.; Locs, J. Local and Systemic Morphofunctional Response of Osteoporotic Rabbits Bone Defect Following Implantation of Strontium Doped Biphasic Ceramic Granules. Solid State Phenomena 2017, 267, 124–131. [Google Scholar] [CrossRef]
- Baofeng, L.; Zhi, Y.; Bei, C.; Guolin, M.; Qingshui, Y.; Jian, L. Characterization of a rabbit osteoporosis model induced by ovariectomy and glucocorticoid. Acta Orthop. 2010, 81, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthopedic Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Calciolari, E.; Donos, N.; Mardas, N. Osteoporotic animal models of bone healing: Advantages and pitfalls. J. Investig. Surg. 2017, 30, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices: Comparative Bone Structure and Common Model Uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Neyt, J.; Buckwalter, J.A.; Carroll, N. Use of animal models in musculoskeletal research. Iowa Orthop. J. 1998, 18, 118e23. [Google Scholar]
- Gilsanz, V.; Roe, T.F.; Gibbens, D.T.; Schulz, E.E.; Carlson, M.E.; Gonzalez, O.; Boechat, M.I. Effect of sex steroids on peak bone density of growing rabbits. Am. J. Physiol. 1988, 255, E416–E421. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mabrey, J.D.; Agrawal, C.M. An interspecies comparison of bone fracture properties. Bio-Med Mater. Eng. 1998, 8, 1–9. [Google Scholar]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal models for bone tissue engineering and modelling disease. Dis. Models Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Bashoura, A.G.; Borden, T.; Baggett, L.S.; Jansen, J.A.; Wong, M.; Mikos, A.G. Development and characterization of a rabbit alveolar bone nonhealing defect model. J. Biomed. Mater. Res. A 2008, 86, 182e94. [Google Scholar] [CrossRef] [PubMed]
- Dagang, G.; Kewei, X.; Yong, H. The influence of Sr doses on the in vitro biocompatibility and in vivo degradability of single-phase Sr-incorporated HAP cement. J. Biomed. Mater. Res. A 2008, 86, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Zhang, X.; Li, L.; Wang, Q.; Yu, X.; Feng, T. Acceleration of segmental bone regeneration in a rabbit model by strontium-doped calcium polyphosphate scaffold through stimulating VEGF and bFGF secretion from osteoblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.G.; Shenoy, S.J.; Babu, S.S.; Varma, H.K.; John, A. Strontium calcium phosphate for the repair of leporine (Oryctolagus cuniculus) ulna segmental defect. J. Biomed. Mater. Res. A 2013, 101, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Chen, F.; Song, W.; Song, Y.; Chen, Y.; Wan, C.; Yu, X.; Zhang, X. In vivo study of porous strontium-doped calcium polyphosphate scaffolds for bone substitute applications. J. Mater. Sci. Mater. Med. 2009, 20, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Xie, X.; Tan, Z.; Yang, J.; Shen, B.; Zhou, Z.; Pei, F. Repairing defect and preventing collapse of femoral head in a steroid-induced osteonecrotic of femoral head animal model using strontium-doped calcium polyphosphate combined BM-MNCs. J. Mater. Sci. Mater. Med. 2015, 26, 80. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. 3D printed tricalcium phosphate bone tissue engineering scaffolds: Effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model. Biomater. Sci. 2013, 1, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Q.; Ye, Q.; Wan, C.; Li, L. Application of K/Sr co-doped calcium polyphosphate bioceramic as scaffolds for bone substitutes. J. Mater. Sci. Mater. Med. 2012, 23, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, L.; Chang, J.; Miron, R.J.; Shi, B.; Yi, S.; Wu, C. Strontium-incorporated mesoporous bioactive glass scaffolds stimulating in vitro proliferation and differentiation of bone marrow stromal cells and in vivo regeneration of osteoporotic bone defects. J. Mater. Chem. B Mater. Biol. Med. 2013, 1, 5711–5722. [Google Scholar] [CrossRef]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Joint Res. 2018, 7, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for Bone Regenerative Engineering. Adv. Healthcare Mater. 2015, 4, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Cardemil, C.; Elgali, I.; Xia, W.; Emanuelsson, L.; Norlindh, B.; Omar, O.; Thomsen, P. Strontium-doped calcium phosphate and hydroxyapatite granules promote different inflammatory and bone remodelling responses in normal and ovariectomised rats. PLoS ONE 2013, 8, e84932. [Google Scholar] [CrossRef] [PubMed]
- Dondossola, E.; Holzapfel, B.M.; Alexander, S.; Filippini, S.; Hutmacher, D.W.; Friedl, P. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat. Biomed. Eng. 2016, 1, 0007. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2007, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Dard, M.; Kokkinopoulou, M.; Markl, J.; Booms, P.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Small-sized granules of biphasic bone substitutes support fast implant bed vascularization. Biomatter 2015, 5, e1056943. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, Y.; Ninomiya, S.; Kim, Y.; Sekikawa, M. Tissue response to porous hydroxyapatite ceramic in the human femoral head. J. Orthop. Sci. 2003, 8, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.; Staudt, P.; Klein, R.; Sommer, U.; Wenz, R.; Grafe, I.; Meeder, P.J.; Nawroth, P.P.; Kasper, C. Strontium enhances osseointegration of calcium phosphate cement: A histomorphometric pilot study in ovariectomized rats. J. Orthop. Surg. Res. 2013, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants-A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Jebahi, S.; Oudadesse, H.; El Feki, H.; Rebai, T.; Keskes, H.; Pellen-Mussi, P.; El Feki, A. Antioxidative/oxidative effects of strontium-doped bioactive glass as bone graft. In vivo assays in ovariectomised rats. J. Appl. Biomed. 2012, 10, 195–209. [Google Scholar] [CrossRef]
- Thormann, U.; Ray, S.; Sommer, U.; Elkhassawna, T.; Rehling, T.; Hundgeburth, M.; Henß, A.; Rohnke, M.; Janek, J.; Lips, K.S.; et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials 2013, 34, 8589–8598. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Dernell, W.S.; Bandyopadhyay, A.; Bose, S. SrO- and MgO-doped microwave sintered 3D printed tricalcium phosphate scaffolds: Mechanical properties and in vivo osteogenesis in a rabbit model. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Stipniece, L.; Salma-Ancane, K.; Loca, D.; Pastare, S. Synthesis of strontium substituted hydroxyapatite through different precipitation routes. Key Eng. Mater. 2016, 674, 3–8. [Google Scholar] [CrossRef]
- Grybauskas, S.; Locs, J.; Salma, I.; Salms, G.; Berzina-Cimdina, L. Volumetric analysis of implanted biphasic calcium phosphate/collagen composite by three-dimensional cone beam computed tomography head model superimposition. J. Cranio Maxill. Surg. 2015, 43, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, J.A. Histological and Histochemical Methods: Theory and Practice; Scion: Bloxham, UK, 2008. [Google Scholar]
- Hsu, S.M.; Raine, L.; Fanger, H. Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAS) procedures. J. Histochem. Cytochem. 1981, 29, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Pilmane, M.; Rumba, I.; Sundler, F.; Luts, A. Patterns of distribution and occurrence of neuroendocrine elements in lungs of humans with chronic lung disease. Proc. Latvian Acad. Sci. Sect. 1998, 52, 144–152. [Google Scholar]
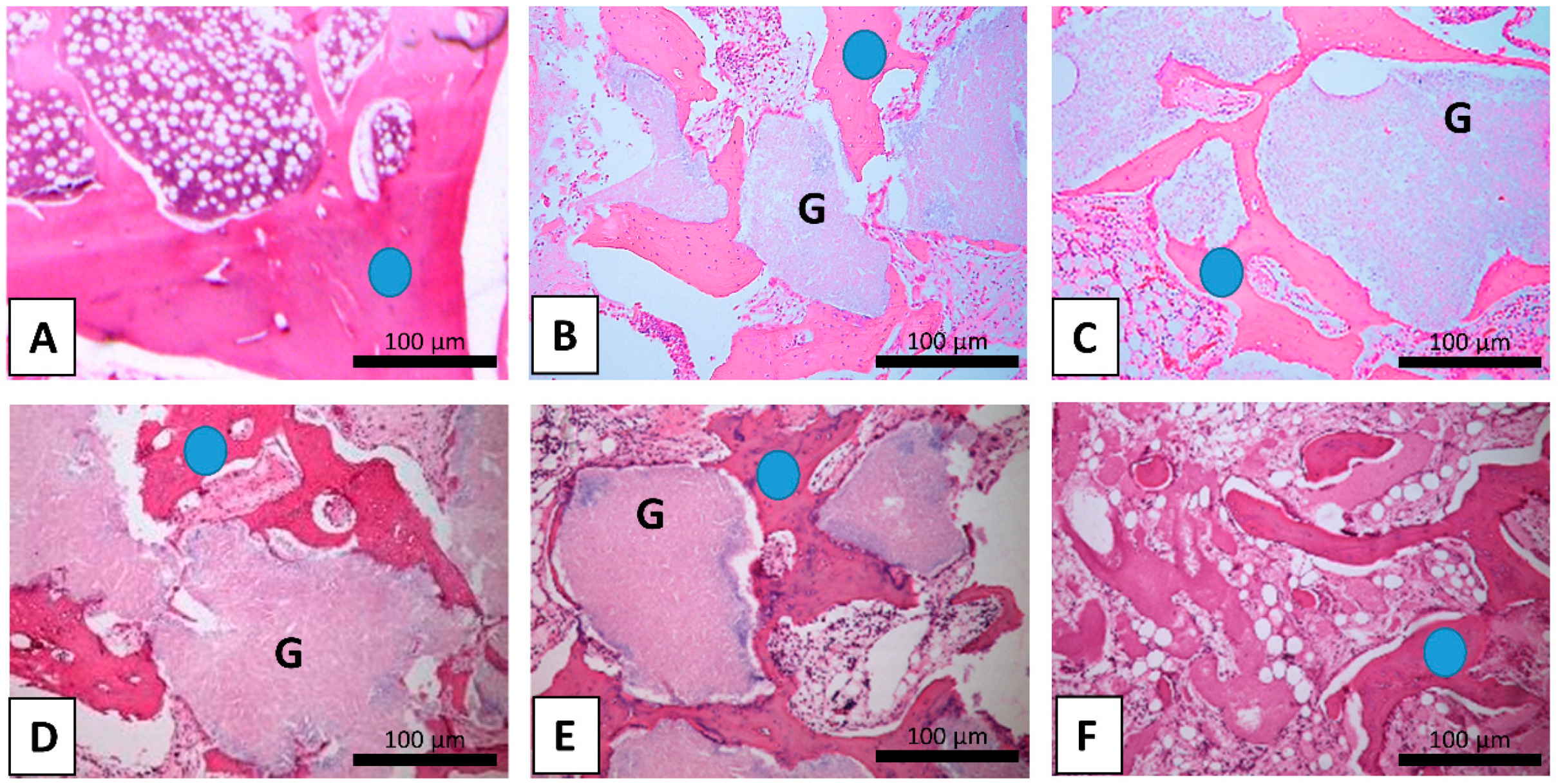
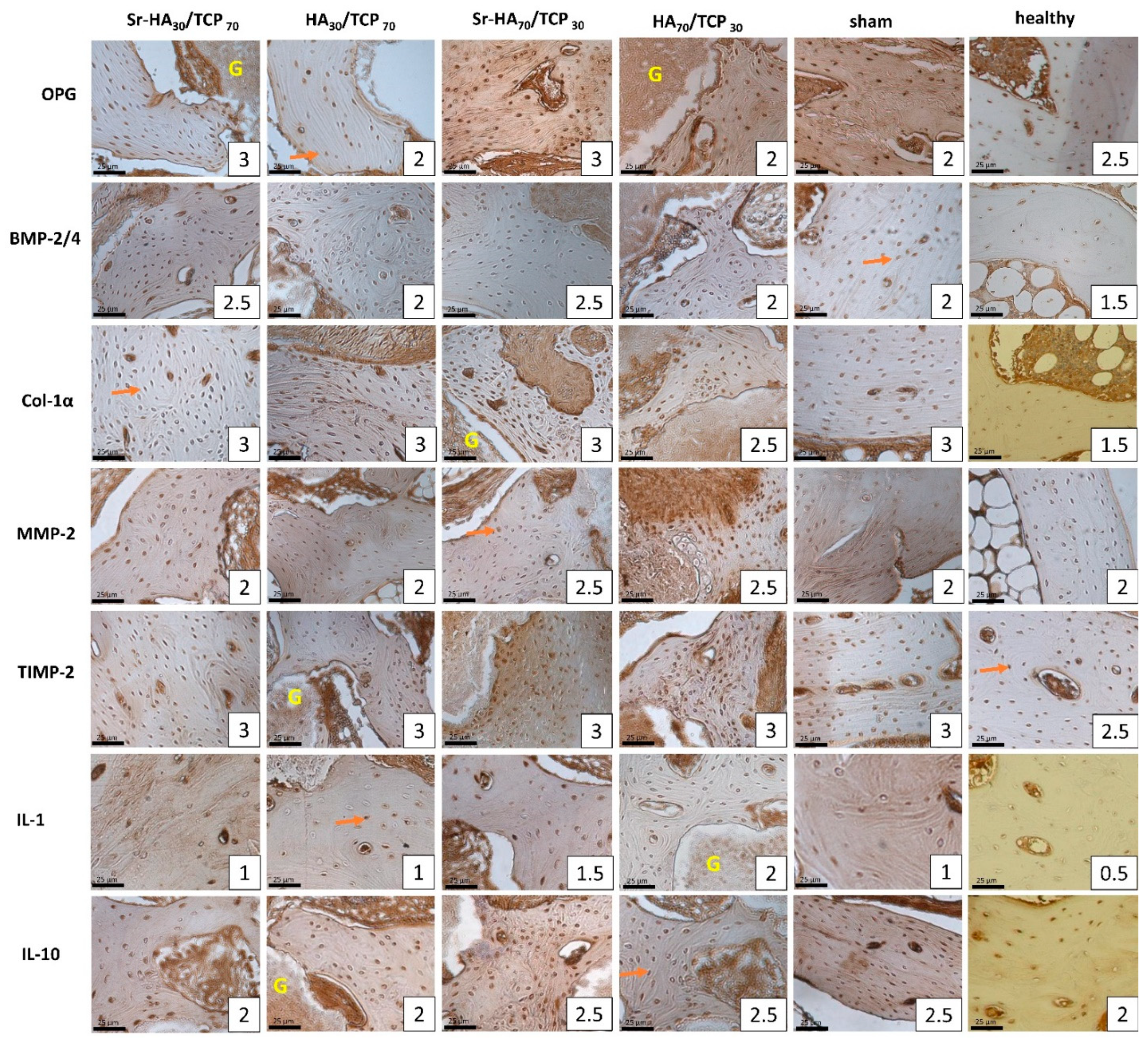
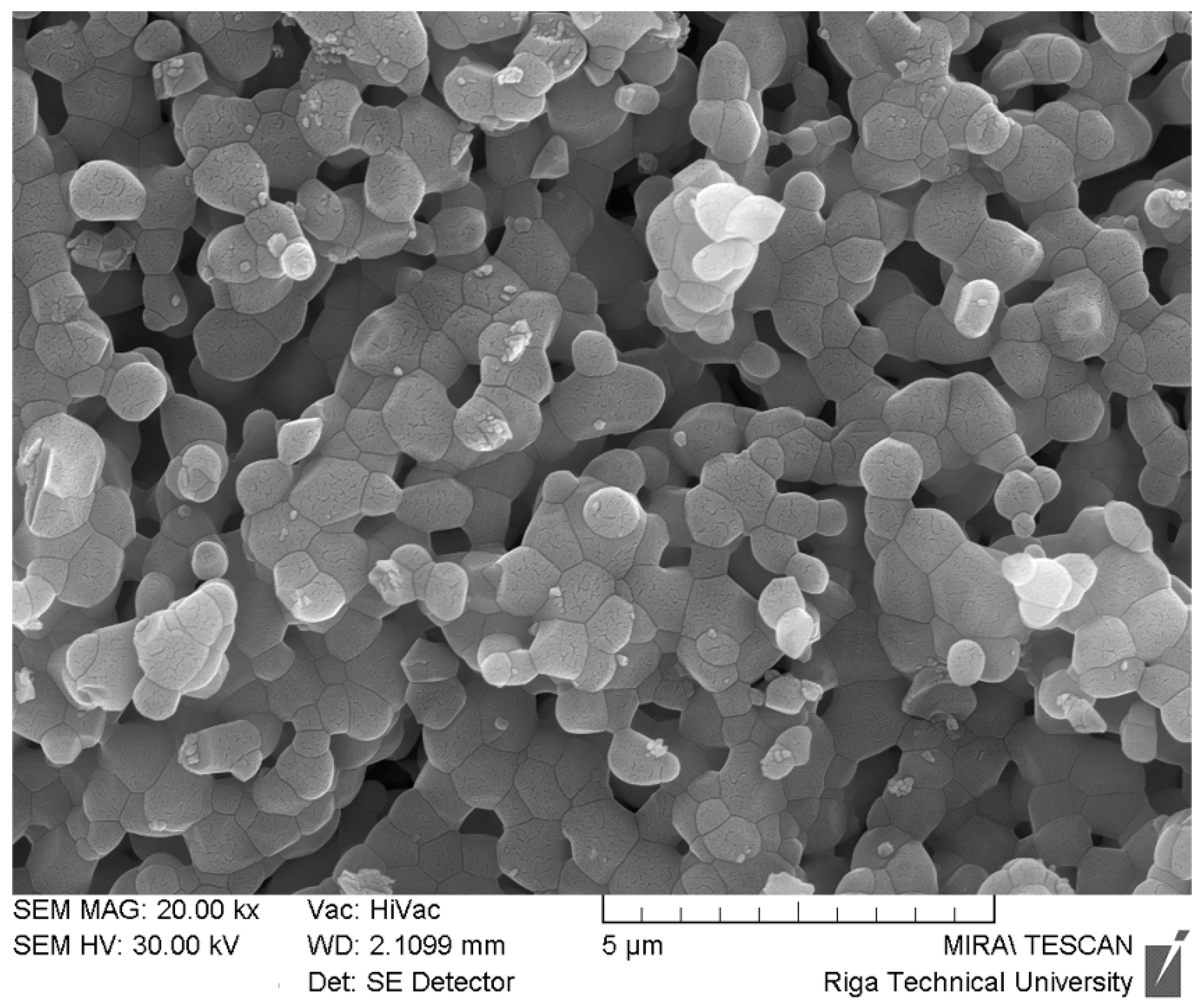
| Variables | TBA, mm2 | OPG | OC | NFkB-105 | BMP-2/4 | Col-1α | MMP-2 | TIMP-2 | IL-1 | IL-10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | |||||||||||
| A | |||||||||||
| Sr-HA30/TCP70 | 0.234 | 3c (2.5–3) | 3c (2–3) | 2c (1.5–2) | 2.5c (1.5–3) | 3c (2–3) | 2 (1–3) | 3 (2.5–3) | 1c (0.5–1.5) | 2 (1.5–2.5) | |
| Intact leg | 0.226 | 2 (2–2.5) | 2 (2–2.5) | 1 (1–1.5) | 1.5 (1–2) | 2 (2–3) | 1 (1–2) | 3 (2–3) | 0.5 (0–1) | 1.5 (1–2) | |
| HA30/TCP70 | 0.226 | 2a (2–3)b | 2.5 (2–3) | 1.5c (1–2) | 2 (1–2) | 3 (2–3) | 2c (1.5–3) | 3 (2–3) | 1 (0–1.5) | 2 (2–2.5) | |
| Intact leg | 0.198 | 2 (1.5–2.5) | 2 (1.5–3) | 1 (0–1.5) | 2 (1–2) | 2.5 (2–3) | 1.5 (1–1.5) | 2.5 (2–3) | 0.5 (0–1) | 2 (1.5–2.5) | |
| B | |||||||||||
| Sr-HA70/TCP30 | 0.206 | 3c (2.5–3) | 3.5c (2.5–4) | 3c (2–3.5) | 2.5c (2–3) | 3c (2–3.5) | 2.5c (2–3) | 3c (2.5–3) | 1.5c (1–2) | 2.5c (2–3) | |
| Intact leg | 0.222 | 2 (1.5–2) | 1.5 (1–3) | 2 (1.5–2) | 0.5 (0.5–1) | 1.5 (1–3) | 1.5 (1–2) | 2 (1.5–2.5) | 0.5 (0.5–1) | 1.5 (1–2) | |
| HA70/TCP30 | 0.223 | 2c (2–3) | 3c (2–4) | 2c (2–3) | 2c (1.5–2.5) | 2.5c (2.5–3) | 2.5c (2.5–3.5) | 3c (2.5–3.5) | 2c (1.5–2.5) | 2.5c (2–3) | |
| Intact leg | 0.212 | 1.5 (1.5–2) | 2 (1–2) | 1.5 (1–2) | 0.5 (0.5–1.5) | 2 (1–2.5) | 1.5 (1–2) | 2 (1.5–2) | 0.5 (0.5–1) | 2 (1.5–2.5) | |
| C | |||||||||||
| Sham | 0.242 | 2 (2–2.5) | 2.5 (2–3) | 1.5 (1–2) | 2 (1.5–2) | 3c (2–3) | 2 (1–3) | 3 (2–3) | 1 (0.5–2) | 2.5 (2–3) | |
| Intact leg | 0.207 | 2 (2–2.5) | 2 (2–2.5) | 1.5 (1–3) | 1.5 (1–2) | 2 (1.5–3) | 1.5 (1–3) | 3 (2–3) | 1 (0.5–2) | 2 (1–3) | |
| D | |||||||||||
| Healthy | 0.393 | 2.5 (2–3) | 3 (2.5–3) | 2 (1.5–2) | 1.5 (1–2) | 1.5 (1–2) | 2 (1–3) | 2.5 (2–2.5) | 0.5 (0–1) | 2 (1–2.5) | |
| Group | Variables | Correlation | Coefficient | p value |
|---|---|---|---|---|
| A | ||||
| Sr-HA30/TCP70 | NFkB-105 and Col-1α | SP | rs = 0.734 | p = 0.046 |
| TBA and Col-1α | VSP | rs = 0.809 | p = 0.028 | |
| HA30/TCP70 | OC and NFkB-105 | VSP | rs = 0.833 | p = 0.020 |
| OC and IL-1 | SP | rs = 0.772 | p = 0.042 | |
| NFkB-105 and Il-1 | VSP | rs= 0.814 | p = 0.026 | |
| MMP-2 and TIMP-2 | SN | rs = −0.764 | p = 0.046 | |
| Col-1α and Il-1 | VSP | rs = 0.814 | p = 0.026 | |
| NFkB-105 and Col-1α | SP | rs = 0.767 | p = 0.044 | |
| OC and TBA | VSN | rs = −0.837 | p = 0.019 | |
| B | ||||
| Sr-HA70/TCP30 | - | - | - | - |
| HA70/TCP30 | OC and MMP-2 | SP | rs = 0.736 | p = 0.037 |
| OPG and IL-10 | VSN | rs = −0.816 | p = 0.013 | |
| OC and Col-1α | SN | rs = −0.732 | p = 0.039 | |
| C | ||||
| Sham | NFkB-105 and MMP-2 | VSP | rs = 0.939 | p = 0.005 |
| NFkB-105 and IL-1 | VSP | rs = 0.833 | p = 0.039 | |
| OPG and IL-10 | VSN | rs= −0.880 | p = 0.021 | |
| D | ||||
| Healthy | BMP 2/4 and Col-1α | VSP | rs = 0.829 | p = 0.003 |
| OC and BMP 2/4 | SP | rs = 0.634 | p = 0.049 | |
| MMP-2 and IL-10 | SP | rs = 0.650 | p = 0.042 | |
| TBA and NFkB-105 | SP | rs = 0.657 | p = 0.039 | |
| NFkB-105 and MMP-2 | SN | rs = −0.670 | p = 0.034 | |
| NFkB-105 and Col-1α | SN | rs = −0.646 | p = 0.044 | |
| TBA and TIMP-2 | SN | rs = −0.690 | p = 0.027 | |
| TBA and IL-1 | SN | rs = −0.783 | p = 0.007 | |
| Author | Status | Number | Defect, mm | Localization | Material | Time, Weeks | New Bone | Bone Remodeling | |
|---|---|---|---|---|---|---|---|---|---|
| E | C | ||||||||
| Dagang [35] | H | 2 | 2 | 2.2 | distal femur | Sr-HA | 24 | similar | increased |
| Gu [36] | H | 12 | 12 | 15 | radius | Sr-CPP | 16 | increased | increased |
| Mohan [37] | H | 6 | 6 | 15 | ulna | Sr-CP | 12 | increased | increased |
| Tian [38] | H | 24 | 24 | 15 | radius | Sr-CPP | 16 | increased | similar |
| Kang [39] | ON | 18 | 18 | 3 | proximal femur | Sr-CPP and MNC | 12 | increased | increased |
| Tarafder [40] | H | 2 | 2 | 5.5 | distal femur | Β-TCP-MgO/SrO | 12 | increased | increased |
| Xie [41] | H | 9 | 9 | 15 | femoral shaft | K/Sr-CPP | 12 | increased | increased |
| Zhang [42] | H | 6 | 6 | 6 | distal femur | Sr-BBG | 8 | increased | increased |
| Californian Female Rabbits | ||||||
|---|---|---|---|---|---|---|
| Group | A | B | C | D | ||
| Status | Sr-HA/TCP | HA/TCP | Sr-HA/TCP | HA/TCP | sham | healthy |
| Ceramic ratio | 30/70 | 30/70 | 70/30 | 70/30 | - | - |
| Number | 7 | 7 | 7 | 8 | 7 | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarins, J.; Pilmane, M.; Sidhoma, E.; Salma, I.; Locs, J. The Role of Strontium Enriched Hydroxyapatite and Tricalcium Phosphate Biomaterials in Osteoporotic Bone Regeneration. Symmetry 2019, 11, 229. https://doi.org/10.3390/sym11020229
Zarins J, Pilmane M, Sidhoma E, Salma I, Locs J. The Role of Strontium Enriched Hydroxyapatite and Tricalcium Phosphate Biomaterials in Osteoporotic Bone Regeneration. Symmetry. 2019; 11(2):229. https://doi.org/10.3390/sym11020229
Chicago/Turabian StyleZarins, Janis, Mara Pilmane, Elga Sidhoma, Ilze Salma, and Janis Locs. 2019. "The Role of Strontium Enriched Hydroxyapatite and Tricalcium Phosphate Biomaterials in Osteoporotic Bone Regeneration" Symmetry 11, no. 2: 229. https://doi.org/10.3390/sym11020229





