Magnetic and Structural Properties of Barium Hexaferrite Nanoparticles Doped with Titanium
Abstract
:1. Introduction
2. Experimental Techniques
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gutfleisch, O.; Willard, M.A.; Bruck, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic materials and devices for the 21st century: Stronger, lighter, and more energy efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.A.; Jafelicci, M., Jr. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Cochardt, A. Recent ferrite magnet developments. J. Appl. Phys. 1966, 37, 1112–1115. [Google Scholar] [CrossRef]
- Sugimoto, M. The past, present, and future of ferrites. J. Am. Ceram. Soc. 1999, 82, 269–280. [Google Scholar] [CrossRef]
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Koutzarova, T.; Kolev, S.; Ghelev, C.; Grigorov, K.; Nedkov, I. Structural and magnetic properties and preparation techniques of nanosized M-type hexaferrite powders. In Advances in Nanoscale Magnetism; Springer: Berlin, Germany, 2009; pp. 183–203. [Google Scholar]
- Maswadeh, Y.; Mahmood, S.H.; Awadallah, A.; Aloqaily, A.N. Synthesis and structural characterization of nonstoichiometric barium hexaferrite materials with Fe:Ba ratio of 11.5–16.16. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Philadelphia, PA, USA, 2015. [Google Scholar]
- Radwan, M.; Rashad, M.M.; Hessien, M.M. Synthesis and characterization of barium hexaferrite nanoparticles. J. Mater. Process. Technol. 2007, 181, 106–109. [Google Scholar] [CrossRef]
- Martirosyan, K.S.; Galstyan, E.; Hossain, S.M.; Wang, Y.-J.; Litvinov, D. Barium hexaferrite nanoparticles: synthesis and magnetic properties. Mater. Sci. Eng. B 2011, 176, 8–13. [Google Scholar] [CrossRef]
- Meng, Y.Y.; He, M.H.; Zeng, Q.; Jiao, D.L.; Shukla, S.; Ramanujan, R.V.; Liu, Z.W. Synthesis of barium ferrite ultrafine powders by a sol–gel combustion method using glycine gels. J. Alloy. Compd. 2014, 583, 220–225. [Google Scholar] [CrossRef]
- Kouřil, K. Local structure of hexagonal ferrites studied by NMR. (2013). Available online: https://www.researchgate.net (accessed on 20 March 2019).
- Wu, M. M-Type barium hexagonal ferrite films. In Advanced Magnetic MaterialsAdv; InTech Open: London UK, 2012. [Google Scholar]
- Awadallah, A.M.; Sami, M. Effects of preparation conditions and metal ion substitutions for barium and iron on the properties of M-type barium hexaferrites. Ph.D. Research Proposal, The University of Jordan, Amman, Jordan, 2012. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, D.; Liu, Z. The law of approach to saturation in ferromagnets originating from the magnetocrystalline anisotropy. J. Magn. Magn. Mater. 2010, 322, 2375–2380. [Google Scholar] [CrossRef]
- Bsoul, I.; Mahmood, S.H. Magnetic and structural properties of BaFe12−xGaxO19 nanoparticles. J. Alloy. Compd. 2010, 489, 110–114. [Google Scholar] [CrossRef]
- Awawdeh, M.; Bsoul, I.; Mahmood, S.H. Magnetic properties and Mössbauer spectroscopy on Ga, Al, and Cr substituted hexaferrites. J. Alloy. Compd. 2014, 585, 465–473. [Google Scholar] [CrossRef]
- Packiaraj, G.; Panchal, N.R.; Jotania, R.B. Structural and Dielectric studies of Cu substituted Barium hexaferrite prepared by Sol-gel auto combustion technique. Solid State Phenom. 2014, 209, 102–106. [Google Scholar] [CrossRef]
- Mahmood, S.H.; Aloqaily, A.N.; Maswadeh, Y.; Awadallah, A.; Bsoul, I.; Juwhari, H. Structural and magnetic properties of Mo-Zn substituted (BaFe12-4xMoxZn3xO19) M-type hexaferrites. Mater. Sci. Res. India 2014, 11, 9–20. [Google Scholar] [CrossRef]
- Haneda, K.; Hiroshi, K. Intrinsic coercivity of substituted BaFe12O19. Japan. J. Appl. Phys. 1973, 12, 355. [Google Scholar] [CrossRef]
- Dushaq, G.H.; Mahmood, S.H.; Bsoul, I.; Juwhari, H.K.; Lahlouh, B.; AlDamen, M.A. Effects of molybdenum concentration and valence state on the structural and magnetic properties of BaFe11.6MoxZn0.4-xO19 hexaferrites. Acta Metall. Sin. (English Letters) 2013, 26, 509–516. [Google Scholar] [CrossRef]
- Ali, I.; Islam, M.U.; Awan, M.S.; Ahmad, M. Effects of Ga–Cr substitution on structural and magnetic properties of hexaferrite (BaFe12O19) synthesized by sol–gel auto-combustion route. J. Alloy. Compd. 2013, 547, 118–125. [Google Scholar] [CrossRef]
- Wang, S.; Ding, J.; Shi, Y.; Chen, Y.J. High coercivity in mechanically alloyed BaFe10Al2O19. J. Magn. Magn. Mater. 2000, 219, 206–212. [Google Scholar] [CrossRef]
- Ali, I.; Islam, M.U.; Awan, M.S.; Ahmad, M.; Ashiq, M.N.; Naseem, S. Effect of Tb3+ substitution on the structural and magnetic properties of M-type hexaferrites synthesized by sol–gel auto-combustion technique. J. Alloy. Compd. 2013, 550, 564–572. [Google Scholar] [CrossRef]
- Sharma, R.; Bisen, D.P.; Shukla, U.; Sharma, B.G. X-ray diffraction: a powerful method of characterizing nanomaterials. Recent Res. Sci. Technol. 2012, 4, 77–79. [Google Scholar]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods at different acquisition geometries for determination of crystallite size in nano-scale materials. Mater. Charact. 2013, 85, 111–123. [Google Scholar] [CrossRef]
- Stuart, B.H. Experimental methods. In Infrared Spectroscopy: Fundamentals And Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 15–44. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Goldstein, J.; Yakowitz, H. Practical Scanning Electron Microscopy: Electron and Ion Microprobe Analysis; Springer: Boston, MA, USA, 1975. [Google Scholar]
- Aravind, A. Synthesis and characterization of 3d-transition metals doped ZnO thin films and nanostructures for possible spintronic applications. Ph.D. Thesis, Cochin University of Science and Technology, Kerala, India, 2012. [Google Scholar]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Mahmood, S.H.; Dushaq, G.H.; Bsoul, I.; Awawdeh, M.A.; Juwhari, H.K.; Lahlouh, B.; AlDamen, M.A. Magnetic properties and hyperfine interactions in M-type BaFe12-2xMoxZnxO19 hexaferrites. J. Appl. Math. Phys. 2014, 2, 77–87. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Yang, H. Synthesis, characterization, and magnetic properties of nanocrystalline BaFe12O19 Ferrite. Curr. Appl. Phys. 2009, 9, 1375–1380. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Kostishyn, V.G.; Panina, L.V.; Trukhanov, A.V.; Turchenko, V.A.; Tishkevich, D.I.; Trukhanova, E.L.; Yakovenko, O.S.; Matzui, L.Y.; et al. Effect of gallium doping on electromagnetic properties of barium hexaferrite. J. Phys. Chem. Solid. 2017, 111, 142–152. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Kostishyn, V.G.; Panina, L.V.; Trukhanov, An.V.; Turchenko, V.A.; Tishkevich, D.I.; Trukhanova, E.L.; Yakovenko, O.S.; Matzui, L.Yu. Investigation into the structural features and microwave absorption of doped barium hexaferrites. Dalton Trans. 2017, 46, 9010–9021. [Google Scholar] [CrossRef] [Green Version]
- Trukhanov, S.V.; Trukhanov, A.V.; Salem, M.M.; Trukhanova, E.L.; Panina, L.V.; Kostishyn, V.G.; Darwish, M.A.; Trukhanov, A.V.; Zubar, T.I.; Tishkevich, D.I.; et al. Preparation and investigation of structure, magnetic and dielectric properties of (BaFe11.9Al0.1O19)1-x-(BaTiO3)x bicomponent ceramics. Ceram. Int. 2018, 44, 21295–21302. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Turchenko, V.A.; Trukhanov, An.V.; Tishkevich, D.I.; Trukhanova, E.L.; Zubar, T.I.; Karpinsky, D.V.; Kostishyn, V.G.; Panina, L.V.; et al. Magnetic and dipole moments in indium doped barium hexaferrites. J. Magn. Magn. Mater. 2018, 457, 83–96. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Turchenko, V.A.; Kostishyn, V.G.; Panina, L.V.; Kazakevich, I.S.; Balagurov, A.M. Structure and magnetic properties of BaFe11.9In0.1O19 hexaferrite in a wide temperature range. J. Alloy. Compd. 2016, 689, 383–393. [Google Scholar] [CrossRef]
- Turchenko, V.A.; Trukhanov, S.V.; Balagurov, A.M.; Kostishyn, V.G.; Trukhanov, A.V.; Panina, L.V.; Trukhanova, E.L. Features of crystal structure and dual ferroic properties of BaFe12-xMexO19 (Me = In3+ and Ga3+; x = 0.1–1.2). J. Magn. Magn. Mater. 2018, 464, 139–147. [Google Scholar] [CrossRef]



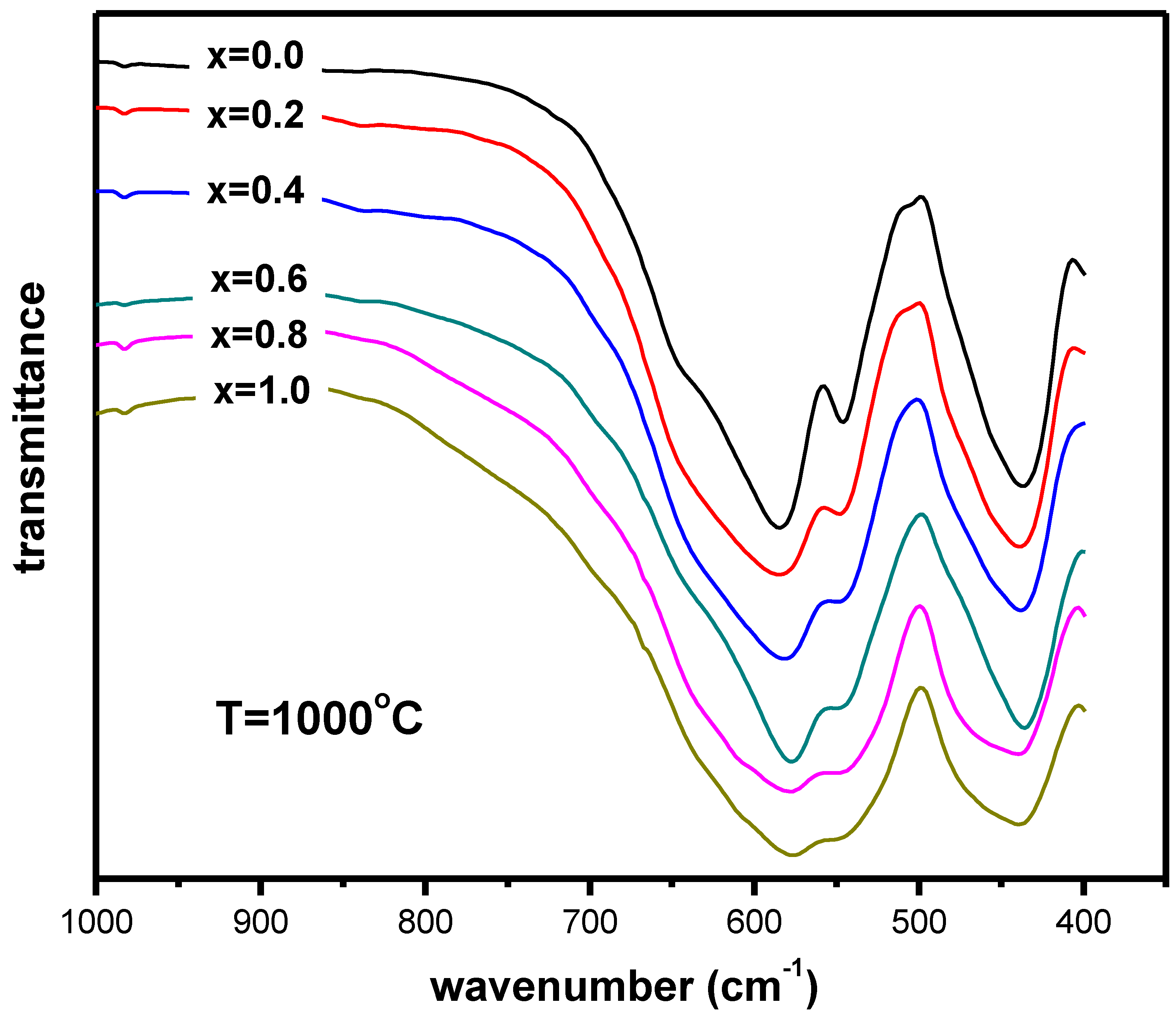



| Sample | (2θ)-Position (Degrees) | Size (nm) | Average Size (nm) |
|---|---|---|---|
(x = 0.0) | 32.19 | 24.8 | 27.2 |
| 34.14 | 29.3 | ||
| 37.1 | 29.6 | ||
| 40.33 | 27 | ||
| 55.1 | 25.3 | ||
(x = 0.2) | 33.1 | 27.3 | 30.3 |
| 35.67 | 33 | ||
| 49.51 | 25.4 | ||
| 54.1 | 35.3 | ||
(x = 0.4) | 33.3 | 34.1 | 34 |
| 35.8 | 35.9 | ||
| 49.65 | 33.3 | ||
| 54.26 | 32.7 | ||
(x = 0.6) | 33.31 | 39 | 36.5 |
| 35.79 | 41 | ||
| 49.69 | 34.6 | ||
| 54.24 | 31.5 | ||
(x= 0.8) | 33.39 | 34.2 | 34 |
| 35.86 | 35.9 | ||
| 49.7 | 33.3 | ||
| 54.3 | 32.7 | ||
(x = 1.0) | 33.31 | 39 | 36.3 |
| 35.69 | 41.3 | ||
| 49.54 | 34.6 | ||
| 54.15 | 30.4 |
| Sample | (2θ)-Position (Degrees) | Size (nm) | Average Size (nm) |
|---|---|---|---|
(x = 0.0) | 32.4 | 30.3 | 34.9 |
| 34.34 | 41 | ||
| 40.55 | 36.4 | ||
| 55.31 | 31.7 | ||
(x = 0.2) | 32.29 | 38.9 | 38.7 |
| 34.23 | 41.1 | ||
| 40.44 | 41.9 | ||
| 55.1 | 32.8 | ||
(x = 0.4) | 32.26 | 43.1 | 41.5 |
| 34.22 | 45.7 | ||
| 40.42 | 46.5 | ||
| 55.19 | 30.6 | ||
(x = 0.6) | 32.29 | 43 | 41 |
| 34.27 | 45.7 | ||
| 40.48 | 39.9 | ||
| 54.23 | 35.3 | ||
(x = 0.8) | 33.27 | 45.6 | 40.5 |
| 34.24 | 43.3 | ||
| 40.96 | 36.5 | ||
| 54.19 | 36.7 | ||
(x = 1.0) | 33.23 | 43.1 | 38 |
| 35.7 | 41.3 | ||
| 40.93 | 32.2 | ||
| 54.19 | 35.3 |
| x | Ms (emu/g) | Mr (emu/g) | Mrs (emu/g) | Hc (kOe) |
|---|---|---|---|---|
| 0.0 | 44.65 | 23.0606 | 0.5165 | 4.51 |
| 0.2 | 45.24 | 19.7474 | 0.4365 | 3.4557 |
| 0.4 | 44.83 | 19.5835 | 0.4368 | 1.6554 |
| 0.6 | 39.99 | 12.9342 | 0.3234 | 0.583 |
| 0.8 | 20.39 | 9.5829 | 0.47 | 1.385 |
| 1.0 | 17.17 | 7.7163 | 0.4494 | 1.11455 |
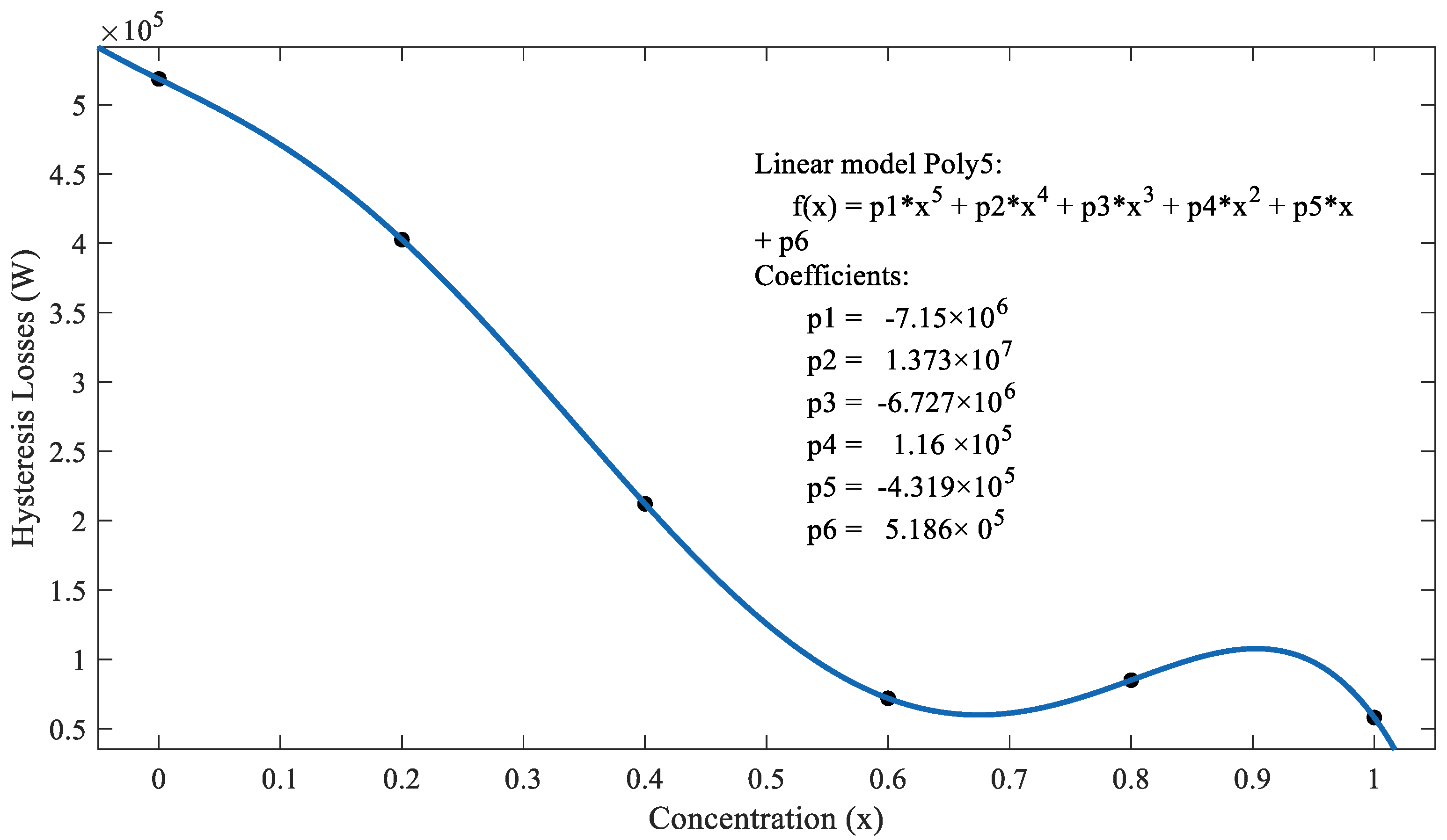
| Concentration (x) | Slope | Hysteresis Losses (W) |
|---|---|---|
| 0.0 | 0.004908 | 518,580 |
| 0.2 | 0.005326 | 402,700 |
| 0.4 | 0.00571 | 212,180 |
| 0.6 | 0.005252 | 71,914 |
| 0.8 | 0.002623 | 84,982 |
| 1.0 | 0.002229 | 58,080 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dairy, A.R.A.; Al-Hmoud, L.A.; Khatatbeh, H.A. Magnetic and Structural Properties of Barium Hexaferrite Nanoparticles Doped with Titanium. Symmetry 2019, 11, 732. https://doi.org/10.3390/sym11060732
Dairy ARA, Al-Hmoud LA, Khatatbeh HA. Magnetic and Structural Properties of Barium Hexaferrite Nanoparticles Doped with Titanium. Symmetry. 2019; 11(6):732. https://doi.org/10.3390/sym11060732
Chicago/Turabian StyleDairy, Abdul Raouf Al, Lina A. Al-Hmoud, and Heba A. Khatatbeh. 2019. "Magnetic and Structural Properties of Barium Hexaferrite Nanoparticles Doped with Titanium" Symmetry 11, no. 6: 732. https://doi.org/10.3390/sym11060732





