The Investigation of Organic Binder Effect on Morphological Structure of Ceramic Membrane Support
Abstract
:1. Introduction
2. Material and Methods
2.1. Natural Raw Clay (Kaolin) Material
2.2. Preparation of Mixture for Ceramic Membrane Supports
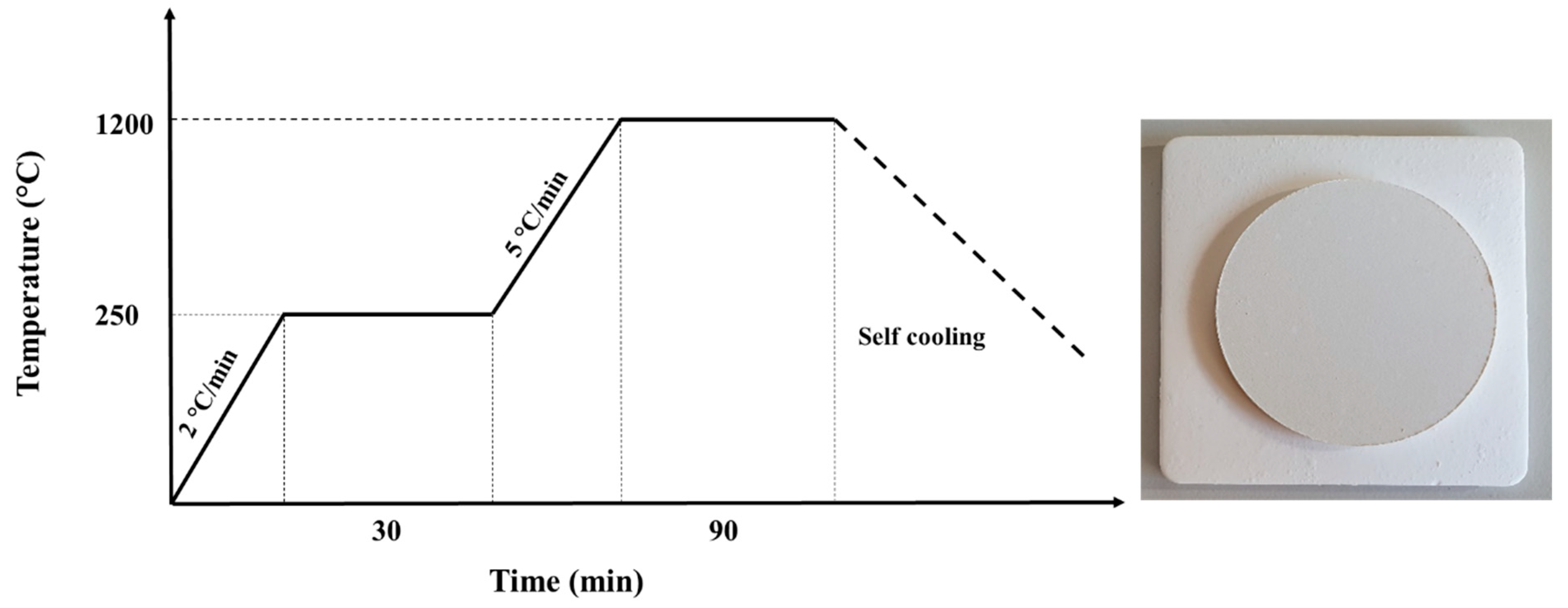
2.3. Sintering Process of Ceramic Membrane Supports
2.4. Characterization Methods
3. Results and Discussion
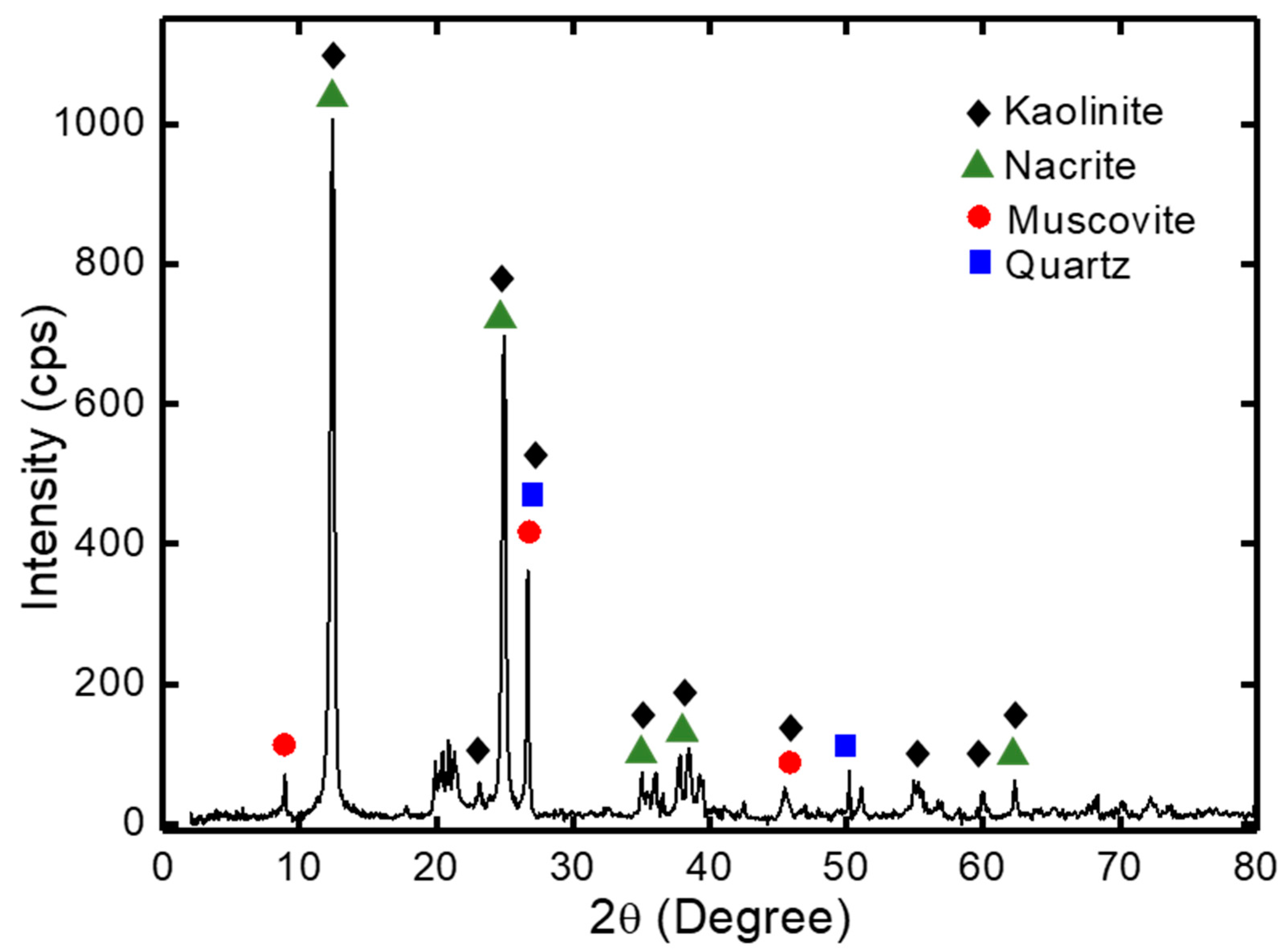
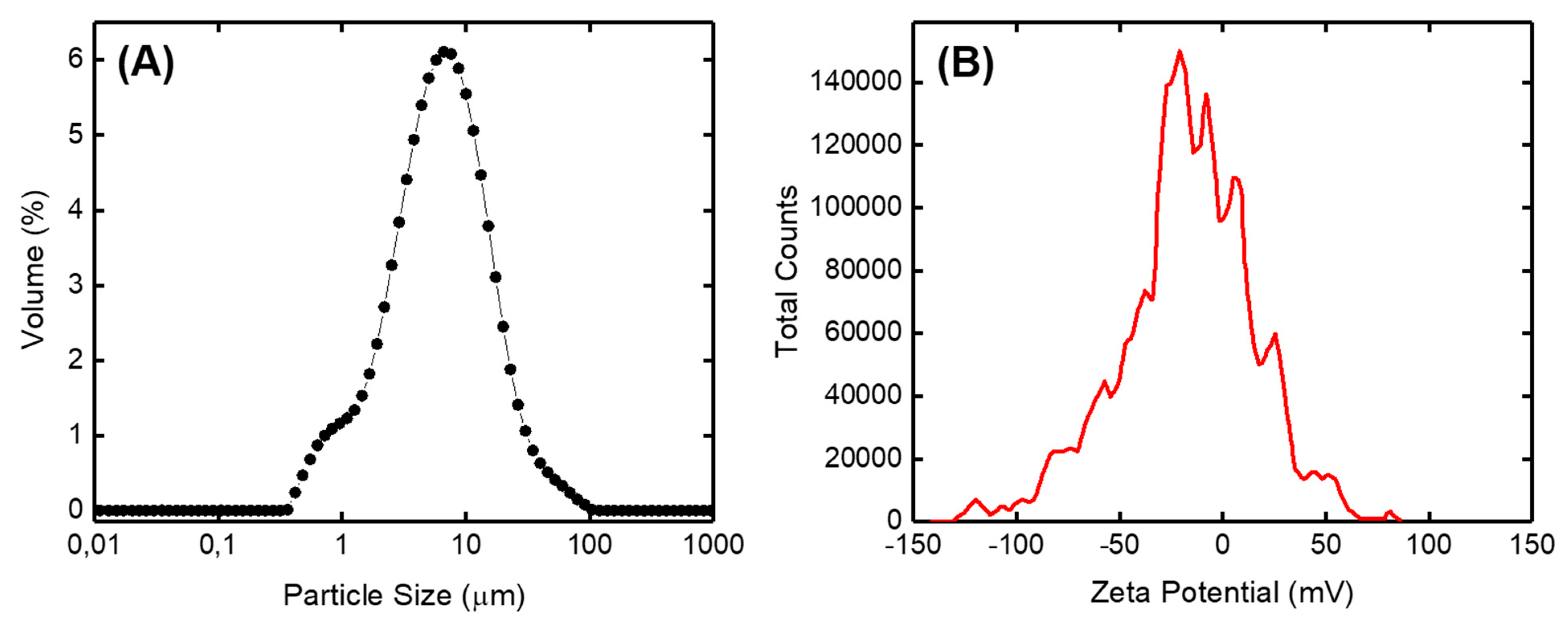
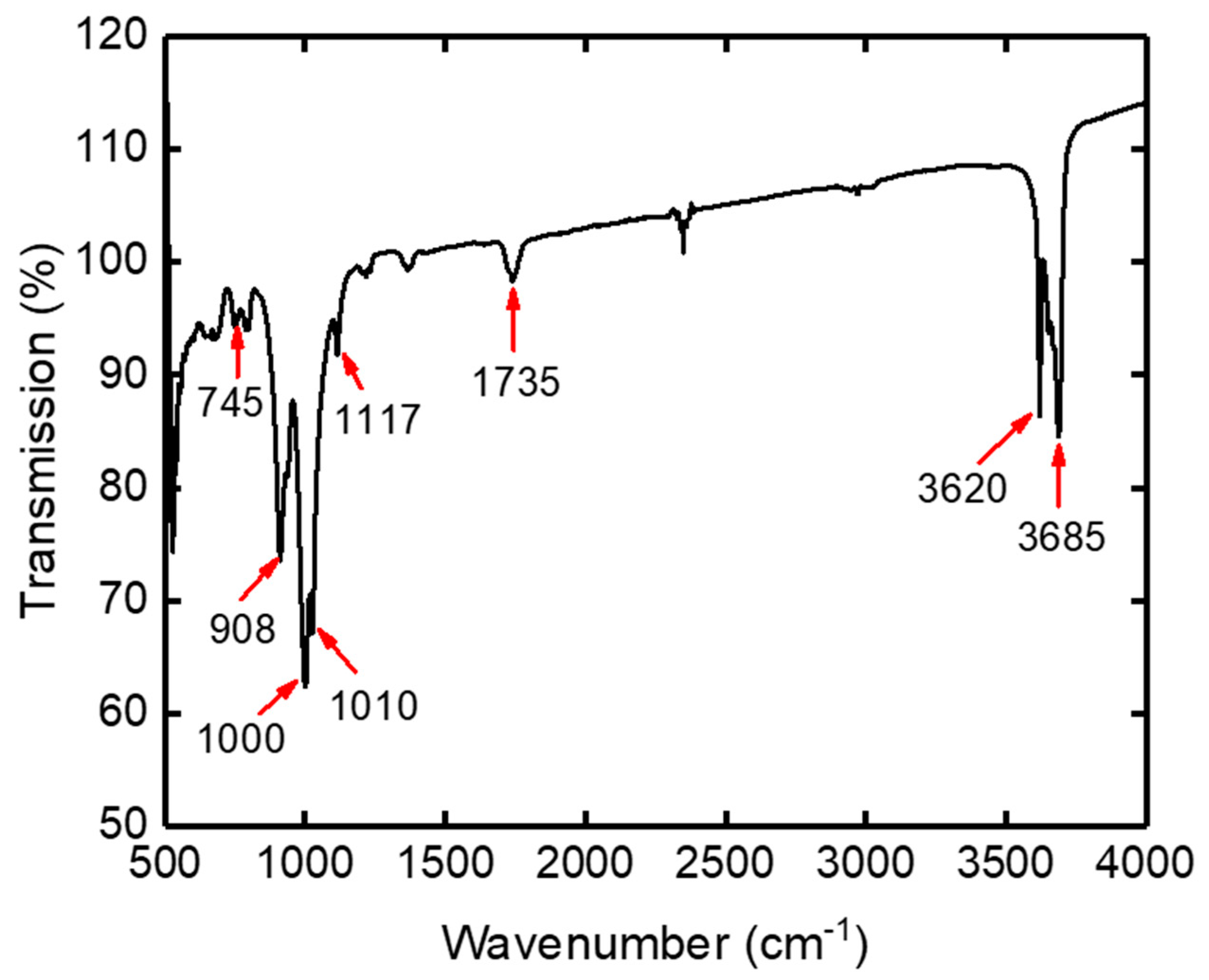
3.1. Characterization of Kaolin Powder
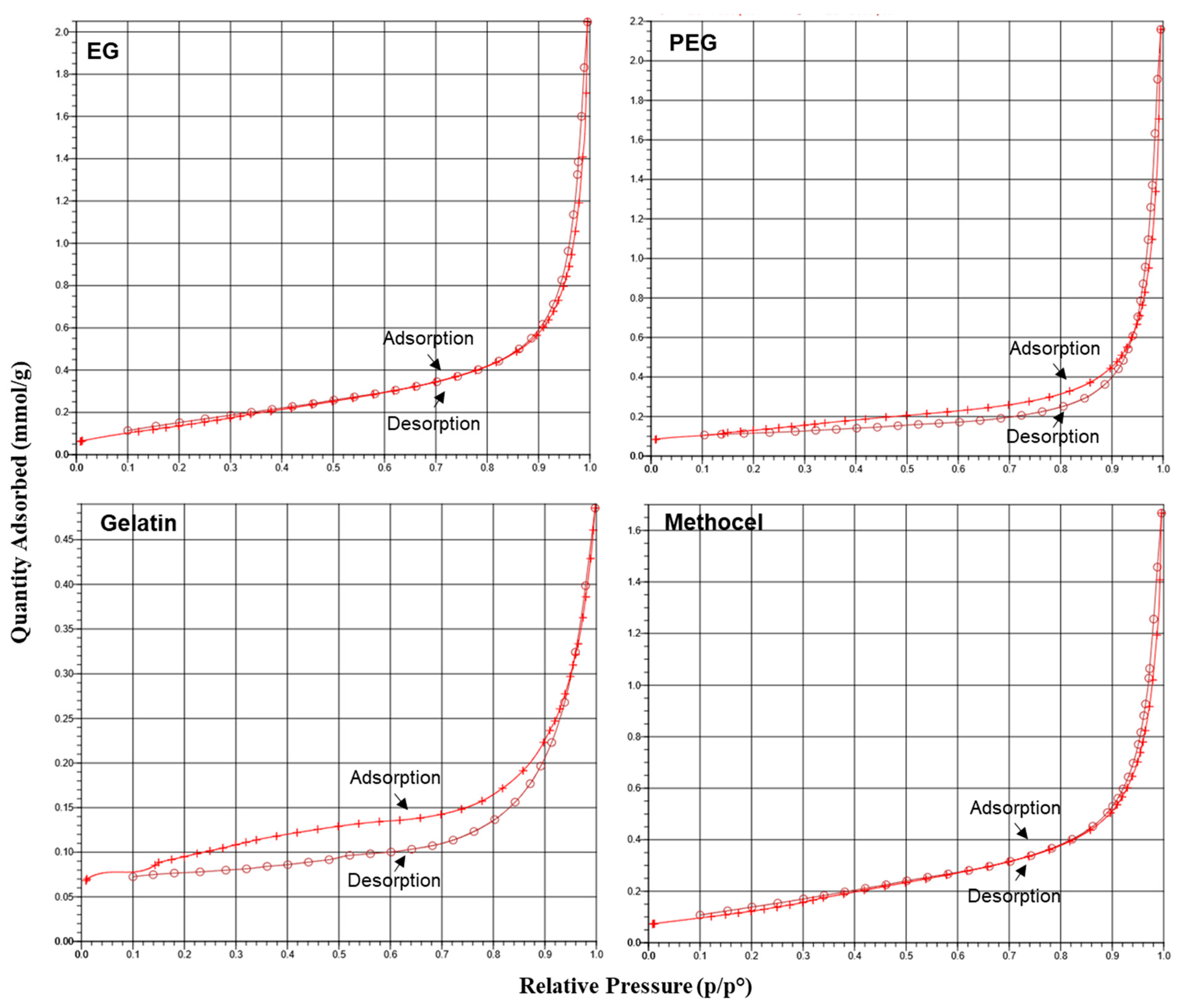
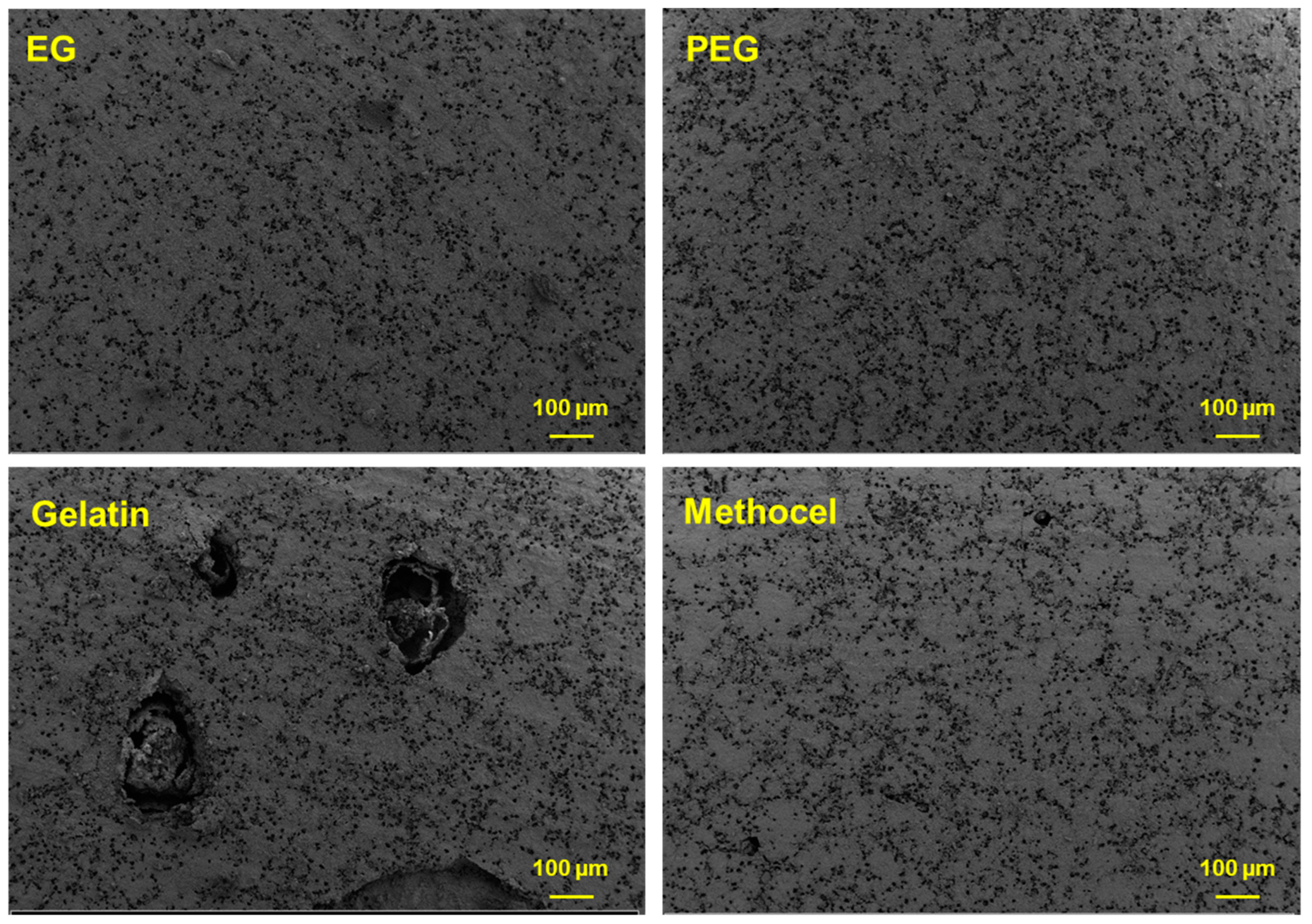
3.2. Characterization of Ceramic Membrane Supports
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Majouli, A.; Younssi, S.A.; Tahiri, S.; Albizane, A.; Loukili, H.; Belhaj, M. Characterization of flat membrane support elaborated from local Moroccan Perlite. Desalination 2011, 277, 61–66. [Google Scholar] [CrossRef]
- Bouzerara, F.; Harabi, A.; Ghouil, B.; Medjemem, N.; Boudaira, B.; Condom, S. Elaboration and Properties of Zirconia Microfiltration Membranes. Procedia Eng. 2012, 33, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Malik, N.; Bulasara, V.K.; Basu, S. Preparation of novel porous ceramic microfiltration membranes from fly ash, kaolin and dolomite mixtures. Ceram. Int. 2020, 46, 6889–6898. [Google Scholar] [CrossRef]
- Rekik, S.B.; Bouaziz, J.; Deratani, A.; Beklouti, S. Study of Ceramic Membrane from Naturally Occurring-Kaolin Clays for Microfiltration Applications. Period. Polytech. Chem. Eng. 2017, 61, 206. [Google Scholar] [CrossRef]
- Kadiri, C.; Harabi, A.; Bouzerara, F.; Foughali, L.; Brihi, N.; Hallour, S.; Guechi, A.; Boudaira, B. Preparation and properties of tubular macroporous ceramic membrane supports based on natural quartz sand and dolomite. J. Aust. Ceram. Soc. 2019. [Google Scholar] [CrossRef]
- Hou, Z.; Cui, B.; Liu, L.; Liu, Q. Effect of the different additives on the fabrication of porous kaolin-based mullite ceramics. Ceram. Int. 2016, 42, 17254–17258. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.; Hanaor, D.; Gurlo, A. Materials and Applications for Low-Cost Ceramic Membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Forano, C.; Hibino, T.; Leroux, F.; Taviot-Guého, C. Layered Double Hydroxides. In Developments in Clay Science; Elsevier: Cambridge, MA, USA, 2006; pp. 1021–1095. ISBN 0080441831. [Google Scholar]
- Murray, H.H. Kaolin Applications. In Applied Clay Mineralogy—Occurrences, Processing and Application of Kaolins, Bentonites, Palygorskite-Sepiolite, and Common Clays; Elsevier: Cambridge, MA, USA, 2006; pp. 85–109. ISBN 9780444517012. [Google Scholar]
- Pak, V.I.; Kirov, S.S.; Nalivaiko, A.Y.; Ozherelkov, D.Y.; Gromov, A.A. Obtaining alumina from kaolin clay via aluminum chloride. Materials 2019, 12, 3938. [Google Scholar] [CrossRef] [Green Version]
- ElDeeb, A.B.; Brichkin, V.N.; Kurtenkov, R.V.; Bormotov, I.S. Extraction of alumina from kaolin by a combination of pyro- and hydro-metallurgical processes. Appl. Clay Sci. 2019, 172, 146–154. [Google Scholar] [CrossRef]
- Xing, W.-H. Ceramic Membranes. In Membrane-Based Separations in Metallurgy; Elsevier: Cambridge, MA, USA, 2017; pp. 357–370. ISBN 9780128034279. [Google Scholar]
- Mestre, S.; Gozalbo, A.; Lorente-Ayza, M.M.; Sánchez, E. Low-cost ceramic membranes: A research opportunity for industrial application. J. Eur. Ceram. Soc. 2019, 39, 3392–3407. [Google Scholar] [CrossRef]
- Sondhi, R.; Bhave, R.; Jung, G. Applications and benefits of ceramic membranes. Membr. Technol. 2003, 2003, 5–8. [Google Scholar] [CrossRef]
- Bose, S.; Das, C. Role of Binder and Preparation Pressure in Tubular Ceramic Membrane Processing: Design and Optimization Study Using Response Surface Methodology (RSM). Ind. Eng. Chem. Res. 2014, 53, 12319–12329. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, Y.; Chang, Q.; Liu, F.; Wang, Y.; Rao, J. Preparation of a High-Performance Porous Ceramic Membrane by a Two-Step Coating Method and One-Step Sintering. Appl. Sci. 2018, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Hubadillah, S.K.; Harun, Z.; Othman, M.H.D.; Ismail, A.F.; Salleh, W.N.W.; Basri, H.; Yunos, M.Z.; Gani, P. Preparation and characterization of low cost porous ceramic membrane support from kaolin using phase inversion/sintering technique for gas separation: Effect of kaolin content and non-solvent coagulant bath. Chem. Eng. Res. Des. 2016, 112, 24–35. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Das, N.; Maiti, H.S. Formatation of pore structure in tape-cast alumina membranes—Effects of binder content and firing temperature. J. Memb. Sci. 1998, 140, 205–212. [Google Scholar] [CrossRef]
- Jana, S.; Purkait, M.K.; Mohanty, K. Preparation and Characterizations of Ceramic Microfiltration Membrane: Effect of Inorganic Precursors on Membrane Morphology. Sep. Sci. Technol. 2010, 46, 33–45. [Google Scholar] [CrossRef]
- Ivanets, A.; Agabekov, V. Preparation and Characterization of Microfiltration Ceramic Membranes Based on Natural Quartz Sand. ChemJMold 2017, 12, 67–73. [Google Scholar] [CrossRef]
- Zheng, M.-P.; Hou, Y.-D.; Ge, H.-Y.; Zhu, M.-K.; Yan, H. Effect of NiO additive on microstructure, mechanical behavior and electrical properties of 0.2PZN–0.8PZT ceramics. J. Eur. Ceram. Soc. 2013, 33, 1447–1456. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Buekenhoudt, A. Stability of Porous Ceramic Membranes. In Membrane Science and Technology; Elsevier: Cambridge, MA, USA, 2008; pp. 1–31. ISBN 9780444530707. [Google Scholar]
- Dewi, R.; Agusnar, H.; Alfian, Z. Tamrin Characterization of technical kaolin using XRF, SEM, XRD, FTIR and its potentials as industrial raw materials. J. Phys. Conf. Ser. 2018, 1116, 042010. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Niaei, A.; Salari, D. Production of γ-Al2O3 from Kaolin. Open J. Phys. Chem. 2011, 1, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Teresa, O.; Choi, C.K. Comparison between SiOC Thin Film by plasma enhance chemical vapor deposition and SiO2 Thin Film by Fourier Transform Infrared Spectroscopy. J. Korean Phys. Soc. 2010, 56, 1150–1155. [Google Scholar]
- Irawati, U.; Sunardi, S.; Suraida, S. SINTESIS DAN KARAKTERISASI GAMMA ALUMINA (γ-Al2O3) DARI KAOLIN ASAL TATAKAN, SELATAN BERDASARKAN VARIASI TEMPERATUR KALSINASI. Molekul 2013, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Shih, K.; Gao, Y.; Li, F.; Wei, L. Dechlorinating transformation of propachlor through nucleophilic substitution by dithionite on the surface of alumina. J. Soils Sediments 2012, 12, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Qabaqous, O.; Tijani, N.; Naciri Bennani, M.; El Krouk, A. Preparation and characterization of supports plans from the (Rhassoul) clay for mineral membranes. J. Mater. Environ. Sci. 2014, 5, 2244–2249. [Google Scholar]
- Benito, J.M.; Conesa, A.; Rubio, F.; Rodríguez, M.A. Preparation and characterization of tubular ceramic membranes for treatment of oil emulsions. J. Eur. Ceram. Soc. 2005, 25, 1895–1903. [Google Scholar] [CrossRef]




 Ethylene glycol (EG) | 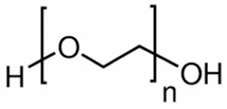 Polyethylene glycol (PEG) |
 Gelatin | 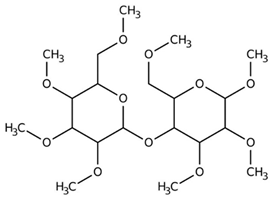 Methocel |
| Compound | Value | Unit |
|---|---|---|
| Loss on ignition | 12.91 | % |
| SiO2 | 47.41 | % |
| Al2O3 | 38.91 | % |
| K2O | 1.37 | % |
| Fe2O3 | 0.66 | % |
| MgO | 0.25 | % |
| Na2O | 0.10 | % |
| CaO | 0.05 | % |
| TiO2 | 0.05 | % |
| P2O5 | 0.02 | % |
| Wavenumber (cm−1) of Our Kaolin | Wavenumber (cm−1) of Observed in Literature | Assignments | References |
|---|---|---|---|
| 3685 | 3650–3695 | νO-H, octahedral | [26] |
| 3620 | 3598–3640 | νO-H of hydration of water | [27] |
| 1735 | 1639–1820 | νO-H buckling vibrations | [27] |
| 1117 | 1108 | Si-O-Si | [28] |
| 1010 | 1010–1022 | strong Si-O-Si | [29] |
| 1000 | 970 | Si-O-Si | [28] |
| 908 | 912–915 | Si-OH | [26] |
| 745 | 621–884 | Al–OH | [30] |
| Organic Binder Name | Pore Width (Å) |
|---|---|
| EG | 149.340 |
| PEG | 204.899 |
| Gelatin | 103.608 |
| Methocel | 137.030 |
| Kaolin powder | 317.984 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boussemghoune, M.; Chikhi, M.; Ozay, Y.; Guler, P.; Ozbey Unal, B.; Dizge, N. The Investigation of Organic Binder Effect on Morphological Structure of Ceramic Membrane Support. Symmetry 2020, 12, 770. https://doi.org/10.3390/sym12050770
Boussemghoune M, Chikhi M, Ozay Y, Guler P, Ozbey Unal B, Dizge N. The Investigation of Organic Binder Effect on Morphological Structure of Ceramic Membrane Support. Symmetry. 2020; 12(5):770. https://doi.org/10.3390/sym12050770
Chicago/Turabian StyleBoussemghoune, Mohamed, Mustapha Chikhi, Yasin Ozay, Pelin Guler, Bahar Ozbey Unal, and Nadir Dizge. 2020. "The Investigation of Organic Binder Effect on Morphological Structure of Ceramic Membrane Support" Symmetry 12, no. 5: 770. https://doi.org/10.3390/sym12050770




