Recent Developments in Heterogeneous Photocatalysts with Near-Infrared Response
Abstract
:1. Introduction
2. Design Strategy of Near-Infrared Photocatalysts with Symmetric/Asymmetric Nanostructures and Properties
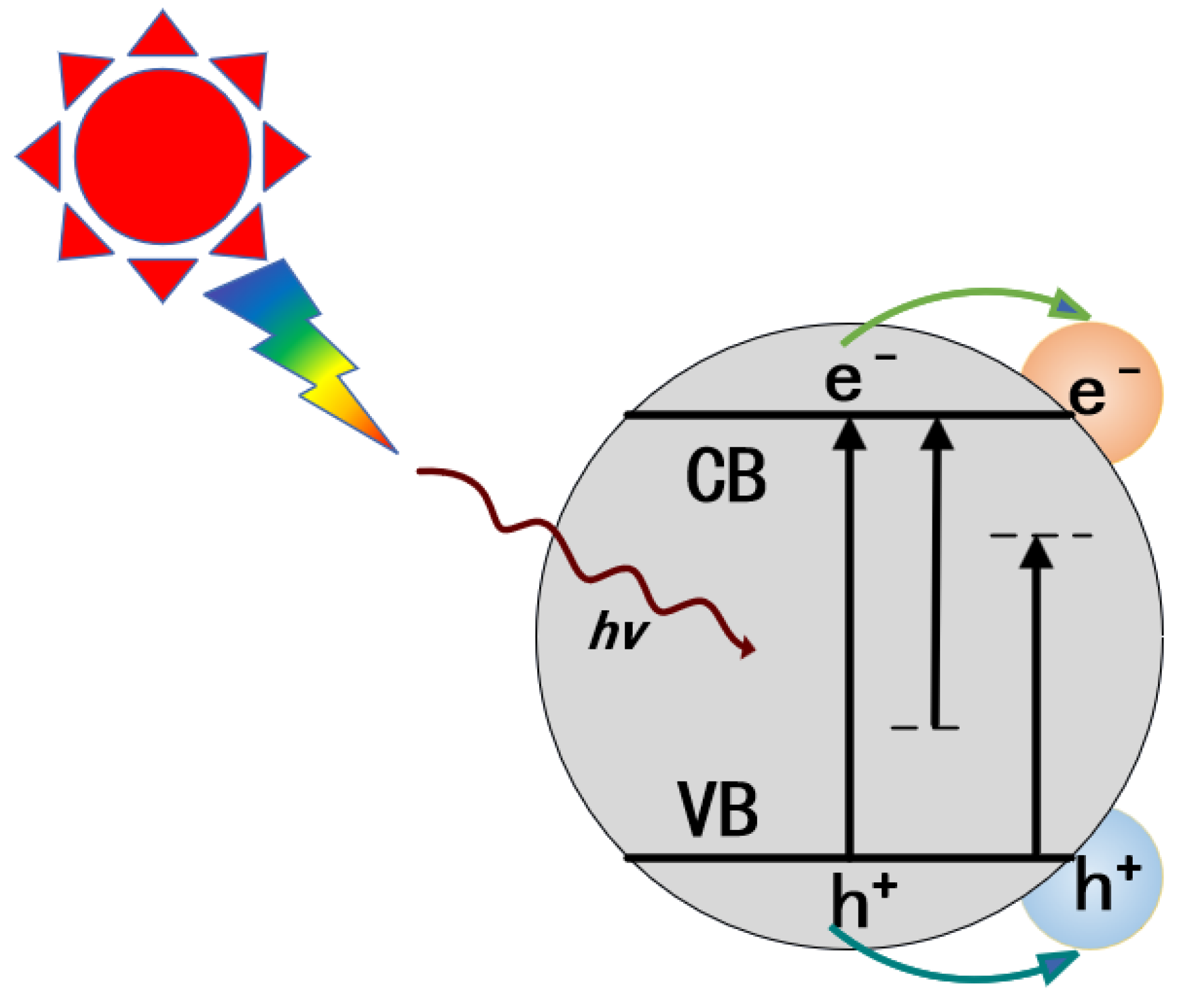
2.1. Materials with Direct NIR Utilization
2.1.1. Narrow-Bandgap Materials
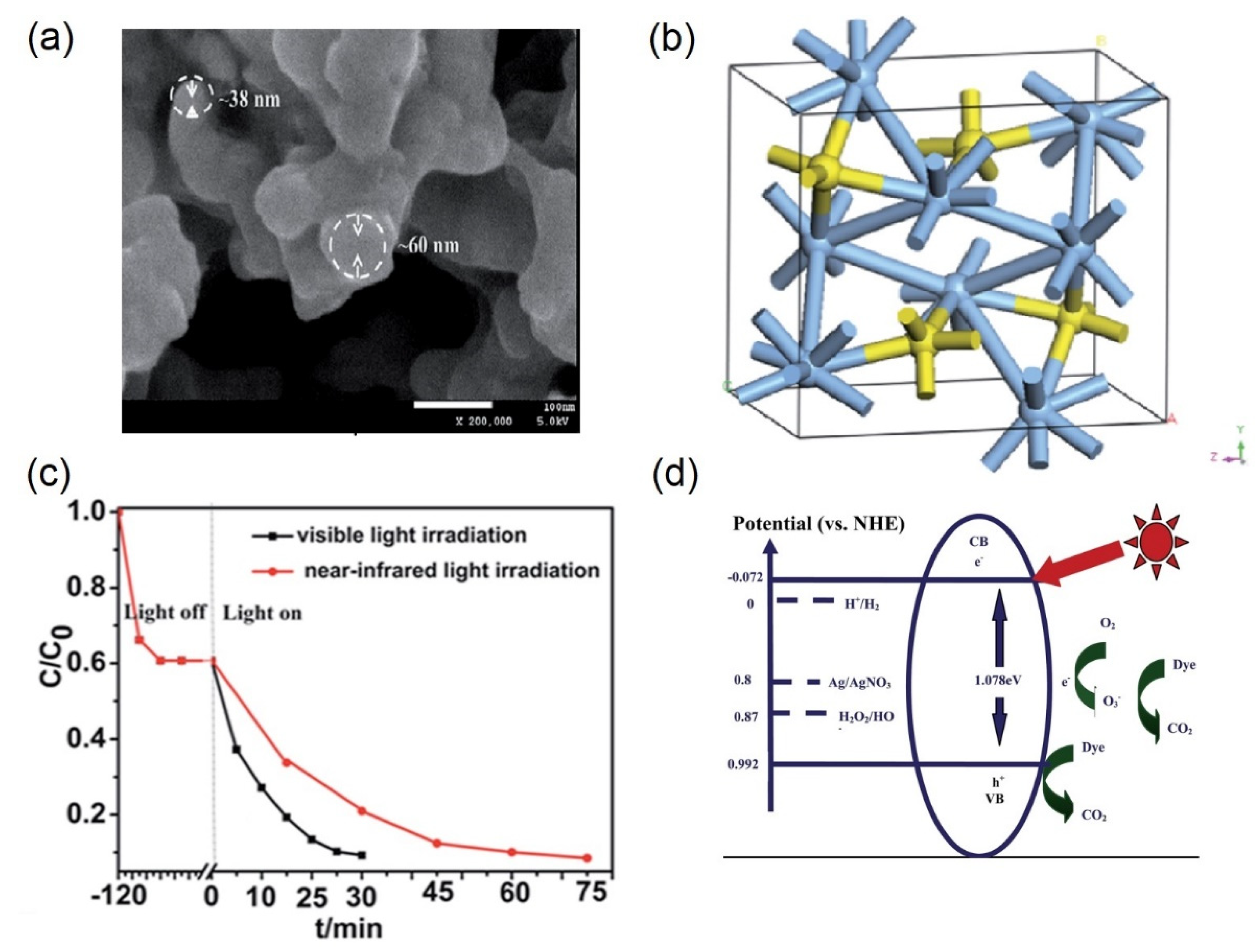
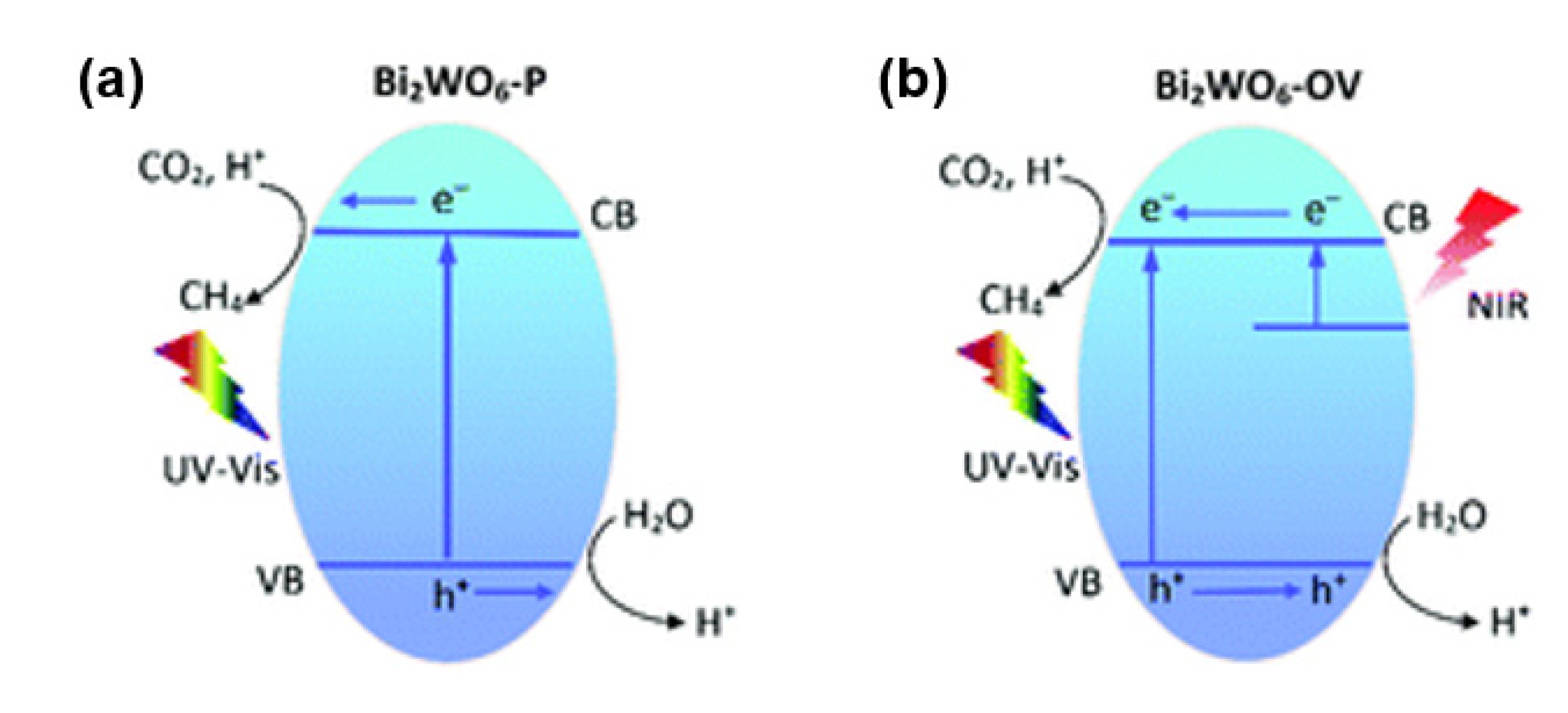
2.1.2. Defect-Engineered Materials
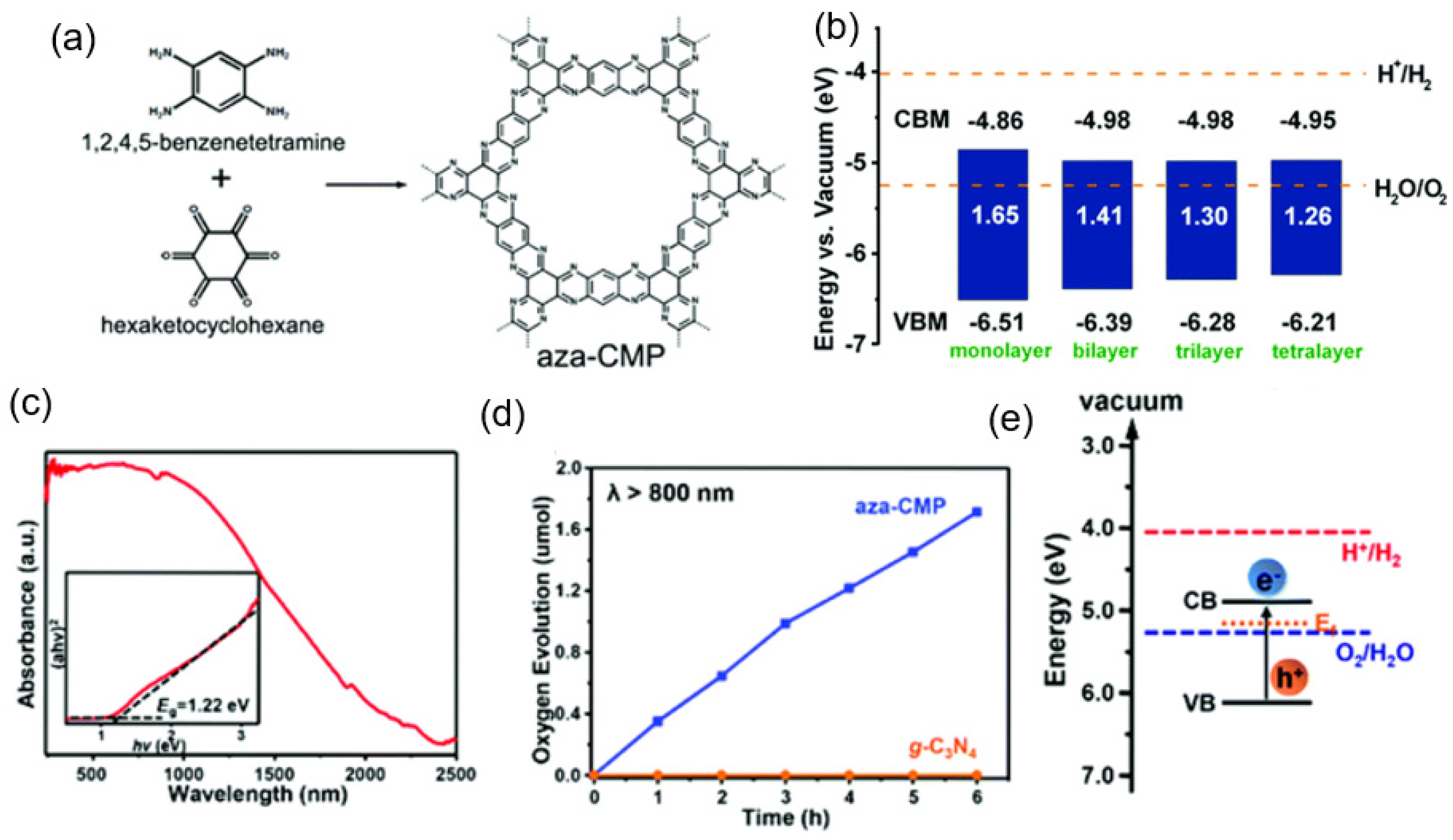
2.1.3. Conjugated Polymeric Materials
2.2. Materials with Indirect NIR Utilization
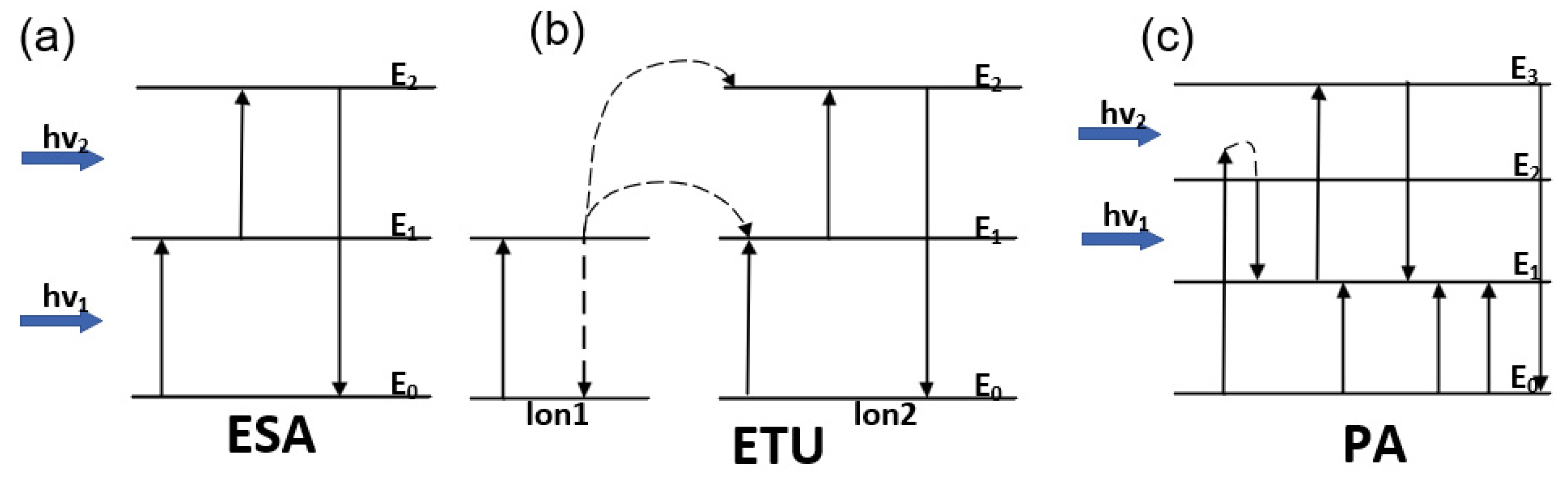
2.2.1. Up-Conversion Luminescent Materials
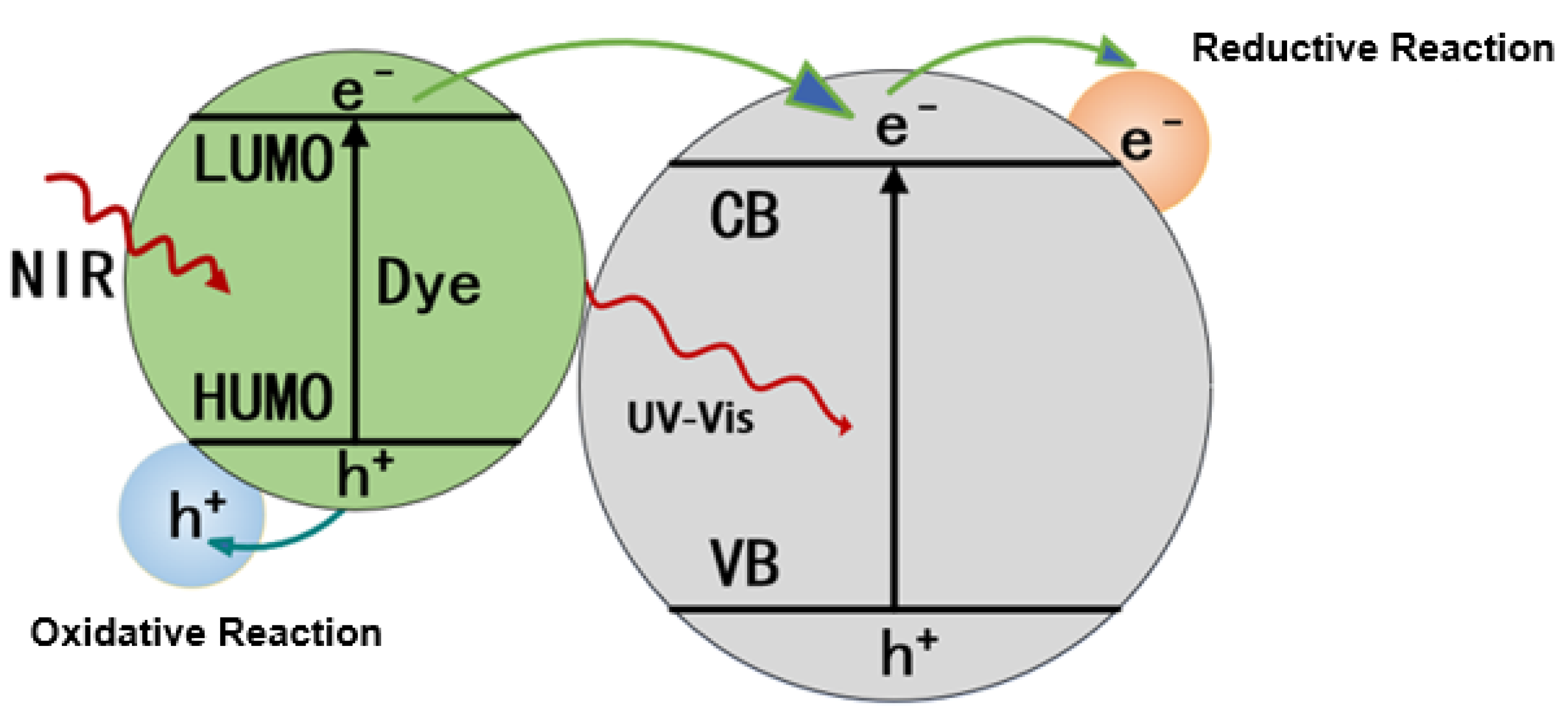
2.2.2. Photosensitization Materials
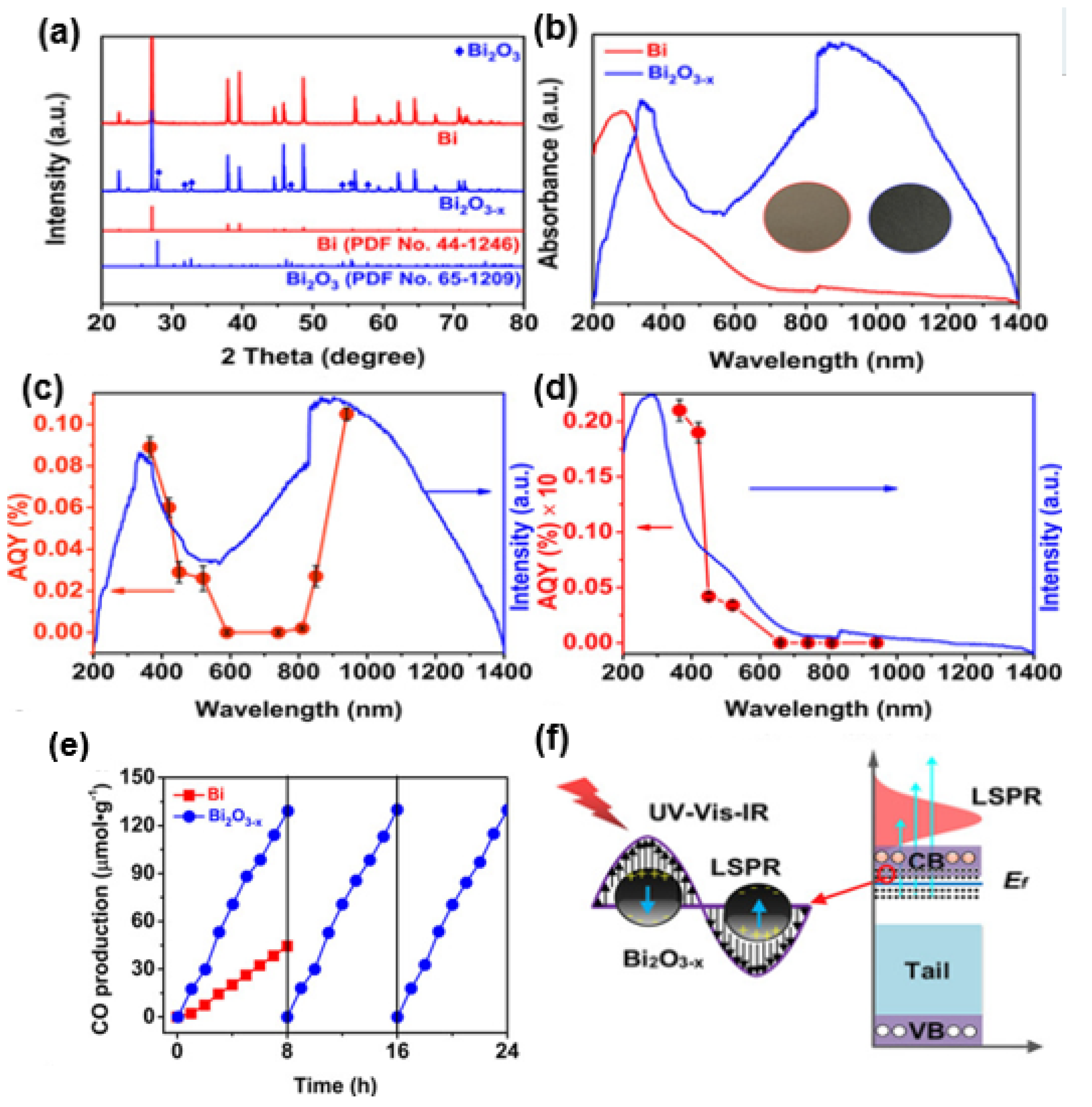
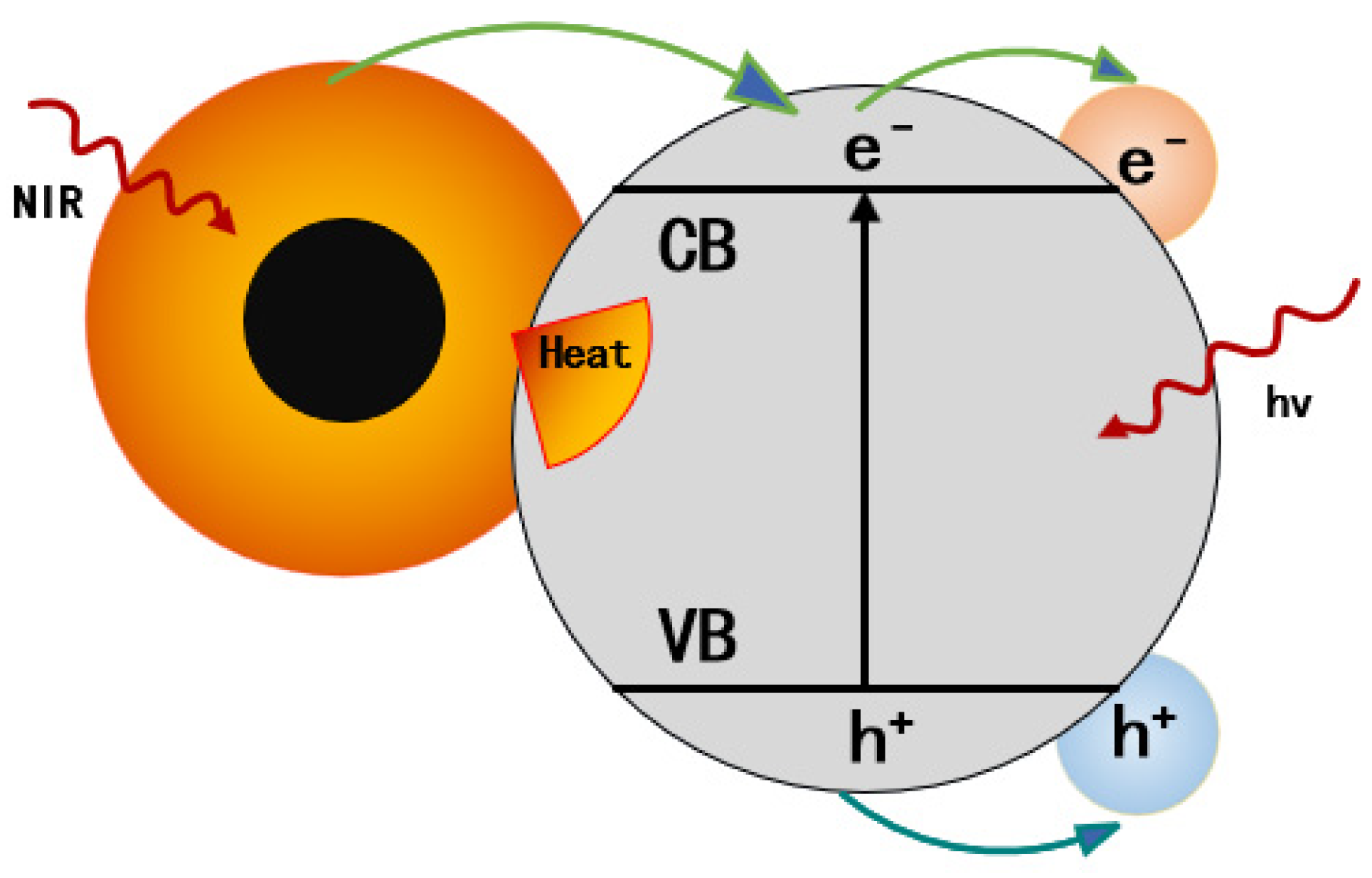
2.3. Noble Metal-Based Photothermal Conversion Materials
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sen, S.; Ganguly, S.; Das, A.; Sen, J.; Dey, S. Renewable energy scenario in India: Opportunities and challenges. J. Afr. Earth. Sci. 2016, 122, 25–31. [Google Scholar] [CrossRef]
- Kreps, B.H. The Rising Costs of Fossil-Fuel Extraction: An Energy Crisis That Will Not Go Away. Am. J. Econ. Sociol. 2020, 79, 695–717. [Google Scholar] [CrossRef]
- Maaß, H.J.; Möckel, H.O. Combined Decarbonization of Electrical Energy Generation and Production of Synthetic Fuels by Renewable Energies and Fossil Fuels. Chem. Eng. Technol. 2020, 43, 111–118. [Google Scholar] [CrossRef]
- Yitao, D.; Yujie, X. Control of selectivity in organic synthesis via heterogeneous photocatalysis under visible light. Nano Res. Energy 2022, 1, e9120006. [Google Scholar]
- CRoberts, A.; Phivilay, S.P.; Wachs, I.E. Nature of surface oxygen intermediates on TiO2 during photocatalytic splitting of water. Chin. Chem. Lett. 2018, 29, 769–772. [Google Scholar] [CrossRef]
- Chen, Q.; Cheng, X.; Long, H.; Rao, Y. A short review on recent progress of Bi/semiconductor photocatalysts: The role of Bi metal. Chin. Chem. Lett. 2020, 31, 2583–2590. [Google Scholar] [CrossRef]
- Gao, Z.; Pan, C.; Choi, C.-H.; Chang, C.-H. Continuous-Flow Photocatalytic Microfluidic-Reactor for the Treatment of Aqueous Contaminants, Simplicity, and Complexity: A Mini-Review. Symmetry 2021, 13, 1325. [Google Scholar] [CrossRef]
- Tan, H.; Kong, P.; Zhang, R.; Gao, M.; Liu, M.; Gu, X.; Liu, W.; Zheng, Z. Controllable Generation of Reactive Oxygen Species on Cyano-Group-Modified Carbon Nitride for Selective Epoxidation of Styrene. Innovation 2021, 2, 100089. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, K.; Wang, L.; Wang, L.; Fan, Z. Porphyrin-based heterogeneous photocatalysts for solar energy conversion. Chin. Chem. Lett. 2022, 33, 33–60. [Google Scholar] [CrossRef]
- Duan, M.; Hu, C.; Duan, D.; Chen, R.; Wang, C.; Wu, D.; Xia, T.; Liu, H.; Dai, Y.; Long, R.; et al. Ppm-level Cu dopant on ultrathin Pd nanosheets/TiO2 for highly enhanced photocatalytic alcoholysis of epoxides. Appl. Catal. B Environ. 2022, 307, 121211. [Google Scholar] [CrossRef]
- He, C.; Xiong, Y.; Shu, D.; Zhu, X. Enhanced Photocatalytic Efficiency of TiO2 by Combining the Modification of Ag Nanoparticles with the Application of Anodic Bias. Chin. Chem. Lett. 2003, 14, 539–542. [Google Scholar]
- Lu, Y.; Zhu, L. Topochemical polymerization of diphenyldiacetylene-based materials and the relevant application in photocatalysis. Chin. Chem. Lett. 2018, 29, 1591–1600. [Google Scholar] [CrossRef]
- Konstantinova, E.; Zaitsev, V.; Marikutsa, A.; Ilin, A. Comparative Study: Catalytic Activity and Rhodamine Dye Luminescence at the Surface of TiO2-Based Nanoheterostructures. Symmetry 2021, 13, 1758. [Google Scholar] [CrossRef]
- Janczarek, M.; Wei, Z.; Mogan, T.R.; Wang, L.; Wang, K.; Nitta, A.; Ohtani, B.; Kowalska, E. Does Symmetry Control Photocatalytic Activity of Titania-Based Photocatalysts? Symmetry 2021, 13, 1682. [Google Scholar] [CrossRef]
- Stefanov, B.I.; Blagoev, B.S.; Österlund, L.; Tzaneva, B.R.; Angelov, G.V. Effects of Anodic Aluminum Oxide Substrate Pore Geometry on the Gas-Phase Photocatalytic Activity of ZnO/Al2O3 Composites Prepared by Atomic Layer Deposition. Symmetry 2021, 13, 1456. [Google Scholar] [CrossRef]
- Wang, G.; Guo, W.; Xu, D.; Liu, D.; Qin, M. Graphene Oxide Hybridised TiO2 for Visible Light Photocatalytic Degradation of Phenol. Symmetry 2020, 12, 1420. [Google Scholar] [CrossRef]
- Dong, B.; Liu, T.; Li, C.; Zhang, F. Species, engineering and characterizations of defects in TiO2 -based photocatalyst. Chin. Chem. Lett. 2018, 29, 671–680. [Google Scholar] [CrossRef]
- You, J.; Zhao, Y.; Wang, L.; Bao, W. Recent developments in the photocatalytic applications of covalent organic frameworks: A review. J. Cleaner Prod. 2021, 291, 125822. [Google Scholar] [CrossRef]
- Yoshimizu, M.; Kobayashi, R.; Saegusa, M.; Takashima, T.; Funakubo, H.; Akiyama, K.; Matsumoto, Y.; Irie, H. Photocatalytic hydrogen evolution over beta-iron silicide under infrared-light irradiation. Chem. Commun. 2015, 51, 2818–2820. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ding, M.; Lu, C.; Ni, Y.; Xu, Z. A study on upconversion UV–vis–NIR responsive photocatalytic activity and mechanisms of hexagonal phase NaYF4:Yb3+,Tm3+@TiO2 core–shell structured photocatalyst. Appl. Catal. B Environ. 2014, 144, 379–385. [Google Scholar] [CrossRef]
- Luo, S.L.; Zhang, E.L.; Su, Y.P.; Cheng, T.M.; Shi, C.M. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.R.; Shen, S.H. Single Metal Atom Photocatalysis. Small Methods 2019, 3, 1800447. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Tian, Q.; Yao, W.; Wu, W.; Jiang, C. NIR light-activated upconversion semiconductor photocatalysts. Nanoscale Horiz. 2019, 4, 10–25. [Google Scholar] [CrossRef]
- Li, B.; Hu, Y.; Shen, Z.; Ji, Z.; Yao, L.; Zhang, S.; Zou, Y.; Tang, D.; Qing, Y.; Wang, S.; et al. Photocatalysis Driven by Near-Infrared Light: Materials Design and Engineering for Environmentally Friendly Photoreactions. ACS ES&T Eng. 2021, 1, 947–964. [Google Scholar]
- Hossain, M.I.; Nanda, S.S.; Selvan, S.T.; Yi, D.K. Recent Insights into NIR-Light-Responsive Materials for Photothermal Cell Treatments. Nanomaterials 2022, 12, 3318. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Hong, Z.L. Synthesis of lanthanide-doped NaYF4@TiO2 core-shell composites with highly crystalline and tunable TiO2 shells under mild conditions and their upconversion-based photocatalysis. Nanoscale 2013, 5, 8930–8933. [Google Scholar] [CrossRef]
- Zhang, X.H.; Yu, L.J.; Zhuang, C.S.; Peng, T.Y.; Li, R.J.; Li, X.G. Highly efficient visible/near-IR-light-driven photocatalytic H2 production over asymmetric phthalocyanine-sensitized TiO2. RSC Adv. 2013, 3, 14363–14370. [Google Scholar] [CrossRef]
- Jiang, W.; Bai, S.; Wang, L.; Wang, X.; Yang, L.; Li, Y.; Liu, D.; Wang, X.; Li, Z.; Jiang, J.; et al. Integration of Multiple Plasmonic and Co-Catalyst Nanostructures on TiO2 Nanosheets for Visible-Near-Infrared Photocatalytic Hydrogen Evolution. Small 2016, 12, 1640–1648. [Google Scholar] [CrossRef]
- Huang, W.; Wu, Z.; Tang, J.; Wei, W.D.; Guo, X. Surface chemistry connecting heterogeneous catalysis, photocatalysis and plasmonic catalysis. Chin. Chem. Lett. 2018, 29, 725–726. [Google Scholar] [CrossRef]
- Wu, S.; Blinco, J.P.; Barner-Kowollik, C. Near-Infrared Photoinduced Reactions Assisted by Upconverting Nanoparticles. Chem. Eur. J. 2017, 23, 8325–8332. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, H.; Cheng, B.; Fan, J.; Yu, J.; Ho, W. Near-Infrared-Responsive Photocatalysts. Small Methods 2021, 5, 2001042. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Cheng, Q.; Dou, S.X.; Du, Y. Near-Infrared-Driven Photocatalysts: Design, Construction, and Applications. Small 2021, 17, 1904107. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Yao, L.; Yang, S.; Cui, Z.; Wei, B.; Cao, M.; Hu, C. Band Gap Engineering of In2TiO5 for H2 Production under Near-infrared Light. ACS Appl. Mater. Interfaces 2015, 7, 20761–20768. [Google Scholar] [CrossRef] [PubMed]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170, 90–123. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Li, L.; Zhuge, Z.; Chen, P.; Li, C.; Gong, Y.; Niu, L.; Liu, J.; Lei, L.; et al. Cu2In2ZnS5/Gd2O2S:Tb for full solar spectrum photoreduction of Cr(VI) and CO2 from UV/vis to near-infrared light. Appl. Catal. B Environ. 2019, 249, 82–90. [Google Scholar] [CrossRef]
- Bai, L.; Jiang, W.; Gao, C.; Zhong, S.; Zhao, L.; Li, Z.; Bai, S. Facet engineered interface design of NaYF4:Yb,Tm upconversion nanocrystals on BiOCl nanoplates for enhanced near-infrared photocatalysis. Nanoscale 2016, 8, 19014–19024. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Liu, H.; Umar, A. Photocatalysis from UV/Vis to Near-Infrared Light: Towards Full Solar-Light Spectrum Activity. ChemCatChem 2015, 7, 559–573. [Google Scholar] [CrossRef]
- Patwari, J.; Chatterjee, A.; Sardar, S.; Lemmens, P.; Pal, S.K. Ultrafast dynamics in co-sensitized photocatalysts under visible and NIR light irradiation. Phys. Chem. Chem. Phys. 2018, 20, 10418–10429. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Menichetti, A.; Guernelli, M.; Montalti, M. Extending photocatalysis to the visible and NIR: The molecular strategy. Nanoscale 2021, 13, 9147–9159. [Google Scholar] [CrossRef]
- Jaque, D.; Maestro, L.M.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Rodriguez, E.M.; Sole, J.G. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Wang, W.K.; Xu, D.F.; Cheng, B.; Yu, J.G.; Jiang, C.J. Hybrid carbon@TiO2 hollow spheres with enhanced photocatalytic CO2 reduction activity. J. Mater. Chem. A 2017, 5, 5020–5029. [Google Scholar] [CrossRef]
- Yang, H.; He, L.-Q.; Hu, Y.-W.; Lu, X.; Li, G.-R.; Liu, B.; Ren, B.; Tong, Y.; Fang, P.-P. Quantitative Detection of Photothermal and Photoelectrocatalytic Effects Induced by SPR from Au@Pt Nanoparticles. Angew. Chem. Int. Ed. 2015, 54, 11462–11466. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, S.; Yang, J.; Wang, H.; Yu, H.; Chen, H.; Zhao, Y.; Yuan, X.; Chu, W.; Li, H. Near-Infrared Light Responsive TiO2 for Efficient Solar Energy Utilization. Adv. Funct. Mater. 2021, 32, 2108977. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Yao, L.; Deng, L.; Bowen, C.; Zhang, Y.; Chen, S.; Lin, Z.; Peng, F.; Zhang, P. Recent advances in metal sulfides: From controlled fabrication to electrocatalytic, photocatalytic and photoelectrochemical water splitting and beyond. Chem. Soc. Rev. 2019, 48, 4178–4280. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, Z.M.; Yue, X.N.; Yuan, S.J.; Lu, H.F.; Liang, B. Photocatalytic performance of Ag2S under irradiation with visible and near-infrared light and its mechanism of degradation. RSC Adv. 2015, 5, 24064–24071. [Google Scholar] [CrossRef]
- Aslam, M.; Ismail, I.M.I.; Chandrasekaran, S.; Qari, H.A.; Hameed, A. How the Dyes Are Degraded/Mineralized in a Photocatalytic System? The Possible Role of Auxochromes. Water Air Soil Pollut. 2015, 226, 70. [Google Scholar] [CrossRef]
- Rodríguez-Peña, M.; Pérez, J.A.B.; Llanos, J.; Sáez, C.; Rodrigo, M.A.; Barrera-Díaz, C.E. New insights about the electrochemical production of ozone. Curr. Opin. Electrochem. 2021, 27, 100697. [Google Scholar] [CrossRef]
- Sang, Y.H.; Zhao, Z.H.; Zhao, M.W.; Hao, P.; Leng, Y.H.; Liu, H. From UV to Near-Infrared, WS2 Nanosheet: A Novel Photocatalyst for Full Solar Light Spectrum Photodegradation. Adv. Mater. 2015, 27, 363–369. [Google Scholar] [CrossRef]
- Wang, W.; Xie, G.; Luo, J. Black phosphorus as a new lubricant. Friction 2018, 6, 116–142. [Google Scholar] [CrossRef] [Green Version]
- Castellanos-Gomez, A. Black Phosphorus: Narrow Gap, Wide Applications. J. Phys. Chem. Lett. 2015, 6, 4280–4291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhang, Y.; Huang, H. Black phosphorus-based heterostructures for photocatalysis and photoelectrochemical water splitting. J. Energy Chem. 2022, 67, 745–779. [Google Scholar] [CrossRef]
- Zhu, M.S.; Kim, S.; Mao, L.; Fujitsuka, M.; Zhang, J.Y.; Wang, X.C.; Majima, T. Metal-Free Photocatalyst for H2 Evolution in Visible to Near-Infrared Region: Black Phosphorus/Graphitic Carbon Nitride. J. Am. Chem. Soc. 2017, 139, 13234–13242. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.A. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen Vacancy Induced Band-Gap Narrowing and Enhanced Visible Light Photocatalytic Activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef]
- Guan, M.; Xiao, C.; Zhang, J.; Fan, S.; An, R.; Cheng, Q.; Xie, J.; Zhou, M.; Ye, B.; Xie, Y. Vacancy Associates Promoting Solar-Driven Photocatalytic Activity of Ultrathin Bismuth Oxychloride Nanosheets. J. Am. Chem. Soc. 2013, 135, 10411–10417. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Shi, R.; Wang, B.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Tuning Oxygen Vacancies in Ultrathin TiO2 Nanosheets to Boost Photocatalytic Nitrogen Fixation up to 700 nm. Adv. Mater. 2019, 31, 1806482. [Google Scholar] [CrossRef]
- Kong, X.Y.; Choo, Y.Y.; Chai, S.P.; Soh, A.K.; Mohamed, A.R. Oxygen vacancy induced Bi2WO6 for the realization of photocatalytic CO2 reduction over the full solar spectrum: From the UV to the NIR region. Chem. Commun. 2016, 52, 14242–14245. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.; Yu, G.; Jiang, H.; Mu, X.; Liu, H. A Bi2WO6-Based Hybrid Photocatalyst with Broad Spectrum Photocatalytic Properties under UV, Visible, and Near-Infrared Irradiation. Adv. Mater. 2013, 25, 5075–5080. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Pan, W.; Zhang, G.; Chen, H. Vacancy-Rich Monolayer BiO2-x as a Highly Efficient UV, Visible, and Near-Infrared Responsive Photocatalyst. Angew. Chem. Int. Ed. 2018, 57, 491–495. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, K.; Wang, G.; Deng, K.; Tang, D. Study on the shape control and photocatalytic activity of high-energy anatase titania. Appl. Catal. B Environ. 2010, 100, 378–385. [Google Scholar] [CrossRef]
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet Effect of Single-Crystalline Ag3PO4 Sub-microcrystals on Photocatalytic Properties. J. Am. Chem. Soc. 2011, 133, 6490–6492. [Google Scholar] [CrossRef]
- Bai, J.; Sun, J.; Zhu, X.; Liu, J.; Zhang, H.; Yin, X.-B.; Liu, L. Enhancement of Solar-Driven Photocatalytic Activity of BiOI Nanosheets through Predominant Exposed High Energy Facets and Vacancy Engineering. Small 2020, 16, 1904783. [Google Scholar] [CrossRef]
- Li, Y.; Wen, M.; Wang, Y.; Tian, G.; Wang, C.; Zhao, J. Plasmonic Hot Electrons from Oxygen Vacancies for Infrared Light-Driven Catalytic CO2 Reduction on Bi2O3-x. Angew. Chem. Int. Ed. 2021, 60, 910–916. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Ma, Q.; Zhang, Q.; Bai, H.; Yi, W.; Liu, J.; Han, J.; Xi, G. A metallic molybdenum dioxide with high stability for surface enhanced Raman spectroscopy. Nat. Commun. 2017, 8, 14903. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, Z.; Fang, Y.; Liu, B.; Huang, J.; Miao, F.; Bao, Y.; Dong, B. IR-Driven strong plasmonic-coupling on Ag nanorices/W18O49 nanowires heterostructures for photo/thermal synergistic enhancement of H2 evolution from ammonia borane. Appl. Catal. B Environ. 2019, 252, 164–173. [Google Scholar] [CrossRef]
- Zhang, X.H.; Peng, T.Y.; Song, S.S. Recent advances in dye-sensitized semiconductor systems for photocatalytic hydrogen production. J. Mater. Chem. A 2016, 4, 2365–2402. [Google Scholar] [CrossRef]
- Park, J. Visible and near infrared light active photocatalysis based on conjugated polymers. J. Ind. Eng. Chem. 2017, 51, 27–43. [Google Scholar] [CrossRef]
- Dai, C.; Liu, B. Conjugated polymers for visible-light-driven photocatalysis. Energy Environ. Sci. 2020, 13, 24–52. [Google Scholar] [CrossRef]
- Li, L.; Lo, W.-y.; Cai, Z.; Zhang, N.; Yu, L. Donor–Acceptor Porous Conjugated Polymers for Photocatalytic Hydrogen Production: The Importance of Acceptor Comonomer. Macromolecules 2016, 49, 6903–6909. [Google Scholar] [CrossRef]
- Xu, Y.; Mao, N.; Zhang, C.; Wang, X.; Zeng, J.; Chen, Y.; Wang, F.; Jiang, J.-X. Rational design of donor-π-acceptor conjugated microporous polymers for photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 228, 1–9. [Google Scholar] [CrossRef]
- Shu, C.; Han, C.; Yang, X.; Zhang, C.; Chen, Y.; Ren, S.; Wang, F.; Huang, F.; Jiang, J.-X. Boosting the Photocatalytic Hydrogen Evolution Activity for D-π-A Conjugated Microporous Polymers by Statistical Copolymerization. Adv. Mater. 2021, 33, 2008498. [Google Scholar] [CrossRef]
- Wang, L.; Wan, Y.; Ding, Y.; Niu, Y.; Xiong, Y.; Wu, X.; Xu, H. Photocatalytic oxygen evolution from low-bandgap conjugated microporous polymer nanosheets: A combined first-principles calculation and experimental study. Nanoscale 2017, 9, 4090–4096. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.-A.; Wang, X. Conjugated Polymers: Catalysts for Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727. [Google Scholar] [CrossRef]
- He, Y.L.; Cao, Y.Y.; Wang, Y.P. Progress on Photothermal Conversion in the Second NIR Window Based on Conjugated Polymers. Asian J. Org. Chem. 2018, 7, 2201–2212. [Google Scholar] [CrossRef]
- Luciani, G.; Imparato, C.; Vitiello, G. Photosensitive Hybrid Nanostructured Materials: The Big Challenges for Sunlight Capture. Catalysts 2020, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Radhika, S.; Thomas, J. Solar light driven photocatalytic degradation of organic pollutants using ZnO nanorods coupled with photosensitive molecules. J. Environ. Chem. Eng. 2017, 5, 4239–4250. [Google Scholar]
- Mateo, D.; Cerrillo, J.L.; Durini, S.; Gascon, J. Fundamentals and applications of photo-thermal catalysis. Chem. Soc. Rev. 2021, 50, 2173–2210. [Google Scholar] [CrossRef]
- Zheng, B.; Zhong, D.; Xie, T.; Zhou, J.; Li, W.; Ilyas, A.; Lu, Y.; Zhou, M.; Deng, R. Near-infrared photosensitization via direct triplet energy transfer from lanthanide nanoparticles. Chem 2021, 7, 1615–1625. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, Z.; Yin, Y.; Sun, Y.; Liu, S. Progress in mechanochromic luminescence of gold(I) complexes. Chin. Chem. Lett. 2021, 32, 3718–3732. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, M.; Yu, C.; Li, X.; Yang, K.; Yang, S.; Dai, W.; Huang, W.; Fan, Q.; Zhu, L. High value-added fluorescence upconversion agents-assisted nano-semiconductors for efficient wide spectral response photocatalysis: Exerting energy transfer effect and applications. J. Rare Earths 2021, 39, 243–260. [Google Scholar] [CrossRef]
- Acosta-Mora, P.; Domen, K.; Hisatomi, T.; Lyu, H.; Méndez-Ramos, J.; Ruiz-Morales, J.C.; Khaidukov, N.M. Shifting the NIR into the UV-blue: Up-conversion boosted photocatalysis. Opt. Mater. 2018, 83, 315–320. [Google Scholar] [CrossRef]
- Liu, F.; Meng, J.; Xu, D.; Yu, W.; Dong, X. Highly efficient NIR responsive and upconversion enhanced BiVO4:Er3+ nanofibers for photocatalytic degradation of RhB. In Proceedings of the 2021 IEEE International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale (3M-NANO), Xian, China, 2–6 August 2021; pp. 373–377. [Google Scholar]
- Naing, H.H.; Wang, K.; Tun, P.P.; Zhang, G. Enhanced broad spectrum (vis-NIR) responsive photocatalytic performance of Ag2O/rectorite nanoarchitectures. Appl. Surf. Sci. 2019, 491, 216–224. [Google Scholar] [CrossRef]
- Chatti, M.; Adusumalli, V.N.; Ganguli, S.; Mahalingam, V. Near-infrared light triggered superior photocatalytic activity from MoS2-NaYF4:Yb(3+)/Er(3+) nanocomposites. Dalton Trans. 2016, 45, 12384–12392. [Google Scholar] [CrossRef]
- Xiong, L.; Tang, J. Strategies and Challenges on Selectivity of Photocatalytic Oxidation of Organic Substances. Adv. Energy Mater. 2021, 11, 2003216. [Google Scholar] [CrossRef]
- Shafiee, A.; Rabiee, N.; Ahmadi, S.; Baneshi, M.; Khatami, M.; Iravani, S.; Varma, R.S. Core-Shell Nanophotocatalysts: Review of Materials and Applications. ACS Appl. Nano Mater. 2022, 5, 55–86. [Google Scholar] [CrossRef]
- Duonghong, D.; Borgarello, E.; Graetzel, M. Dynamics of light-induced water cleavage in colloidal systems. J. Am. Chem. Soc. 1981, 103, 4685–4690. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Bai, J.W.; Li, T.R.; Yang, Y.M.; Peng, Y.; Wang, B.D. Gram-scale synthesis of aligned C3N4-polypyrrole heterojunction aerogels with tunable band structures as efficient visible and near infrared light-driven metal-free photocatalysts. J. Mater. Chem. A 2017, 5, 24920–24928. [Google Scholar] [CrossRef]
- Sa, B.S.; Li, Y.L.; Qi, J.S.; Ahuja, R.; Sun, Z.M. Strain Engineering for Phosphorene: The Potential Application as a Photocatalyst. J. Phys. Chem. C 2014, 118, 26560–26568. [Google Scholar] [CrossRef] [Green Version]
- Takanabe, K.; Kamata, K.; Wang, X.C.; Antonietti, M.; Kubota, J.; Domen, K. Photocatalytic hydrogen evolution on dye-sensitized mesoporous carbon nitride photocatalyst with magnesium phthalocyanine. Phys. Chem. Chem. Phys. 2010, 12, 13020–13025. [Google Scholar] [CrossRef]
- Zhang, X.H.; Yu, L.J.; Zhuang, C.S.; Peng, T.Y.; Li, R.J.; Li, X.G. Highly Asymmetric Phthalocyanine as a Sensitizer of Graphitic Carbon Nitride for Extremely Efficient Photocatalytic H2 Production under Near-Infrared Light. ACS Catal. 2014, 4, 162–170. [Google Scholar] [CrossRef]
- Li, D.S.; Huang, Y.; Li, S.M.; Wang, C.H.; Li, Y.Y.; Zhang, X.T.; Liu, Y.C. Thermal coupled photoconductivity as a tool to understand the photothermal catalytic reduction of CO2. Chin. J. Catal. 2020, 41, 154–160. [Google Scholar] [CrossRef]
- Rej, S.; Mascaretti, L.; Santiago, E.Y.; Tomanec, O.; Kment, Š.; Wang, Z.; Zbořil, R.; Fornasiero, P.; Govorov, A.O.; Naldoni, A. Determining Plasmonic Hot Electrons and Photothermal Effects during H2 Evolution with TiN-Pt Nanohybrids. ACS Catal. 2020, 10, 5261–5271. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Yin, Z.; Xiao, D.; Ma, D. Principles and applications of photothermal catalysis. Chem Catal. 2022, 2, 52–83. [Google Scholar] [CrossRef]
- Sarina, S.; Zhu, H.-Y.; Xiao, Q.; Jaatinen, E.; Jia, J.; Huang, Y.; Zheng, Z.; Wu, H. Viable Photocatalysts under Solar-Spectrum Irradiation: Nonplasmonic Metal Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 2935–2940. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Nanogold plasmonic photocatalysis for organic synthesis and clean energy conversion. Chem. Soc. Rev. 2014, 43, 7188–7216. [Google Scholar] [CrossRef] [Green Version]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, W.Z.; Gao, E.P.; Sun, S.M.; Zhang, L. Photocatalysis Coupled with Thermal Effect Induced by SPR on Ag-Loaded Bi2WO6 with Enhanced Photocatalytic Activity. J. Phys. Chem. C 2012, 116, 25898–25903. [Google Scholar] [CrossRef]
- Hu, X.; Tian, J.; Xue, Y.; Li, Y.; Cui, H. Bi2WO6 Nanosheets Decorated with Au Nanorods for Enhanced Near-Infrared Photocatalytic Properties Based on Surface Plasmon Resonance Effects and Wide-Range Near-Infrared Light Harvesting. ChemCatChem 2017, 9, 1511–1516. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, N.; Xi, M.; Li, X.; Zheng, J.; Qian, L.; Dai, Y.; Song, X.; Hu, S. Recent Developments in Heterogeneous Photocatalysts with Near-Infrared Response. Symmetry 2022, 14, 2107. https://doi.org/10.3390/sym14102107
Cao N, Xi M, Li X, Zheng J, Qian L, Dai Y, Song X, Hu S. Recent Developments in Heterogeneous Photocatalysts with Near-Infrared Response. Symmetry. 2022; 14(10):2107. https://doi.org/10.3390/sym14102107
Chicago/Turabian StyleCao, Nan, Meilan Xi, Xiaoli Li, Jinfang Zheng, Limei Qian, Yitao Dai, Xizhong Song, and Shengliang Hu. 2022. "Recent Developments in Heterogeneous Photocatalysts with Near-Infrared Response" Symmetry 14, no. 10: 2107. https://doi.org/10.3390/sym14102107





