The Prospects of Utilizing Geometrical Visual Illusions as Tools for Neuroscience
Abstract
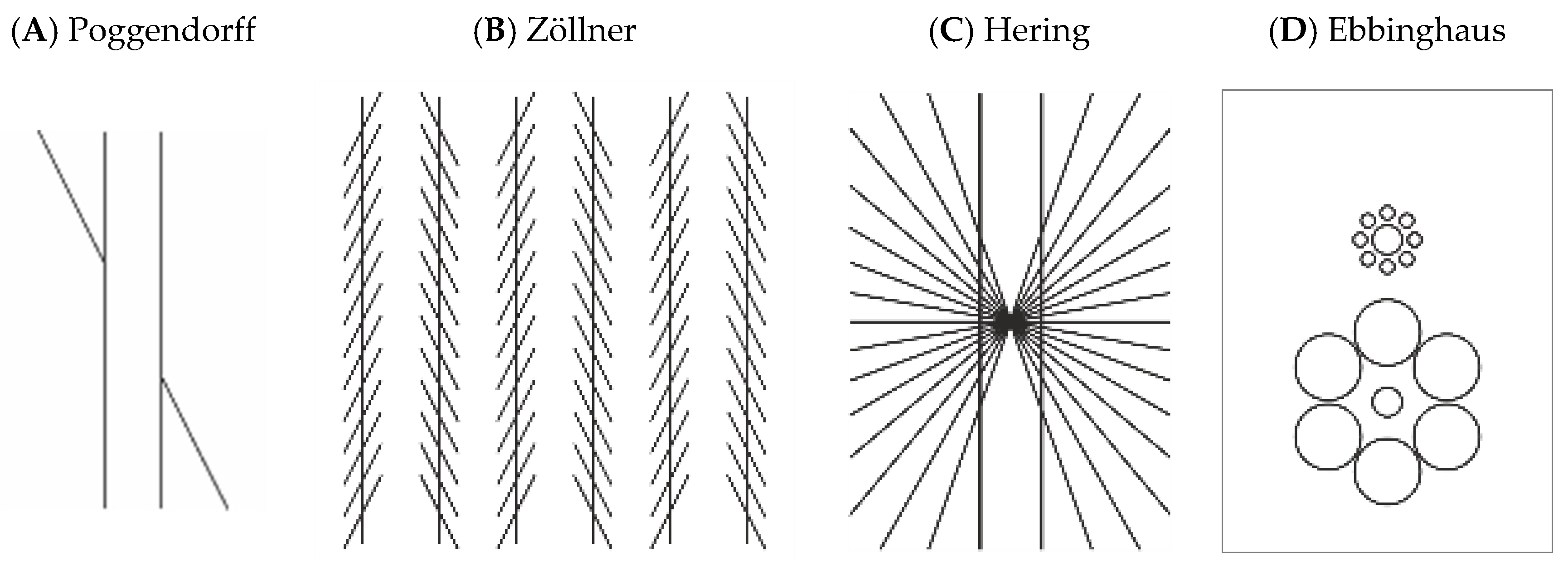
1. Introduction
2. Psychophysics
2.1. The T-Illusion
2.1.1. Ts in Contexts
2.1.2. Modified Ts
2.1.3. Interactions of Different Illusions within a Single Illusion Figure
2.2. Other Illusion Figures
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eagleman, D.M. Visual illusions and neurobiology. Nat. Rev. Neurosci. 2001, 2, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, K. Visual Illusions Based on Processes: New Classification System Needed. Perception 2016, 45, 588–595. [Google Scholar] [CrossRef]
- Fermüller, C.; Malm, H. Uncertainty in visual processes predicts geometrical optical illusions. Vis. Res. 2004, 44, 727–749. [Google Scholar] [CrossRef]
- Franceschiello, B.; Sarti, A.; Citti, G. A neuromathematical model for geometrical optical illusions. J. Math. Imaging Vis. 2018, 60, 94–108. [Google Scholar] [CrossRef]
- Axelrod, V.; Schwarzkopf, D.S.; Gilaie-Dotan, S.; Rees, G. Perceptual similarity and the neural correlates of geometrical illusions in human brain structure. Sci. Rep. 2017, 7, 39968. [Google Scholar] [CrossRef]
- Ward, L.M.; Porac, C.; Coren, S.; Girgus, J.S. The Case for Misapplied Constancy Scaling: Depth Associations Elicited by Illusion Configurations. Am. J. Psychol. 1977, 90, 609–620. [Google Scholar] [CrossRef]
- Coren, S.; Girgus, J.S.; Erlichman, H.; Hakstian, A.R. An empirical taxonomy of visual illusions. Percept. Psychophys. 1976, 20, 129–137. [Google Scholar] [CrossRef]
- Macmillan, N.A.; Creelman, C.D. Detection Theory: A User’s Guide, 2nd ed.; Erlbaum: Mahwah, NJ, USA, 2005. [Google Scholar]
- Morgan, M.J.; Hole, G.J.; Glennerster, A. Biases and sensitivities in geometrical illusions. Vis. Res. 1990, 30, 1793–1810. [Google Scholar] [CrossRef]
- Urban, F.M. The Application of Statistical Methods to the Problems of Psychophysics; The Psychological Clinic Press: Philadelphia, PA, USA, 1908. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- World Medical Association (1964/2013). WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: http://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects (accessed on 3 January 2022).
- Pastore, N. Selective History of Theories of Visual Perception: 1650–1950; Oxford University Press: New York, NY, USA, 1971. [Google Scholar]
- Schumann, F. Beiträge zur Analyse der Gesichtswahrnehmungen. Zweite Abhandlung. Zur Schätzung räumlicher Größen Contributions to the analysis of visual perceptions. Second treatise. On the estimation of spatial extents. Z. Psychol. Physiol. Sinnesorgane 1900, 24, 1–33. [Google Scholar]
- Titchener, E.B. Experimental Psychology; Macmillan: New York, NY, USA, 1901; Volume 1. [Google Scholar]
- Finger, F.W.; Spelt, D.K. The illustration of the horizontal-vertical illusion. J. Exp. Psychol. 1947, 37, 243–250. [Google Scholar] [CrossRef]
- Künnapas, T.M. An analysis of the “vertical-horizontal illusion”. J. Exp. Psychol. 1955, 49, 134–140. [Google Scholar] [CrossRef]
- Cormack, E.O.; Cormack, R.H. Stimulus configuration and line orientation in the horizontal-vertical illusion. Percept. Psychophys. 1974, 16, 208–212. [Google Scholar] [CrossRef]
- Landwehr, K. Titchener’s ⊥ in context 1—Delimited, discrete monomotif patterns, line arrangements, and branching patterns. Atten. Percept. Psychophys. 2016, 78, 278–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ungerleider, L.G.; Mishkin, M. Two cortical visual systems. In Analysis of Visual Behavior; Ingle, D.J., Goodale, M.A., Mansfield, R.J.W., Eds.; MIT Press: Cambridge, MA, USA, 1982; pp. 549–586. [Google Scholar]
- Vishton, P.M.; Rea, J.G.; Cutting, J.E.; Nuñez, L.N. Comparing effects of the horizontal-vertical illusion on grip scaling and judgment: Relative versus absolute, not perception versus action. J. Exp. Psychol. Hum. Percept. Perform. 1999, 25, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Smeets, J.B.J.; Brenner, E. A New View on Grasping. Mot. Control 1999, 3, 237–271. [Google Scholar] [CrossRef]
- Landwehr, K. Visual and visually mediated haptic illusions with Titchener’s ⊥. Atten. Percept. Psychophys. 2014, 76, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, K. Titchener’s ⊥ dissected. Atten. Percept. Psychophys. 2015, 77, 2145–2152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, C.H.C.; Pallier, C.; Wu, D.H.; Nakamura, K.; Jobert, A.; Kuo, W.-J.; Dehaene, S. Adaptation of the human visual system to the statistics of letters and line configurations. NeuroImage 2015, 120, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, K. The ⊥-Illusion Is Not a T-Illusion. Symmetry 2020, 12, 1330. [Google Scholar] [CrossRef]
- Changizi, M.A.; Zhang, Q.; Ye, H.; Shimojo, S. The Structures of Letters and Symbols throughout Human History Are Selected to Match Those Found in Objects in Natural Scenes. Am. Nat. 2006, 167, E117–E139. [Google Scholar] [CrossRef]
- Shevelev, I.A.; Lazareva, N.A.; Novikova, R.V.; Tikhomirov, A.S.; Sharaev, G.A.; Cuckiridze, D.Y. Tuning to Y-like figures in the cat striate neurons. Brain Res. Bull. 2001, 54, 543–551. [Google Scholar] [CrossRef]
- Hubel, D.H.; Wiesel, T.N. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. 1962, 160, 106–154. [Google Scholar] [CrossRef] [PubMed]
- Hubel, D.H.; Wiesel, T.N. Receptive fields and functional architecture in two non-striate visual areas (18 and 19) of the cat. J. Neurophysiol. 1965, 28, 229–289. [Google Scholar] [CrossRef]
- Wurtz, R.H. Recounting the impact of Hubel and Wiesel. J. Physiol. 2009, 587, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- Priebe, N.J.; Ferster, D. Mechanisms of Neuronal Computation in Mammalian Visual Cortex. Neuron 2012, 75, 194–208. [Google Scholar] [CrossRef]
- Gilbert, C.D.; Wiesel, T.N. The influence of contextual stimuli on the orientation selectivity of cells in primary visual cortex of the cat. Vis. Res. 1990, 30, 1689–1701. [Google Scholar] [CrossRef]
- Joo, S.J.; Boynton, G.M.; Murray, S.O. Long-range, pattern-dependent contextual effects in early human visual cortex. Curr. Biol. 2012, 22, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, K. Titchener’s ⊥ with its lines tilted—A partial replication and extension of Cormack and Cormack (1974). Atten. Percept. Psychophys. 2017, 79, 223–229. [Google Scholar] [CrossRef]
- Fisher, G.H. Gradients of Distortion Seen in the Context of the Ponzo Illusion and Other Contours. Q. J. Exp. Psychol. 1968, 20, 212–217. [Google Scholar] [CrossRef]
- Jaeger, T. Ebbinghaus illusions: Size contrast or contour interaction phenomena? Percept. Psychophys. 1978, 24, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Todorović, D.; Jovanović, L. Is the Ebbinghaus illusion a size contrast illusion? Acta Psychol. 2018, 185, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Pollack, R.H.; Jaeger, T.B. The effect of lightness contrast on the colored Müller-Lyer illusion. Percept. Psychophys. 1991, 50, 225–229. [Google Scholar] [CrossRef]
- Hamburger, K.; Hansen, T.; Gegenfurtner, K.R. Geometric-optical illusions at isoluminance. Vis. Res. 2007, 47, 2361–2380. [Google Scholar] [CrossRef]
- Morgan, M.J. The Poggendorff illusion: A bias in the estimation of the orientation of virtual lines by second-stage filters. Vis. Res. 1999, 39, 2361–2380. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landwehr, K. The Prospects of Utilizing Geometrical Visual Illusions as Tools for Neuroscience. Symmetry 2022, 14, 1687. https://doi.org/10.3390/sym14081687
Landwehr K. The Prospects of Utilizing Geometrical Visual Illusions as Tools for Neuroscience. Symmetry. 2022; 14(8):1687. https://doi.org/10.3390/sym14081687
Chicago/Turabian StyleLandwehr, Klaus. 2022. "The Prospects of Utilizing Geometrical Visual Illusions as Tools for Neuroscience" Symmetry 14, no. 8: 1687. https://doi.org/10.3390/sym14081687
APA StyleLandwehr, K. (2022). The Prospects of Utilizing Geometrical Visual Illusions as Tools for Neuroscience. Symmetry, 14(8), 1687. https://doi.org/10.3390/sym14081687





