Catalytic Asymmetric Synthesis of C-Chiral Phosphonates
Abstract
:1. Introduction
2. Methods for the Synthesis of Chiral Phosphonates
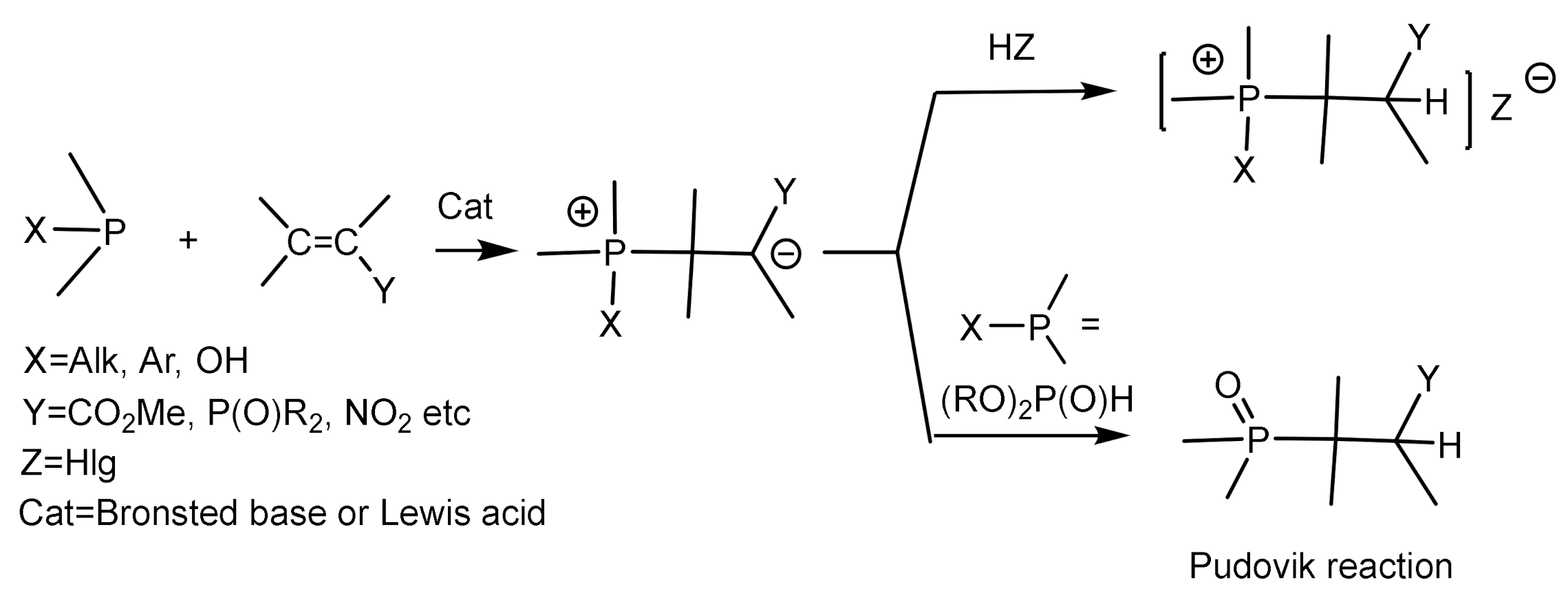
2.1. Addition of Phosphorus Nucleophiles to C=X Compounds
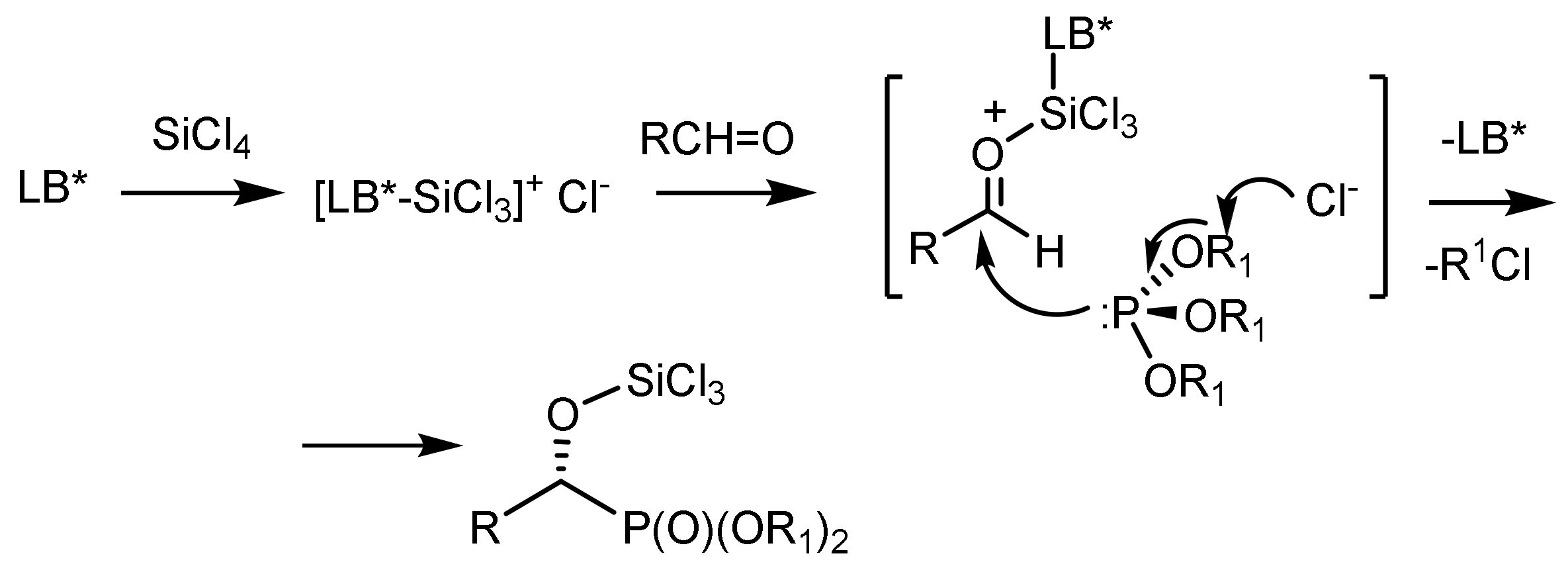
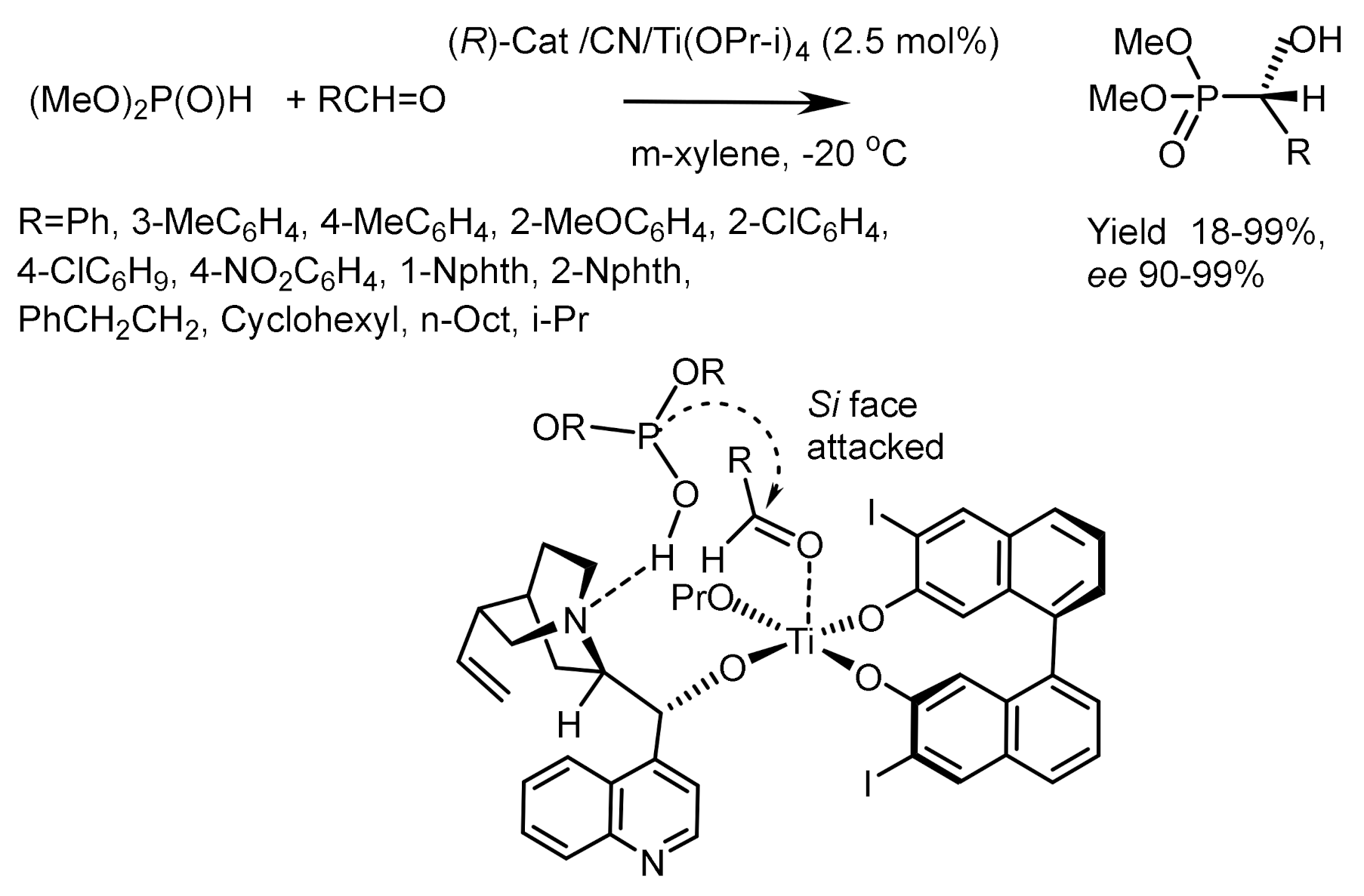
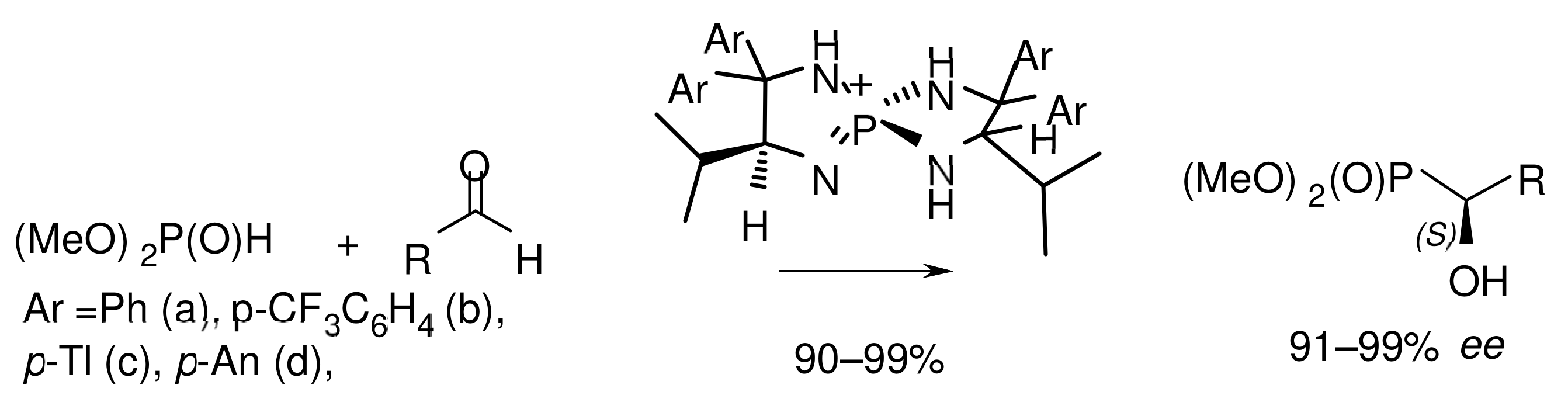
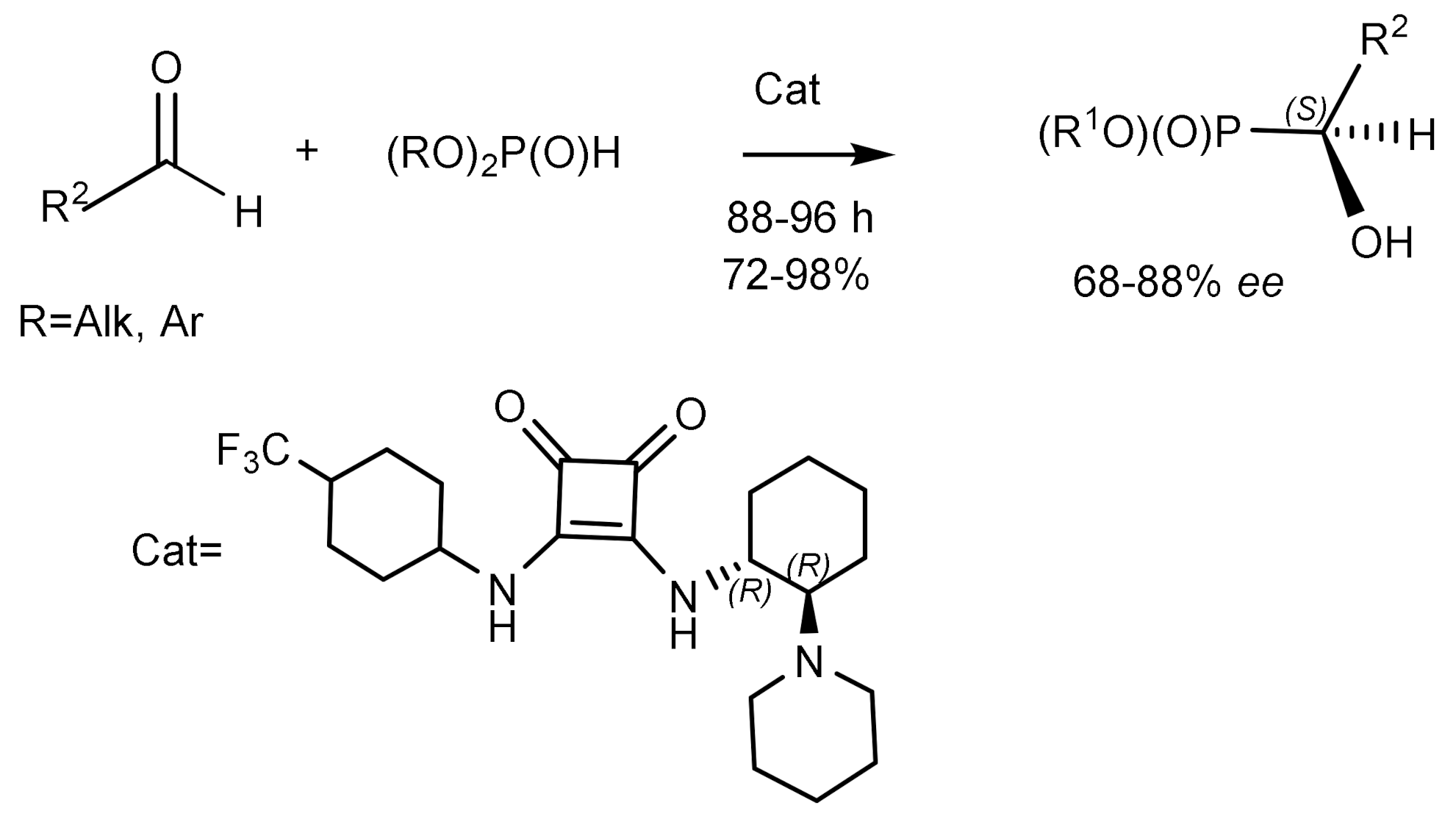
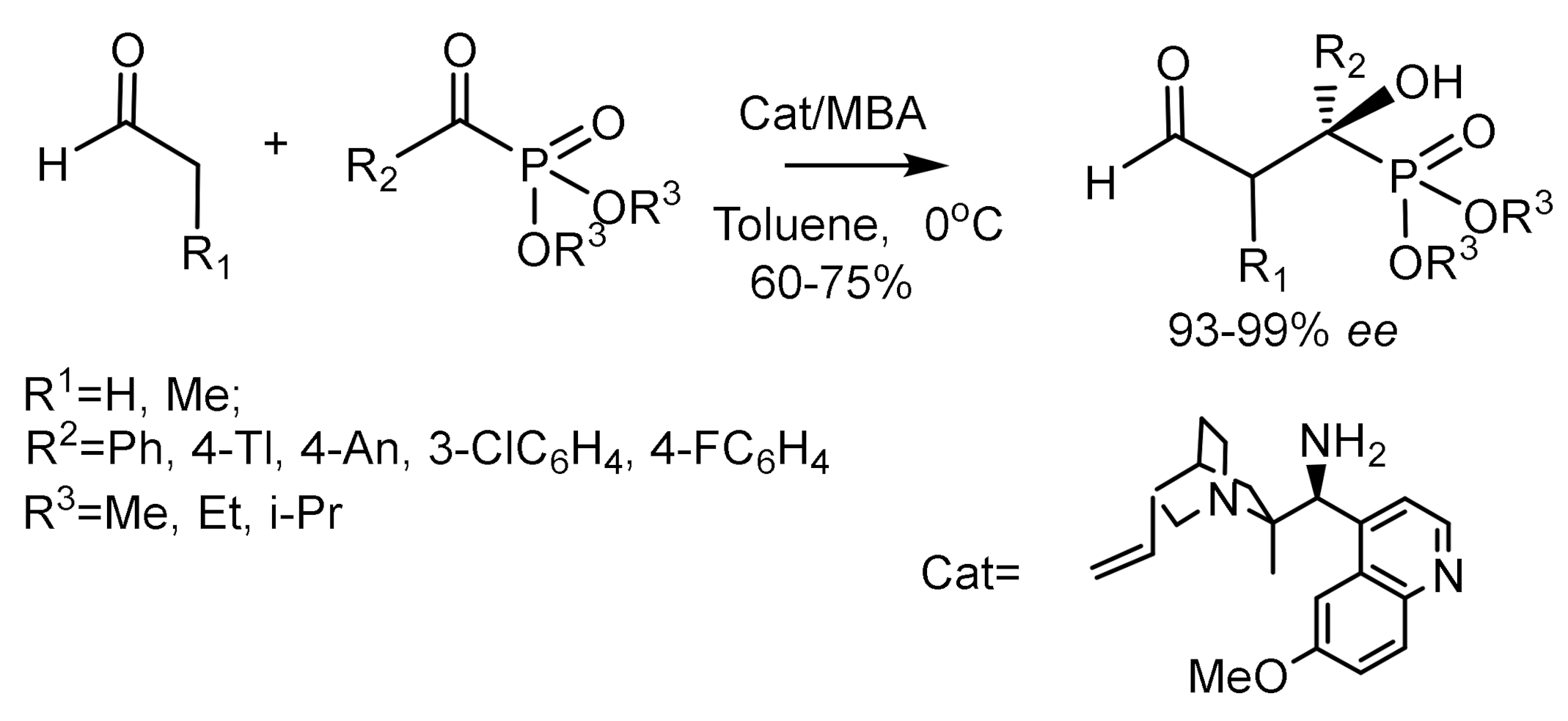
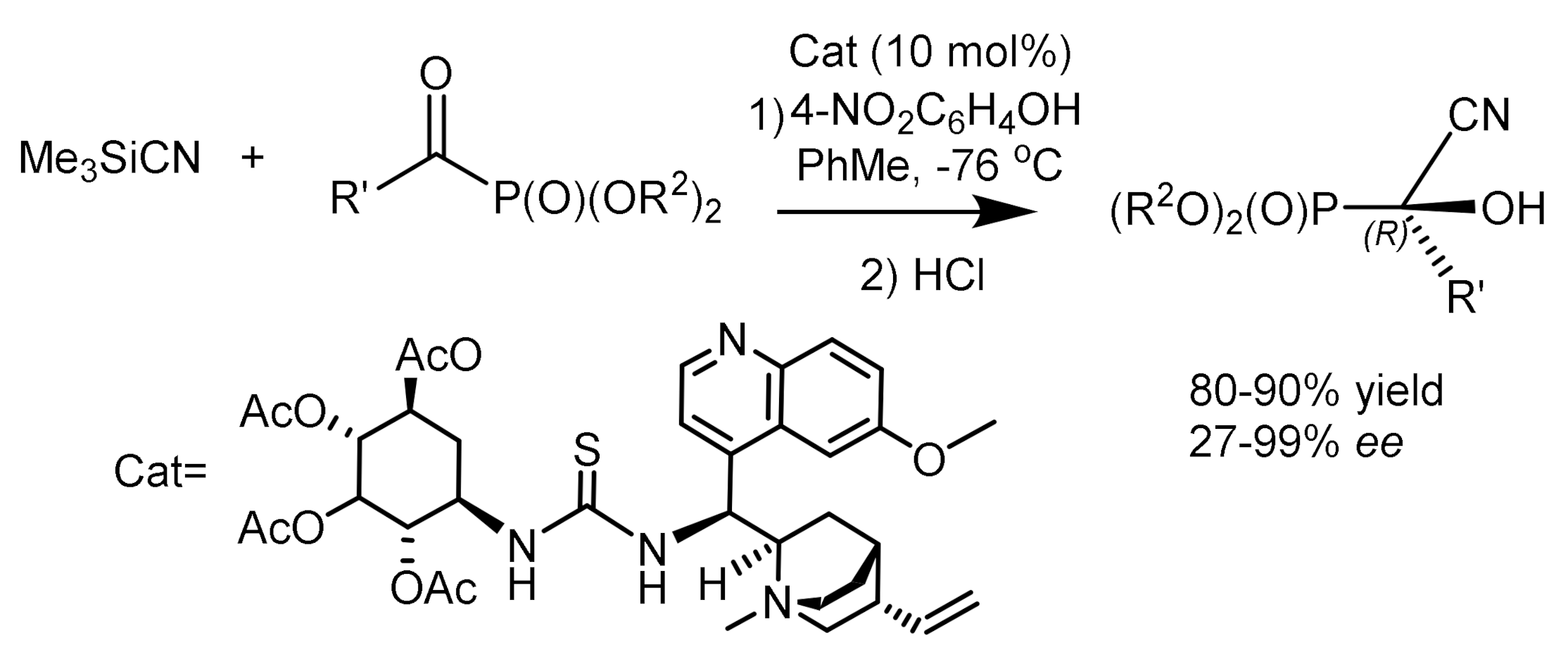
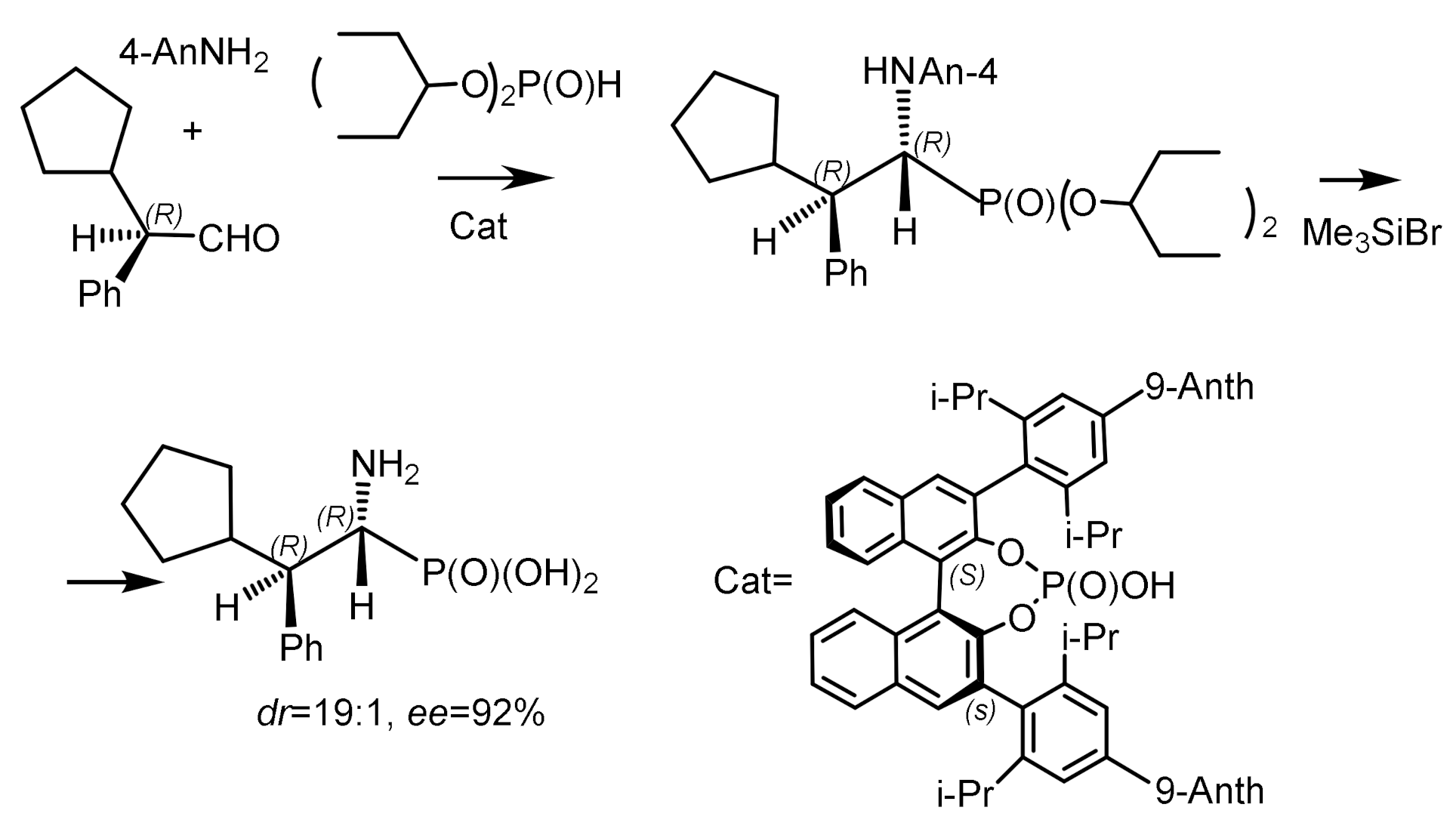
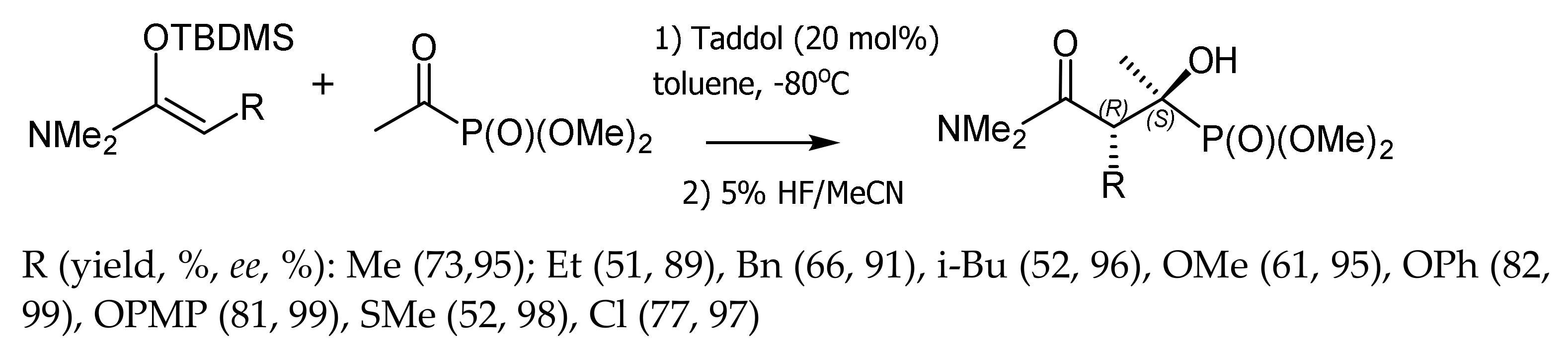
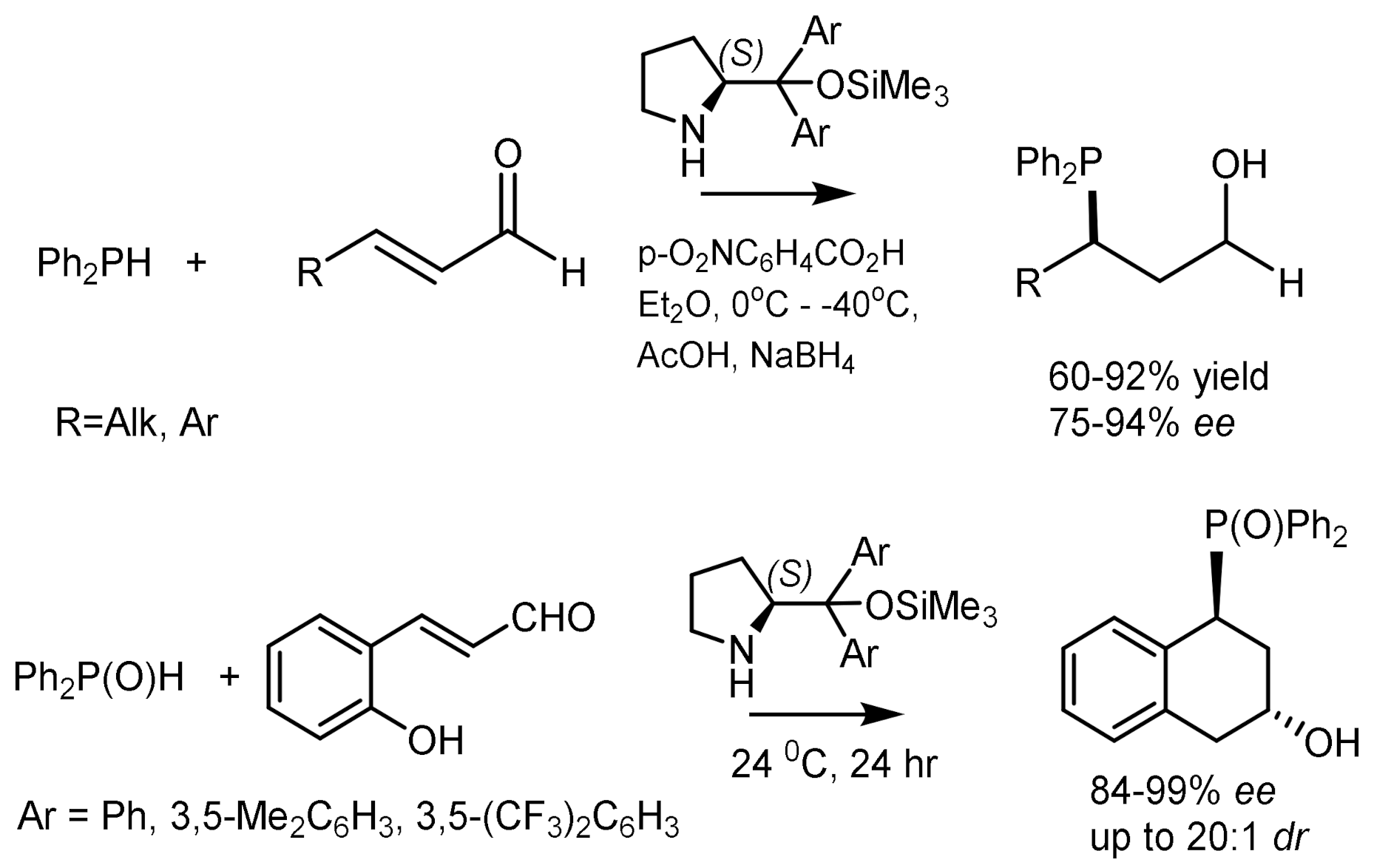
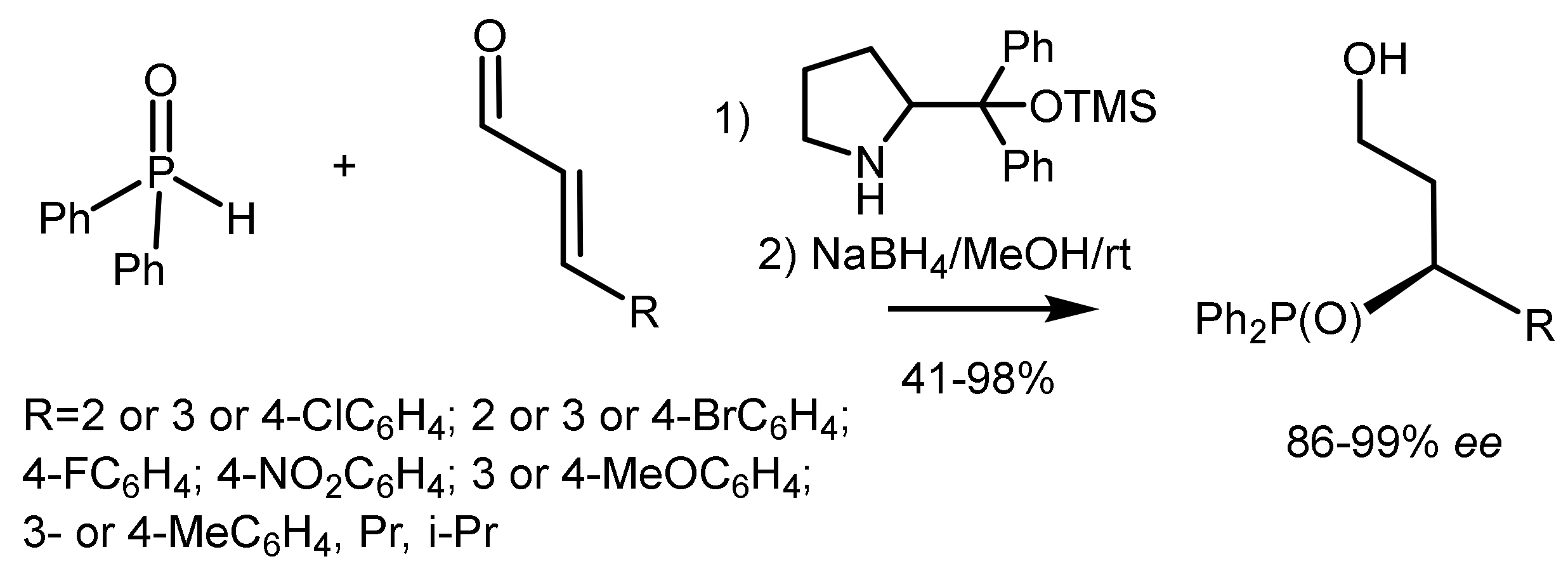
2.1.1. Phospha-Aldol Reaction
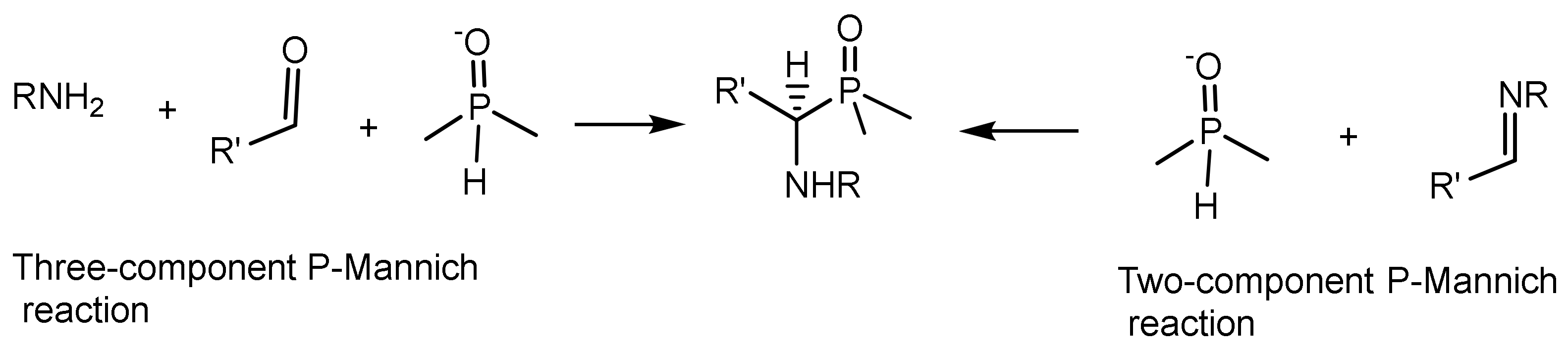
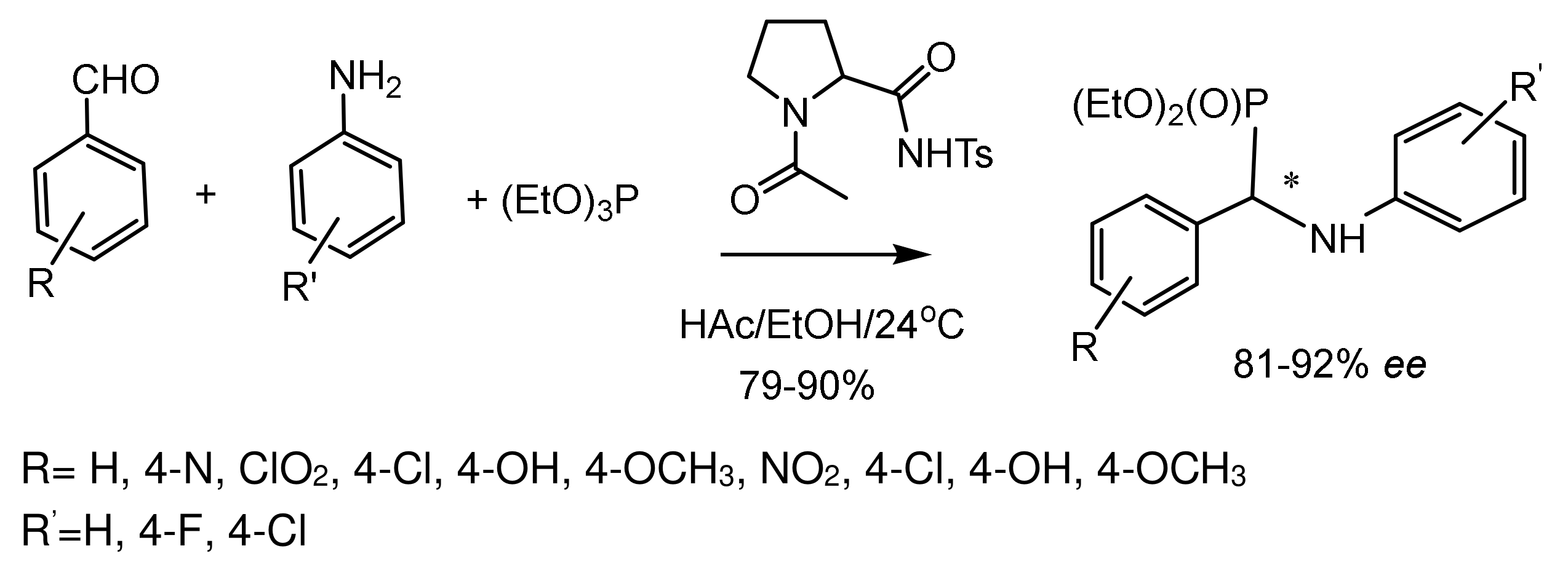
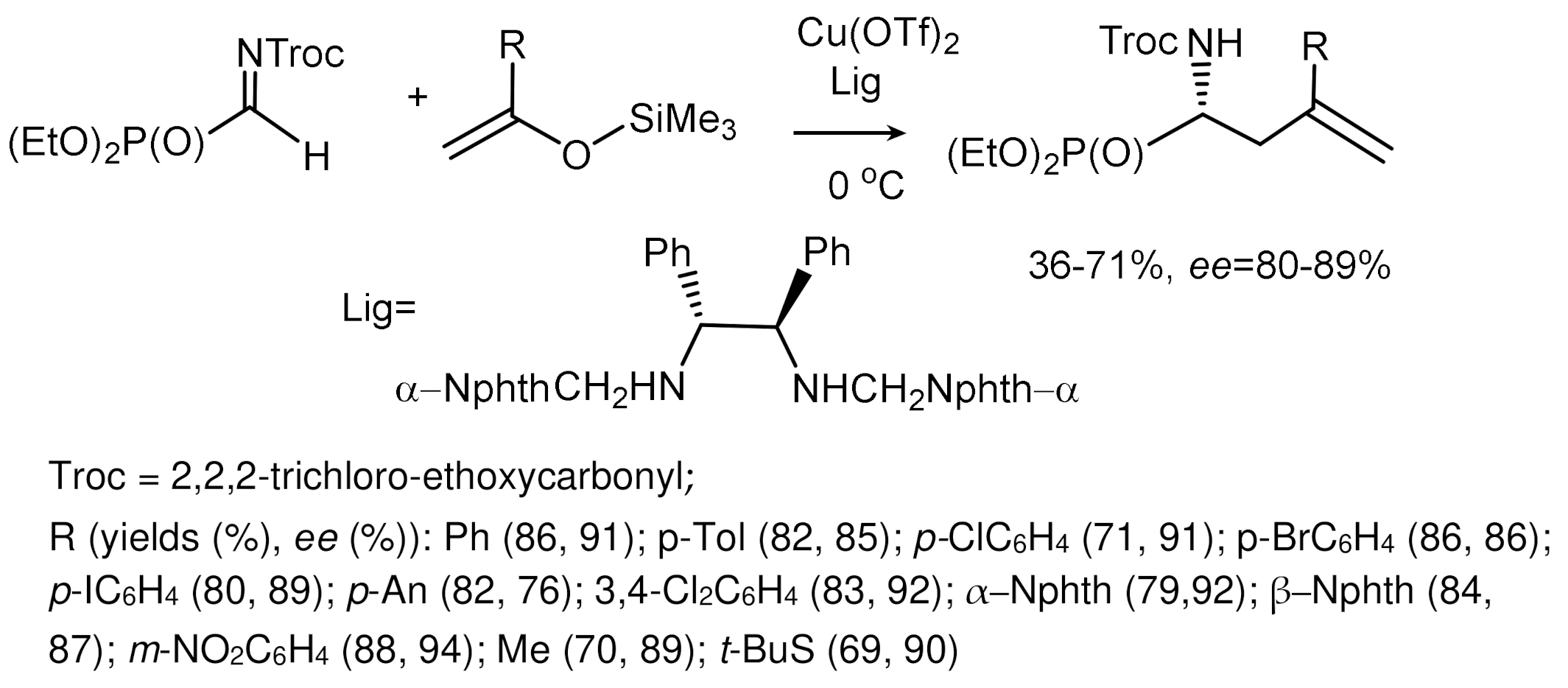
2.1.2. Phospha-Mannich Reaction
2.1.3. Phospha–Mukayama Reaction
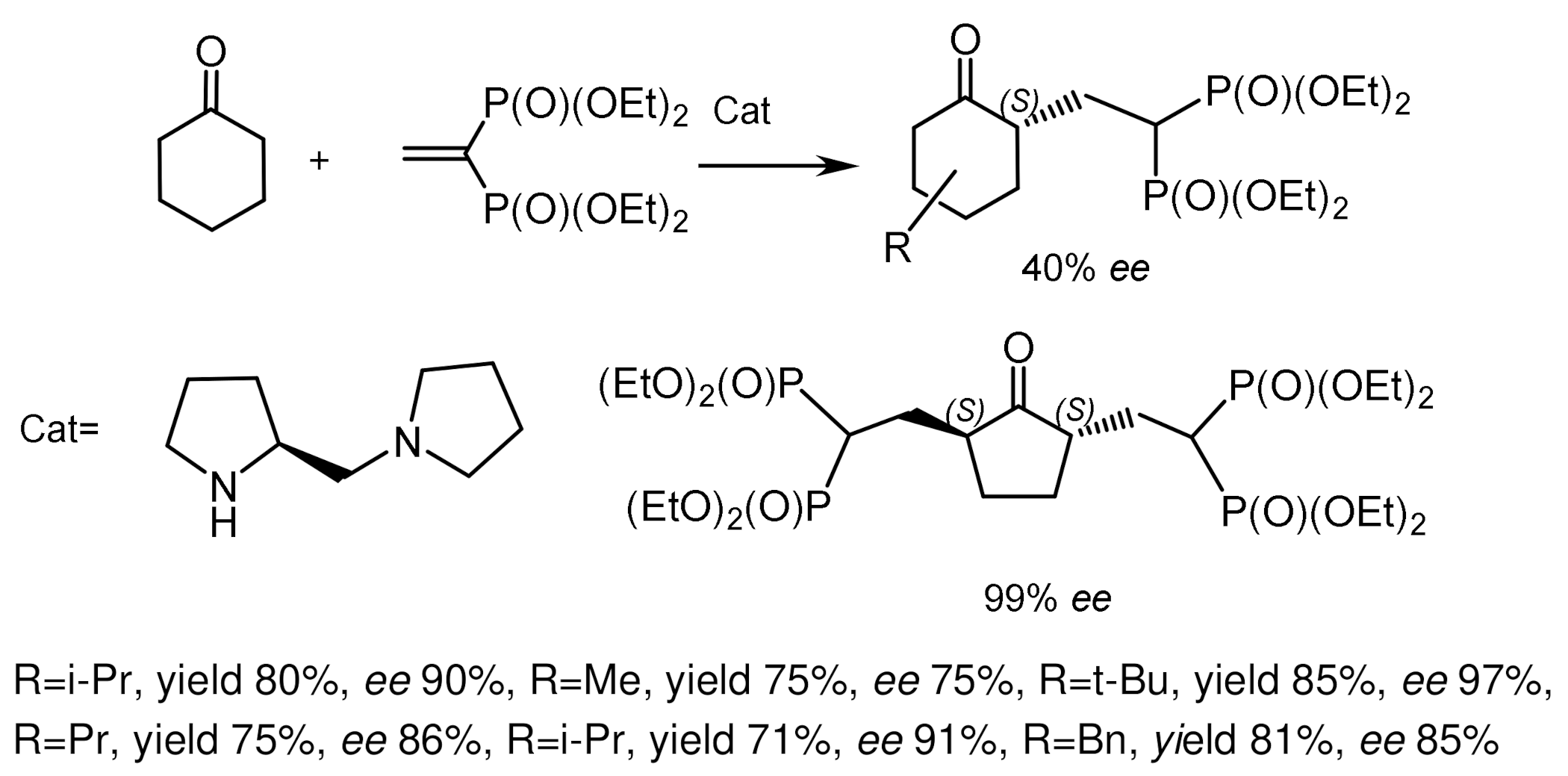
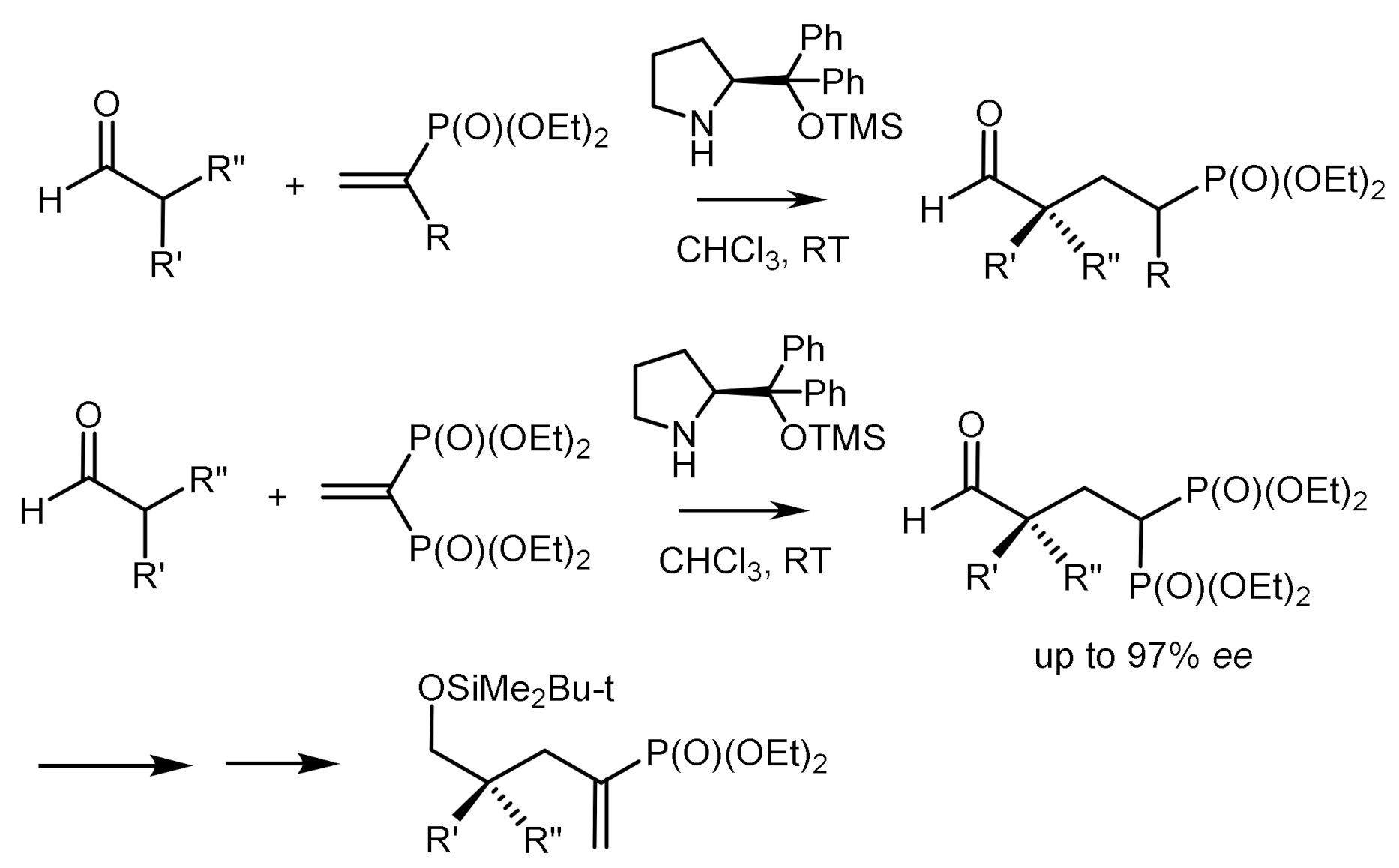
2.1.4. Phospha-Michael Reaction
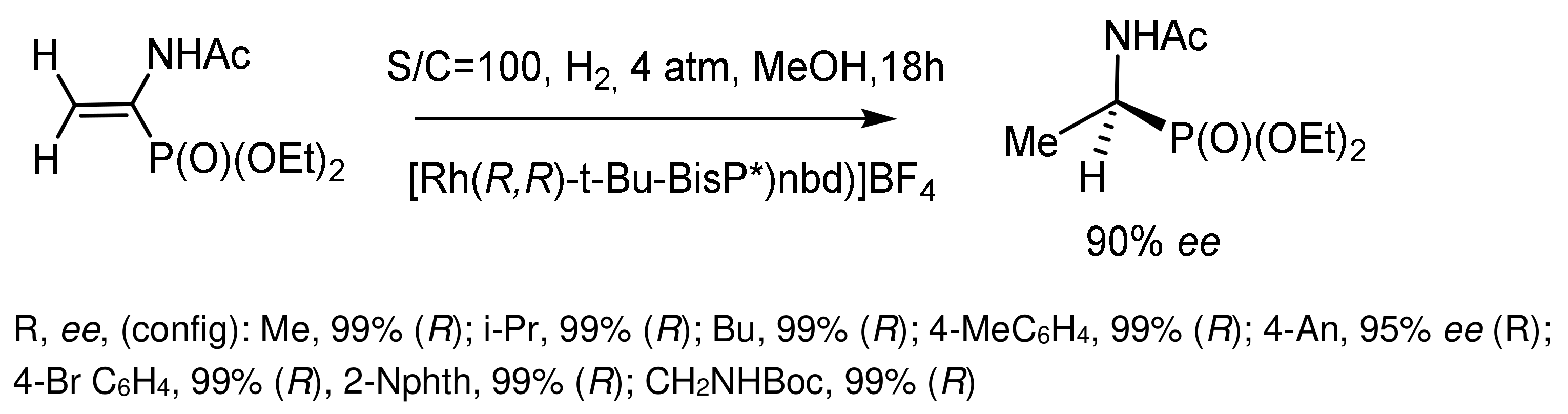
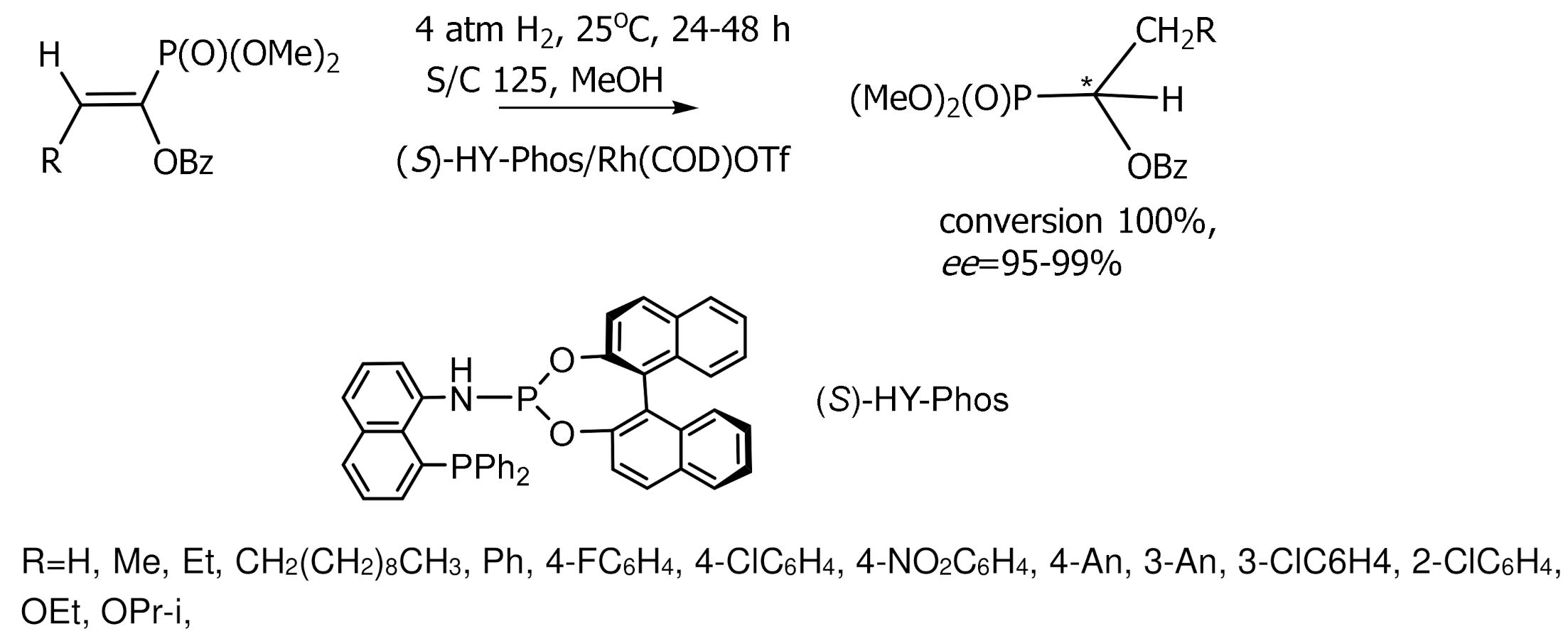
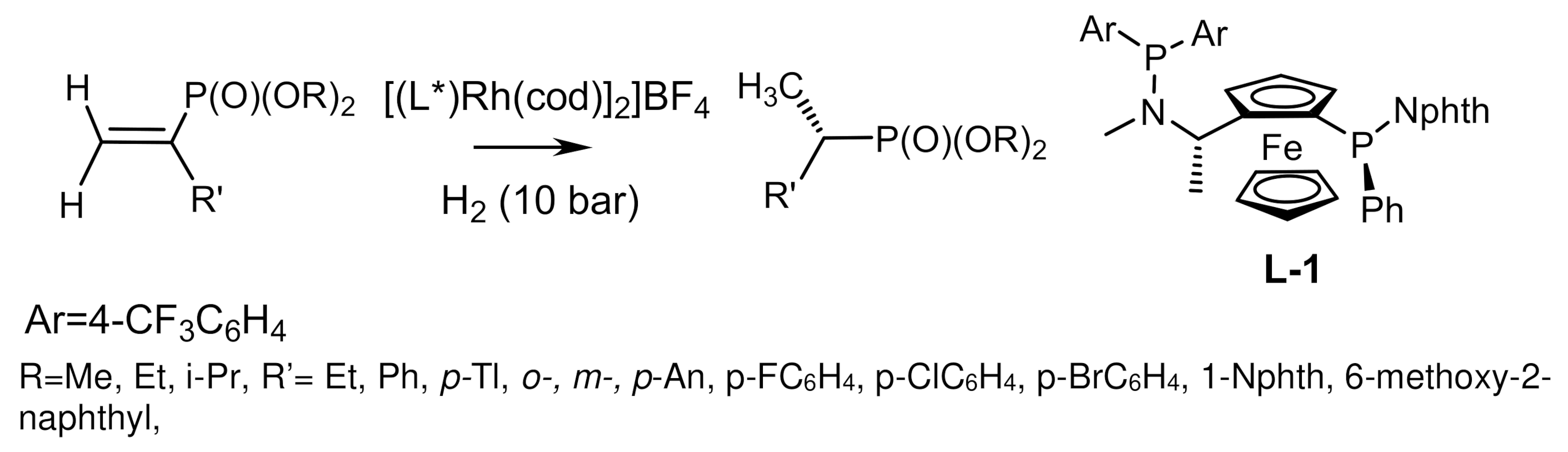
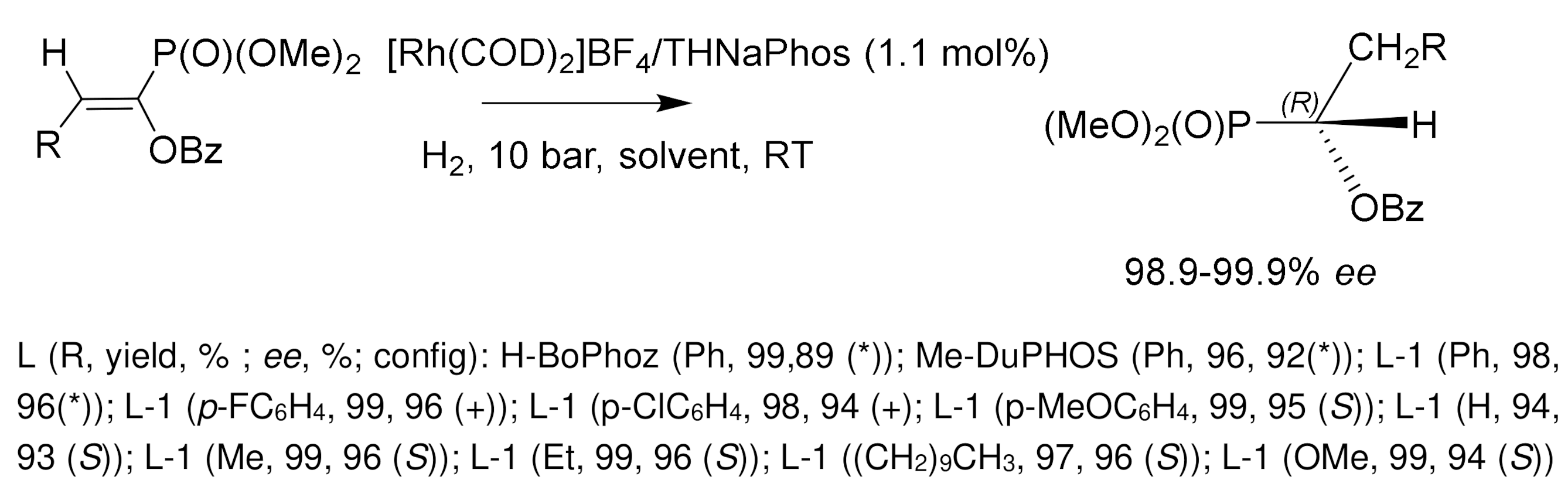
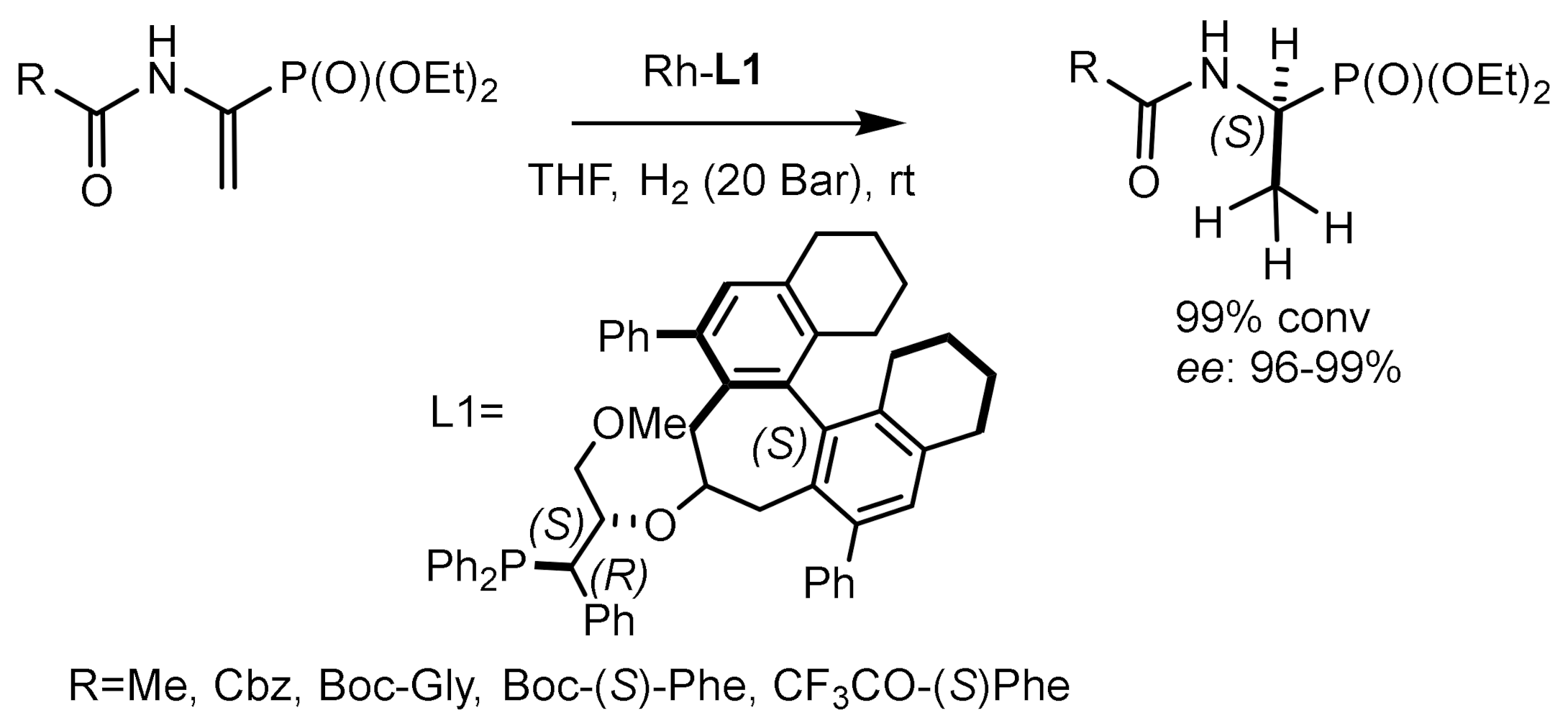
3. Asymmetric Hydrogenation of Unsaturated Phosphonates
3.1. Asymmetric Hydrogenation of Alkenephosphonates
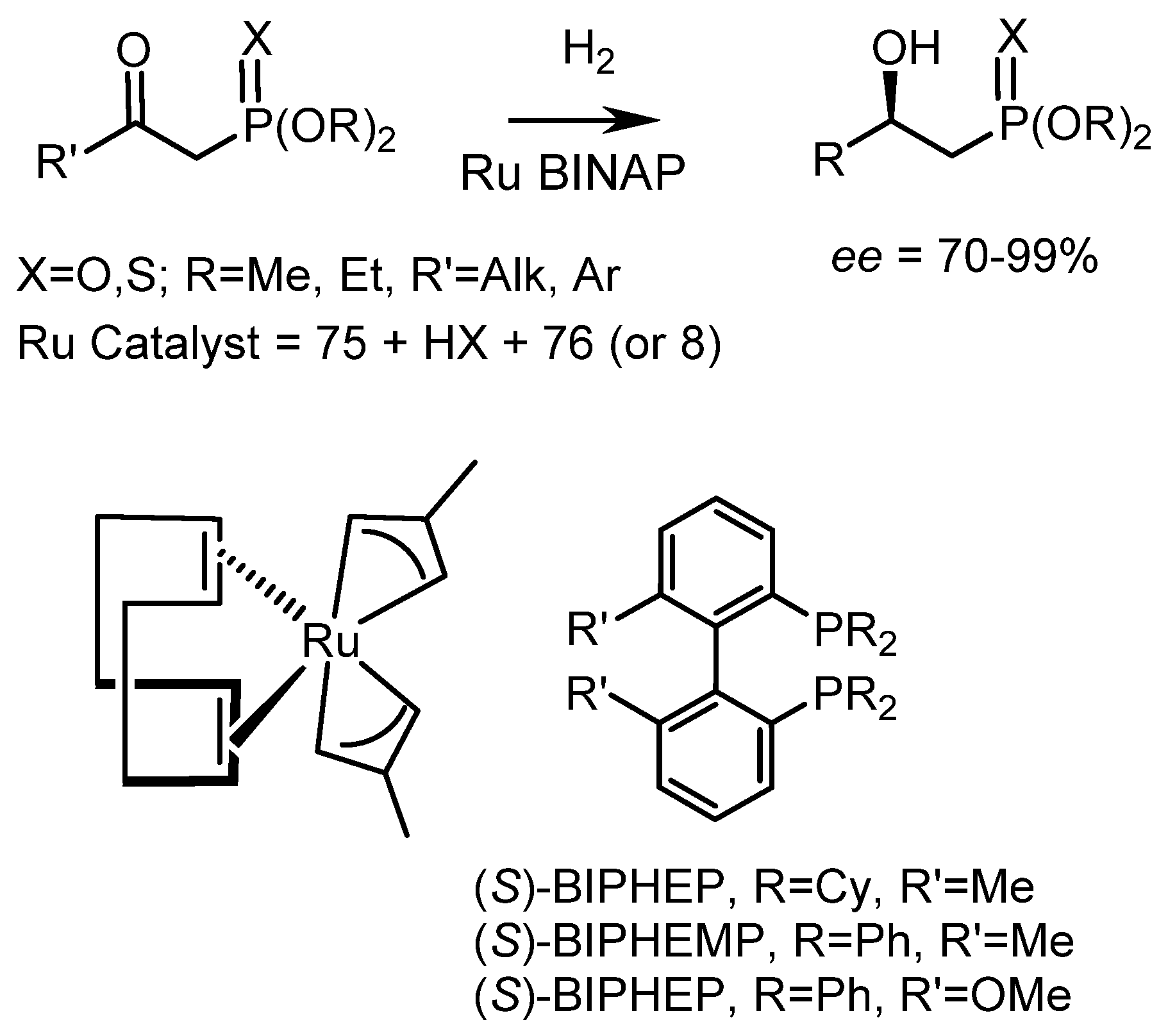
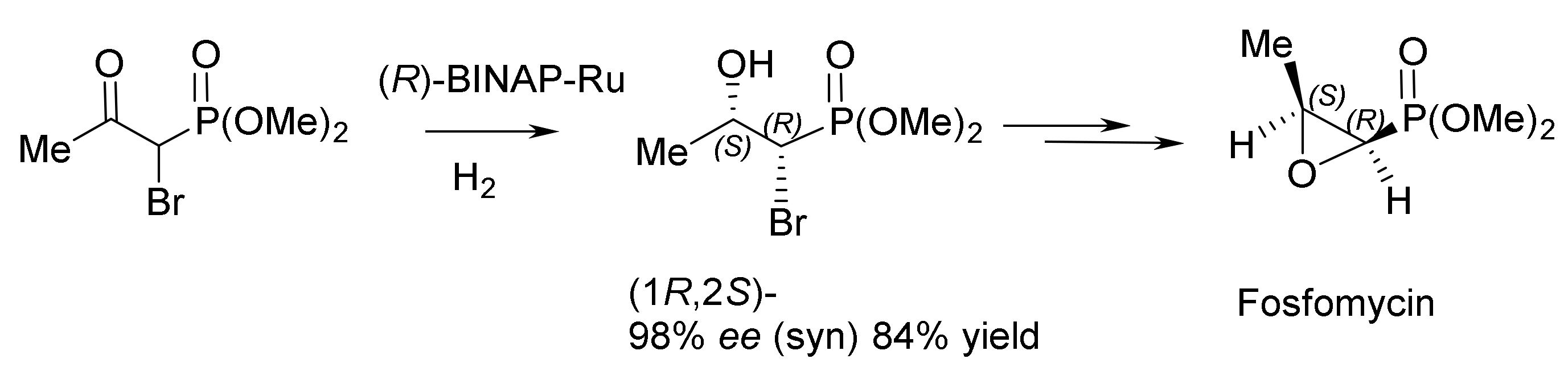
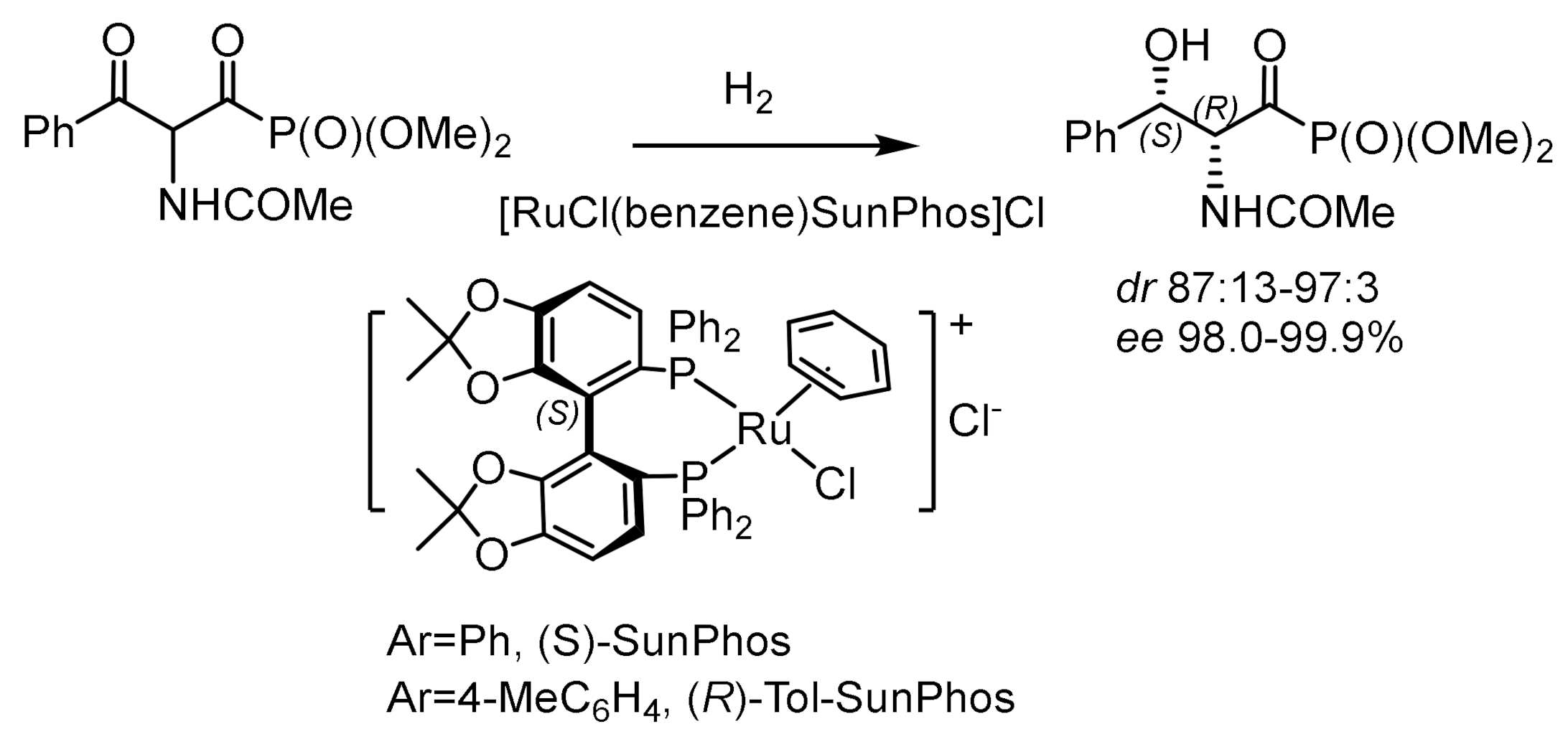
3.2. Asymmetric Hydrogenation and Reduction of Ketophosphonates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Berlicki, L.; Kafarski, P. Computer-Aided Analysis and Design of Phosphonic and Phosphinic Enzyme Inhibitors as Potential Drugs and Agrochemicals. Curr. Org. Chem. 2005, 9, 1829–1850. [Google Scholar] [CrossRef]
- Gbubele, J.D.; Olszewski, T.K. Asymmetric synthesis of organophosphorus compounds using H–P reagents derived from chiral alcohols. Org. Biomol. Chem. 2021, 19, 2823–2846. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Kandatsu, M. Isolation of 2-Aminoethane. Phosphonic Acid from Rumen Protozoa. Nature 1959, 184, 901–902. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, R.L. The Role of Phosphonates in Living Systems, 1st ed.; Taylor and Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2017; ISBN1 10:1315897377. ISBN2 13:978-1315897370. [Google Scholar]
- Kafarski, P. Phosphonates: Their Natural Occurrence and Physiological Role. Contemporary Topics about Phosphorus in Biology and Materials; Lee, W., Abdillah, A., Palma, J., Churchill, D.G., Eds.; IntechOpen: London, UK, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Kolodiazhnyi, O.I. Phosphorus compounds of natural origin: Prebiotic, Stereochemistry, Application. Symmetry 2021, 13, 889. [Google Scholar] [CrossRef]
- Kolodiazhnyi, O.I. Asymmetric Synthesis in Organophosphorus Chemistry: Synthetic Methods, Catalysis and Application; Wiley-VCH: Weinheim, Germany, 2016; ISBN 978-3-527-34150-4. [Google Scholar]
- Takeuchi, M.; Nakajima, M.; Ogita, T.; Inukai, M.; Kodama, K.; Furuya, K.; Nagaki, H.; Haneishi, T. Fosfonochlorin, a new antibiotic with spheroplast forming activity. J. Antibiot. 1989, 42, 198–205. [Google Scholar] [CrossRef]
- Leonard, P.G.; Satani, N.; Maxwell, D.; Lin, Y.-H.; Hammoudi, N.; Peng, Z.F. SF2312 is a natural phosphonate inhibitor of enolase. Nat. Chem. Biol. 2016, 12, 1053–1058. [Google Scholar] [CrossRef]
- Falagas, M.E.; Vouloumanou, K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef]
- Chofor, R.; Sooriyaarachchi, S.; Risseeuw, M.D.P.; Bergfors, T.; Pouyez, J.; Johny, C.; Haymon, A.; Everaert, A.; Dowd, C.S.; Maes, L.; et al. Synthesis and Bioactivity of β-Substituted Fosmidomycin Analogues Targeting 1-Deoxy-D-xylulose-5-phosphate Reductoisomerase. J. Med. Chem. 2015, 58, 2988–3001. [Google Scholar] [CrossRef]
- Kafarski, P. Phosphonopeptides containing free phosphonic groups: Recent advances. RSC Adv. 2020, 10, 25898–25910. [Google Scholar] [CrossRef]
- Hidaka, T.; Seto, H.; Imai, S. Biosynthesis mechanism of carbon-phosphorus bond formation. Isolation of carboxyphosphonoenolpyruvate and its conversion to phosphinopyruvate. J. Am. Chem. Soc. 1989, 111, 8012–8013. [Google Scholar] [CrossRef]
- Peck, S.C.; van der Donk, W. Phosphonate biosynthesis and catabolism: A treasure trove for unusual enzymology. Curr. Opin. Chem. Biol. 2013, 17, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T. The Chemical Biology of Phosphorus; RSC: Cambridge, UK, 2020; 546p. [Google Scholar] [CrossRef]
- Mikołajczyk, M. Phosphonate reagents and building blocks in the synthesis of bioactive compounds, natural products and medicines. Pure Appl. Chem. 2019, 91, 811–838. [Google Scholar] [CrossRef]
- Kolodiazhnyi, O.I.; Kukhar, V.P.; Kolodiazhna, A.O. Asymmetric catalysis as a method for the synthesis of chiral organophosphorus compounds. Tetrahedron Asymmetry 2014, 25, 865–922. [Google Scholar] [CrossRef]
- Kolodiazhnyi, O.I.; Kolodiazhna, A.O. Multiple stereoselectivity in organophosphorus chemistry. Phosph. Sulf. Silicon Relat. Elem. 2016, 191, 444–458. [Google Scholar] [CrossRef]
- Feringa, B.L. Chiral phosphorus compounds by catalytic asymmetric C–P coupling. Nat. Catal. 2022, 5, 8–9. [Google Scholar] [CrossRef]
- Numan, A.; Brichacek, M. Asymmetric Synthesis of Stereogenic Phosphorus P(V) Centers Using Chiral Nucleophilic Catalysis. Molecules 2021, 26, 3661. [Google Scholar] [CrossRef]
- Kolodyazhna, A.O.; Kolodyazhnyi, O.I. Stereoselective Synthesis of Organophosphorus Compounds; Naukova Dumka Pbl: Kyiv, Ukraine, 2017; 342p, ISBN 978-966-00-1599-9. [Google Scholar]
- Rádai, Z.; Keglevich, G. Synthesis and Reactions of Hydroxyphosphonates. Molecules 2018, 23, 1493. [Google Scholar] [CrossRef]
- Enders, D.; Saint-Dizier, A.; Lannou, M.-I.; Lenzen, A.; Lenzen, A. The Phospha-Michael Addition in Organic Synthesis. Eur. J. Org. Chem. 2006, 1, 29–49. [Google Scholar] [CrossRef]
- Guo, H.; Chiao, Y.; Zhanhu, F.; Yang, S.; Kwon, W.O. Phosphine Organocatalysis. Chem. Rev. 2018, 118, 10049–10293. [Google Scholar] [CrossRef]
- Phillips, A.M.F. Organocatalytic Asymmetric Synthesis of Chiral Phosphonates. Mini-Rev. Org. Chem. 2014, 11, 164–185. [Google Scholar] [CrossRef]
- Ordóñez, M.; Viveros-Ceballos, J.L.; Romero-Estudillo, I. Stereoselective Synthesis of α-Aminophosphonic Acids through Pudovik and Kabachnik-Fields Reaction; IntechOpen: Zagreb, Croatia, 2017. [Google Scholar]
- Shibazaki, M.; Sasai, H. Advances in Asymmetric Synthesis Direct Catalytic Asymmetric Three Component Kabachnik-Fields Reaction; Hassner, A., Ed.; Stamford; JAi Press: London, UK, 1998; Volume 3, pp. 191–233. [Google Scholar]
- Yamagishi, T.; Yokomatsu, T.; Suemune, K.; Shibuya, S. Enantioselective synthesis of a-hydroxyphosphinic acid derivatives through hydrophosphinylation of aldehydes catalyzed by Al-Li-BINOL complex. Tetrahedron 1999, 55, 12125–12136. [Google Scholar] [CrossRef]
- Nakajima, M.; Kotani, S.; Ishizuka, T.; Hashimoto, S. Chiral phosphine oxide BINAPO as a catalyst for enantioselective allylation of aldehydes with allyltrichlorosilanes. Tetrahedron Lett. 2005, 46, 157–159. [Google Scholar] [CrossRef]
- Nakanishi, K.; Kotani, S.; Sugiura, M.; Nakajima, M. First asymmetric Abramov-type phosphonylation of aldehydes with trialkyl phosphites catalyzed by chiral Lewis bases. Tetrahedron 2008, 64, 6415–6419. [Google Scholar] [CrossRef]
- Ayad, T.; Gernet, A.; Pirat, J.-L.; Virieux, D. Enantioselective reactions catalyzed by phosphine oxides. Tetrahedron 2019, 75, 4385–4418. [Google Scholar] [CrossRef]
- Kolodiazhna, A.O.; Kukhar, V.P.; Chernega, A.N.; Kolodiazhnyi, O.I. Double and triple asymmetric induction in phosphaaldol reactions. Tetrahedron Asymmetry 2004, 15, 1961–1963. [Google Scholar] [CrossRef]
- Laborde, C.; Wei, M.-M.; Van der Lee, A.; Deydier, E.; Daran, J.-C.; Volle, J.-N.; Poli, R.; Pirat, J.-L.; Manoury, E.; Virieux, D. Double [3 + 2]-dimerisation cascade synthesis of bis(triazolyl)bisphosphanes, a new scaffold for bidentate bisphosphanes. J. Chem. Soc. Dalton Trans. 2015, 44, 12539–12545. [Google Scholar] [CrossRef]
- Sevrain, N.; Volle, J.-N.; Pirat, J.-L.; Ayad, T.; Virieux, D. Chiral Bisdiphenylphosphine Dioxides Bearing a Bis(triazolyl) Backbone as Promising Lewis Bases for Asymmetric Organocatalysis. Eur. J. Org. Chem. 2018, 2018, 2267–2272. [Google Scholar] [CrossRef]
- Sevrain, N.; Volle, J.-N.; Pirat, J.-L.; Ayad, T.; Virieux, D. 1,1′-Dibenzyl-bis-(triazolyl)diphenylphosphine dioxide: A new efficient organocatalyst for silicon tetrachloride-mediated enantioselective Abramov-type phosphonylation of aldehydes with trialkyl phosphites. RSC Adv. 2017, 7, 52101–52104. [Google Scholar] [CrossRef]
- Gou, S.; Zhou, X.; Wang, J.; Liu, X.; Feng, X. Asymmetric hydrophosphonylation of aldehydes catalyzed by bifunctional chiral Al(III) complexes. Tetrahedron 2008, 64, 2864–2870. [Google Scholar] [CrossRef]
- Guin, J.; Wang, Q.; van Gemmeren, M.; List, B. The Catalytic Asymmetric Abramov Reaction. Angew. Chem. Int. Ed. 2015, 54, 355–358. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, D.; Lan, J.; Xi, P.; Yang, L.; Xiang, S.; You, J. Self-Assembled Bifunctional Catalysis Induced by Metal Coordination Interactions: An Exceptionally Efficient Approach to Enantioselective Hydrophosphonylation. Angew. Chem. Int. Ed. 2008, 47, 5646–5649. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, L.-L.; Baia, J.-F.; Ji, L.-N.; Yang, Q.-C.; Huang, Q.-C. Highly effective and nantioselective Phospho-Aldol reaction of diphenyl phosphite with N-alkylated isatins catalyzed by quinine. Tetrahedron Lett. 2011, 52, 1157–1160. [Google Scholar] [CrossRef]
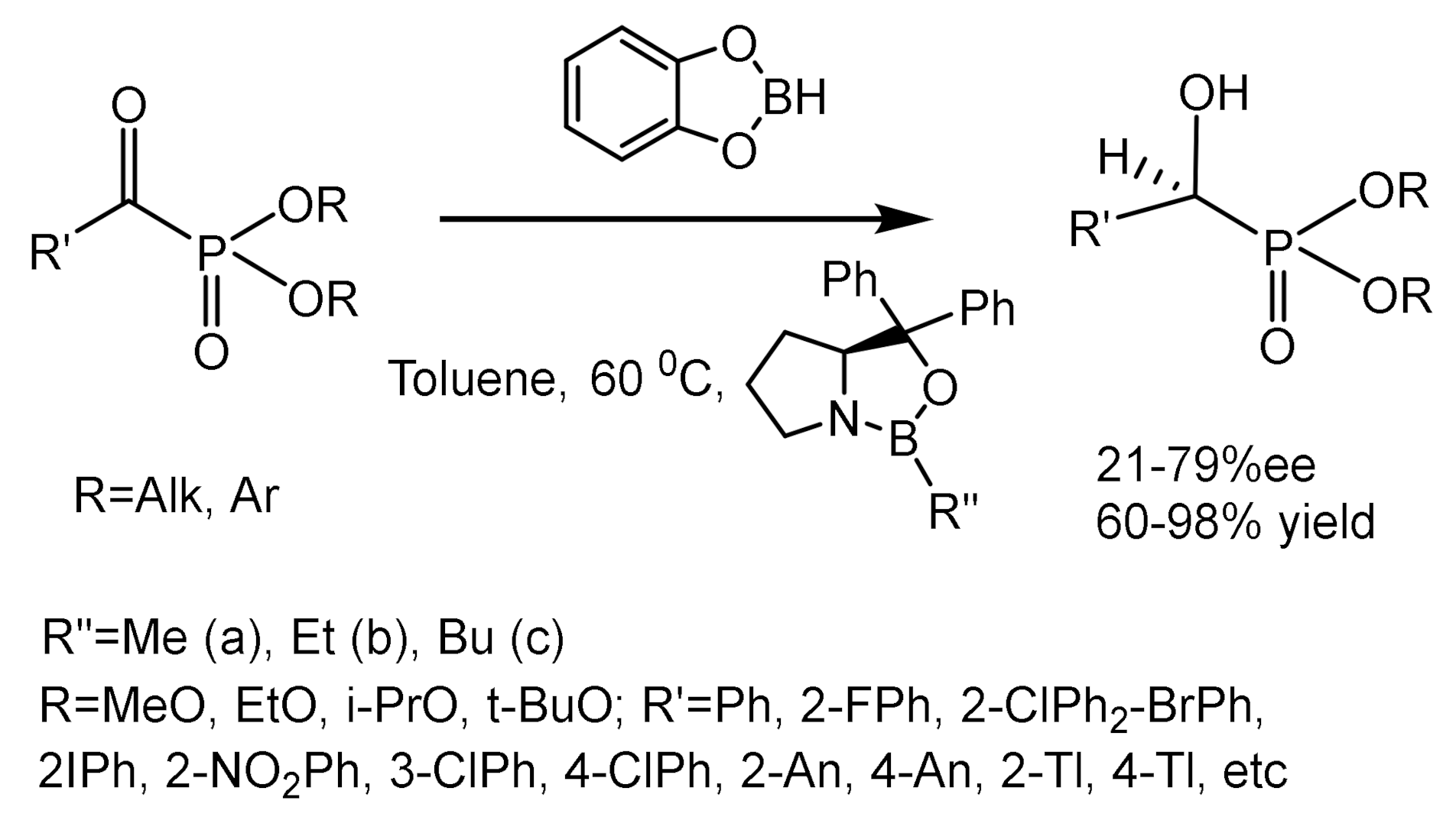
- Suyama, K.; Sakai, Y.; Matsumoto, K.; Saito, B.; Katsuki, T. Highly Enantioselective Hydrophosphonylation of Aldehydes: Base-Enhanced Aluminum-salalen Catalysis. Angew. Chem. Int. Ed. 2010, 49, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Guin, J.; Wang, Q.; Gemmeren, M.V.; List, B. Die katalytische asymmetrische Abramov-Reaktion. Angew. Chem. 2015, 127, 362–365. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Yang, X. Highly Enantioselective Hydrophosphonylation of Aldehydes Catalyzed by Tridentate Schiff Base Aluminum(III) Complexes. Angew. Chem. Int. Ed. 2008, 47, 392–394. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Chang, L. Highly Efficient Synthesis of Quaternary a-Hydroxy Phosphonates via Lewis Acid-Catalyzed Hydrophosphonylation of Ketones. Adv. Synth. Catal. 2009, 351, 2567–2572. [Google Scholar] [CrossRef]
- Ying, S.; Huang, X.; Guo, X.; Yang, S.; Zhao, J.; Shang, D.; Liu, X.; Lin, L.; Feng, X. The sequential C–H oxidation/asymmetric phosphonylation of primary alcohols to synthesize α-hydroxyphosphonates. Green Synth. Catal. 2021, 2, 315–319. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Q.; Hui, Y.; Chen, W.; Jiang, J.; Lin, L.; Liu, X.; Feng, X. Catalytic Asymmetric Synthesis of Quaternary a-Hydroxy Trifluoromethyl Phosphonate via Chiral Aluminum(III) Catalyzed Hydrophosphonylation of Trifluoromethyl Ketones. Org. Lett. 2010, 12, 4296–4299. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Cui, X.; Xiong, Y.; Zhou, X.; Feng, X. Asymmetric Hydrophosphonylation of α–Ketoesters Catalyzed by CinchonaDerived Thiourea Organocatalysts. Chem. Eur. J. 2009, 15, 589–592. [Google Scholar] [CrossRef]
- Abell, J.P.; Yamamoto, H. Catalytic Enantioselective Pudovik Reaction of Aldehydes and Aldimines with Tethered Bis(8-quinolinato) (TBOx) Aluminum Complex. J. Am. Chem. Soc. 2008, 130, 10521–10523. [Google Scholar] [CrossRef]
- Uraguchi, D.; Ito, T.; Ooi, T. Generation of Chiral Phosphonium Dialkyl Phosphite as a Highly Reactive P-Nucleophile: Application to Asymmetric Hydrophosphonylation of Aldehydes. J. Am. Chem. Soc. 2009, 131, 3836–3837. [Google Scholar] [CrossRef] [PubMed]
- Simón, L.; Paton, R.S. Origins of Asymmetric Phosphazene Organocatalysis: Computations Reveal a Common Mechanism for Nitro- and Phospho-Aldol Additions. J. Org. Chem. 2015, 80, 2756–2766. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shi, M. Applications of Chiral Phosphine-Based Organocatalysts in Catalytic Asymmetric Reactions. Chem. Asian J. 2014, 9, 2720–2734. [Google Scholar] [CrossRef] [PubMed]
- Kolodyazhnaya, A.O.; Kukhar, V.P.; Kolodyazhnyi, O.I. Organic Catalysis of Phospha-Aldol Condensation. Russ. J. Gen. Chem. 2008, 78, 2043–2051. [Google Scholar] [CrossRef]
- Alegre-Requena, J.V.; Marques-Lopez, E.; Sanz Miguel, P.J.; Herrera, R.P. Organocatalytic enantioselective hydrophosphonylation of aldehydes. Org. Biomol. Chem. 2014, 12, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Naganaboina, V.K.; Wang, L.; Zhang, B.; Guo, Q.; Rout, L.; Zhao, C.-G. Organocatalytic Highly Enantioselective Synthesis of β-Formyl-ahydroxyphosphonates. Adv. Synth. Catal. 2011, 353, 1729–1734. [Google Scholar] [CrossRef]
- Vicario, J.; Ezpeleta, J.M.; Palaciosa, F. Asymmetric Cyanation of a-Ketiminophosphonates Catalyzed by Cinchona Alkaloids: Enantioselective Synthesis of Tetrasubstituted a-Aminophosphonic Acid Derivatives from Trisubstituted α-Aminophosphonates. Adv. Synth. Catal. 2012, 354, 2641–2647. [Google Scholar] [CrossRef]
- Kong, S.; Fan, W.; Wu, G.; Miao, Z. Enantioselective Synthesis of Tertiary a-Hydroxy Phos. phonates Catalyzed by Carbohydrate I Cinchona Alkaloid Thiourea Organocatalysts. Angew. Chem. Int. Ed. 2012, 51, 8864–8867. [Google Scholar] [CrossRef]
- George, J.; Sridhar, B.; Reddy, B.V.S. First example of quinine-squaramide catalyzed enantioselective addition of diphenyl phosphite to ketimines derived from isatins. Org. Biomol. Chem. 2014, 12, 1595–1602. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, V.; Kaur, J.; Kumar, V.; Mahaja, S.; Mahajan, S. Cinchona-derived Thiourea Catalyzed Hydrophosphonylation of Ketirnines—An Enantioselective Synthesis of a-Amino Phosphonates. Tetrahedron Lett. 2014, 70, 7044–7049. [Google Scholar] [CrossRef]
- Yao, Q.; Yuan, C.A. A Synthetic Study of Chiral α–Hydroxy-H-Phosphinates Based on Proline Catalysis. Chem. Eur. J. 2013, 19, 6080–6088. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, I.; Mohammadi, L.; Heravi, M.M. Organocatalyzed Asymmetric Mannich Reaction: An Update. Chem. Sel. 2021, 6, 1008–1066. [Google Scholar] [CrossRef]
- Albrecht, Q.; Albrecht, A.; Krawczyk, H.; Jorgensen, K.A. Organocatalytic Asymmetric Synthesis of Organophosphorus Compounds. Chem. Eur. J. 2010, 16, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Saranya, N.; Ann Harry, K.; Krishnan, K.; Anilkumar, G. Recent Developments and Perspectives in the Asymmetric Mannich Reaction. Asian J. Org. Chem. 2018, 7, 613–633. [Google Scholar] [CrossRef]
- Yin, Z.; Guo, J.; Zhang, R.; Hu, X.; Borovkov, V. Direct Asymmetric Three-Component Mannich Reaction Catalyzed by Chiral Counteranion-Assisted Silver. J. Org. Chem. 2020, 85, 10369–10377. [Google Scholar] [CrossRef] [PubMed]
- Kafarski, P.; Gorny vel Gorniak, M.; Andrasiak, I. Kabachnik-Fields Reaction Under Green Conditions—A Critical Overview. Curr. Green Chem. 2015, 2, 218–222. [Google Scholar] [CrossRef]
- Keglevich, G.; Bálint, E. The Kabachnik–Fields Reaction: Mechanism and Synthetic Use. Molecules 2012, 17, 12821. [Google Scholar] [CrossRef]
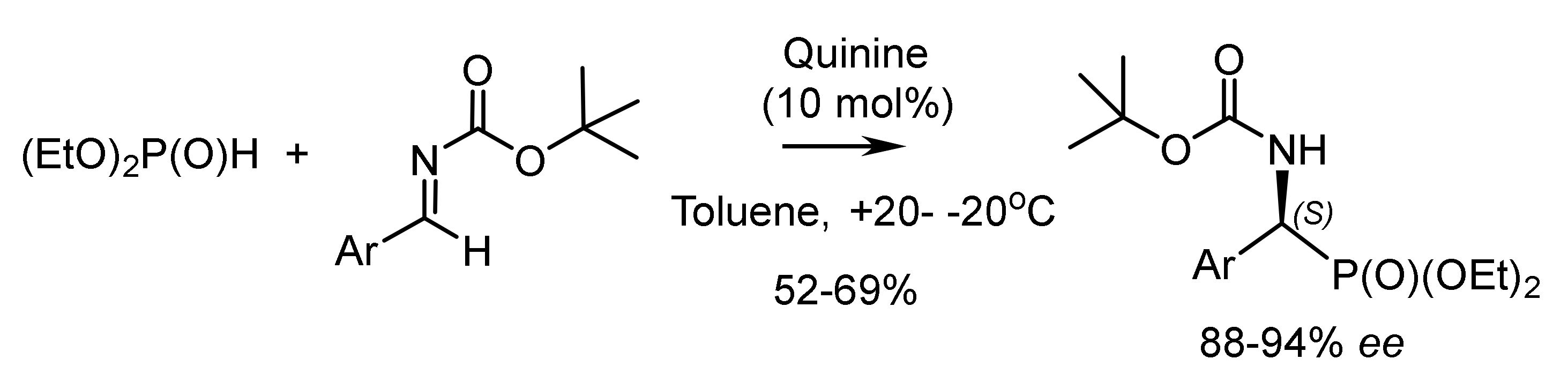
- Boratynski, P.J.; Skarzewski, J.; Sidorowicz, L. Stereochemistry of hydrophosphonylation of 9-aminoquinine Schiff bases. Arkivoc 2012, 4, 204–215. [Google Scholar] [CrossRef]
- Chen, W.; Hui, Y.; Zhou, X.; Jiang, J.; Cai, Y.; Liu, X.; Lin, L.; Feng, X. Chiral N,N′-dioxide-Yb(III) complexes catalyzed enantioselective hydrophosphonylation of aldehydes. Tetrahedron Lett. 2010, 51, 4175–4178. [Google Scholar] [CrossRef]
- Iwanejko, J.; Brol, A.; Szyja, B.M.; Daszkiewicz, M.; Wojaczynska, E.; Olszewski, T.K. Aminophosphonates and aminophosphonic acids with tetrasubstituted stereogenic center: Diastereoselective synthesis from cyclic ketimines. Org. Biomol. Chem. 2019, 17, 7352–7359. [Google Scholar] [CrossRef]
- Cheng, X.; Goddard, R.; Buth, G.; List, B. Direct Catalytic Asymmetric Three-Component Kabachnik-Fields Reaction. Angew. Chem. Int. Ed. 2008, 47, 5079–5081. [Google Scholar] [CrossRef] [PubMed]
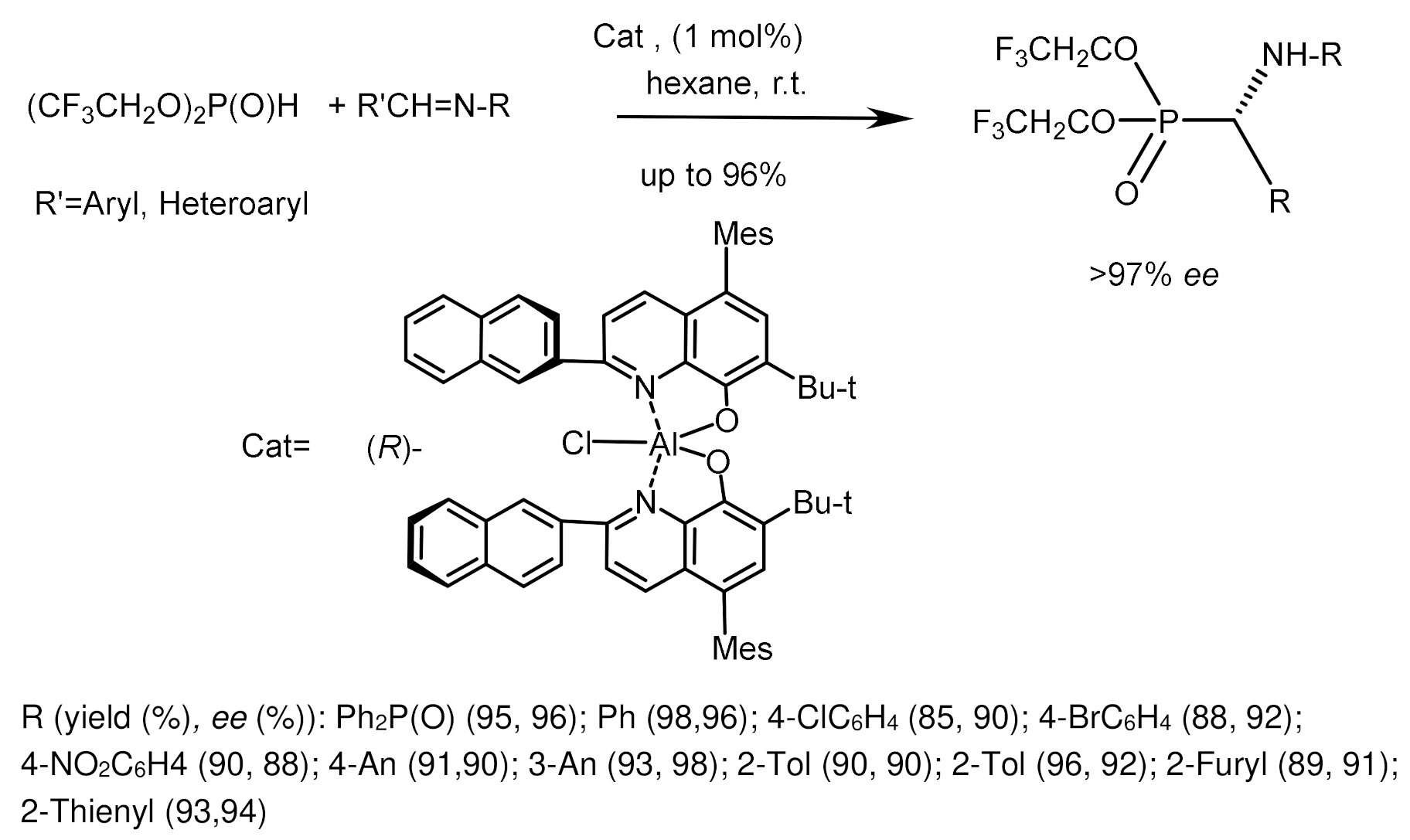
- Akiyama, T.; Morita, H.; Itoh, J.; Fuchibem, K. Chiral Brnnsted Acid Catalyzed Enantioselective Hydrophosphonylation of Imines: Asymmetric Synthesis of a-Amino Phosphonates. Org. Lett. 2005, 7, 2583–2585. [Google Scholar] [CrossRef] [PubMed]
- Pedroni, J.; Cramer, N. Enantioselective C–H Functionalization—Addition Sequence Delivers Densely Substituted 3-Azabicyclo [3.1.0]hexanes. J. Am. Chem. Soc. 2017, 139, 12398–12401. [Google Scholar] [CrossRef] [PubMed]
- Saito, B.; Egami, H.; Katsuki, T. Synthesis of an Optically Active Al(salalen) Complex and Its Application to Catalytic Hydrophosphonylation of Aldehydes and Aldimines. J. Am. Chem. Soc. 2007, 129, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, D.; Marcolini, M.; Bernardi, L.; Fini, F.; Herrera, R.P.; Sgarzani, V.; Ricci, A. Direct Access to Enantiomerically Enriched α-Amino Phosphonic Acid Derivatives by Organocatalytic Asymmetric Hydrophosphonylation of Imines. J. Org. Chem. 2006, 71, 6269–6272. [Google Scholar] [CrossRef]
- Kadota, T.; Sawa, M.; Kondo, Y.; Morimoto, H.; Ohshima, T. Catalytic Enantioselective Strecker Reaction of Isatin-Derived N-Unsubstituted Ketimines. Org. Lett. 2021, 23, 4553–4558. [Google Scholar] [CrossRef]
- Cao, W.L.; Liu, X.L.; Feng, X. Chiral organobases: Properties and applications in asymmetric catalysis. Chin. Chem. Lett. 2018, 29, 1201–1208. [Google Scholar] [CrossRef]
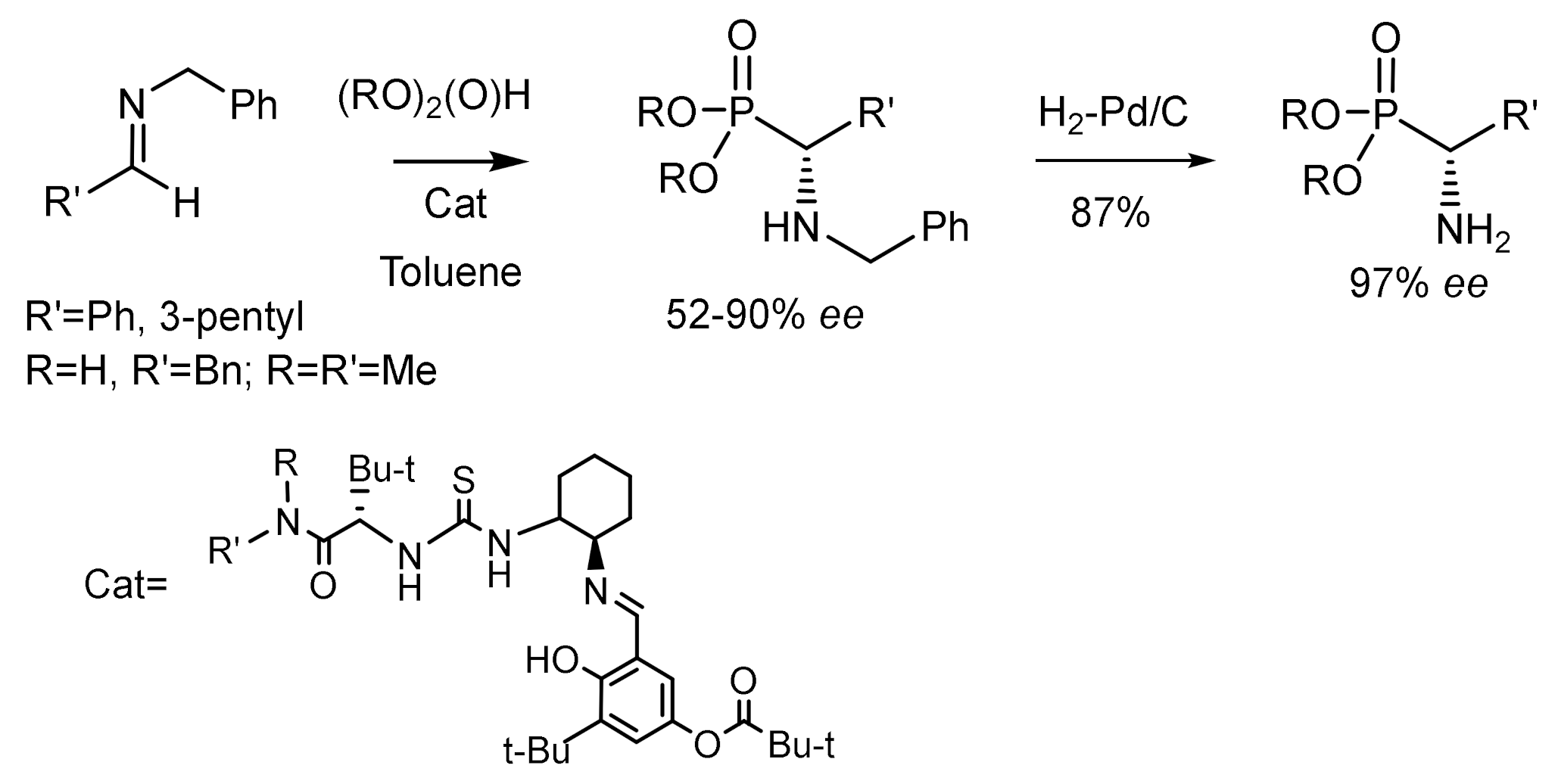
- Joly, G.D.; Jacobsen, E.N. Thiourea-Catalyzed Enantioselective Hydrophosphonylation of Imines: Practical Access to Enantiomerically Enriched α-Amino Phosphonic Acids. J. Am. Chem. Soc. 2004, 126, 4102–4103. [Google Scholar] [CrossRef]
- Guang, J.; Guo, Q.; Zhao, J.C.-G. Acetylphosphonate as a Surrogate of Acetate or Acetamide in Organocatalyzed Enantioselective Aldol Reactions. Org. Lett. 2012, 14, 3174–3177. [Google Scholar] [CrossRef]
- Fu, X.; Loh, W.-T.; Zhang, Y.; Chen, T.; Ma, T.; Liu, H.; Wang, J.; Tan, C.-H. Chiral Guanidinium Salt Catalyzed Enantioselective Phospha-Mannich Reactions. Angew. Chem. Int. Ed. 2009, 48, 7387–7390. [Google Scholar] [CrossRef]
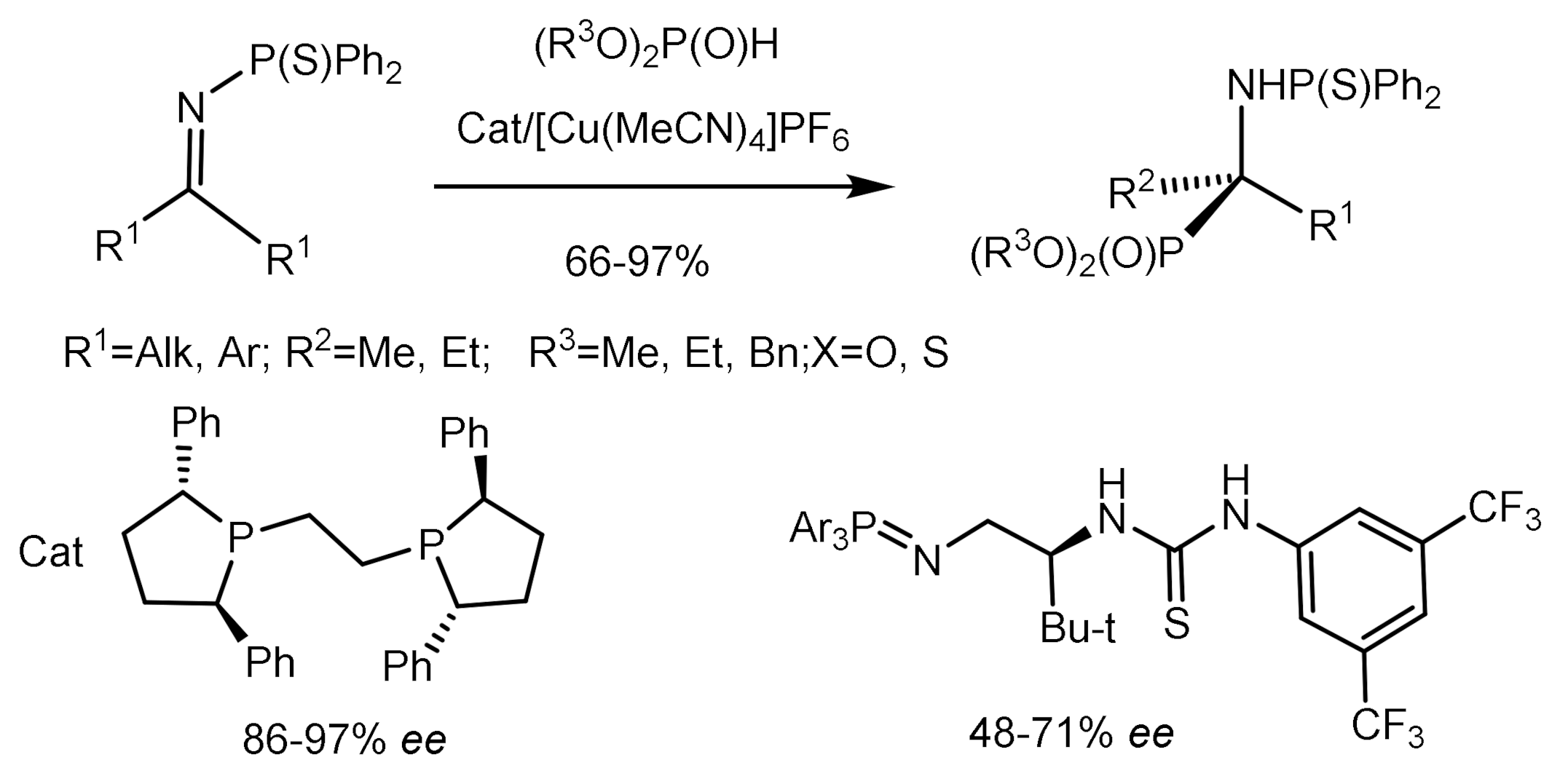
- Lin, S.; Otsuka, Y.; Yin, L.; Kumagai, N.; Shibasaki, M. Catalytic Enantioselective Addition of Diethyl Phosphite to N-Thiophosphinoyl Ketimines: Preparation of (R)-Diethyl (1-Amino-1-phenylethyl)phosphonate. Org. Synth. 2017, 94, 313–331. [Google Scholar] [CrossRef]
- Thorat, P.B.; Goswami, S.V.; Magar, R.L.; Patil, B.R.; Bhusare, S.R. An efficient organocatalysis: A one-pot highly enantioselective synthesis of α-aminophosphonates. Eur. J. Org. Chem. 2013, 24, 5509–5516. [Google Scholar] [CrossRef]
- Vellalath, S.; Čorić, I.; List, B. N-Phosphinyl Phosphoramide—A Chiral Brønsted Acid Motif for the Direct Asymmetric N,O-Acetalization of Aldehydes. Angew. Chem. Int. Ed. 2010, 49, 9749–9752. [Google Scholar] [CrossRef] [PubMed]
- Mukaiyama, T.; Narasaka, K.; Banno, K. New Aldol type reaction. Chem. Lett. 1973, 2, 1011–1014. [Google Scholar] [CrossRef]
- Gondi, V.B.; Hagihara, K.; Rawal, V.H. Diastereoselective and Enantioselective Synthesis of Tertiary α–Hydroxy Phosphonates through Hydrogen-Bond Catalysis. Angew. Chem. Int. Ed. 2009, 48, 776–779. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kiyohara, H.; Nakamura, Y.; Matsubara, R. Catalytic Asymmetric Synthesis of α-Amino Phosphonates Using Enantioselective Carbon-Carbon Bond-Forming Reactions. J. Am. Chem. Soc. 2004, 126, 6558–6559. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Liu, W.; Zheng, Y.; He, Z. Organocatalytic asymmetric cascade cyclization reaction of o-hydroxy cinnamaldehydes with diphenylphosphine oxide. Chin. Chem. Lett. 2018, 29, 1625–1628. [Google Scholar] [CrossRef]
- Heravi, M.M.; Hajiabbasi, P. Recent advances in C-heteroatom bond forming by asymmetric Michael addition. Mol. Divers. 2014, 18, 411–439. [Google Scholar] [CrossRef]
- Zhao, D.; Mao, L.; Yang, D.; Wang, R. Zinc-mediated asymmetric additions of dialkylphosphine oxides to α,β-unsaturated ketones and N-sulfinylimines. J. Org. Chem. 2010, 75, 6756–6763. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Mao, L.; Wang, R. Highly enantioselective conjugate additions of phosphites to α,β-unsaturated N-acylpyrroles and imines: A practical approach to enantiomerically enriched amino phosphonates. Chem. Eur. J. 2009, 15, 10983–10987. [Google Scholar] [CrossRef]
- Bartoli, G.; Bosco, M.; Carlone, A.; Locatelli, M.; Mazzanti, A.; Sambri, L.; Melchiorre, P. Organocatalytic asymmetric hydrophosphination of nitroalkenes. J. Chem. Soc. Chem. Commun. 2007, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Carlone, A.; Bartoli, G.; Bosco, M.; Sambri, L.; Melchiorre, P. Organocatalytic Asymmetric Hydrophosphination of α,β-unsaturated Aldehydes. Angew. Chem. Int. Ed. 2007, 46, 4504–4506. [Google Scholar] [CrossRef] [PubMed]
- Ibrahem, I.; Rios, R.; Vesely, J.; Hammar, P.; Eriksson, L.; Himo, F.; Cordova, A. Enantioselective organocatalytic hydrophosphination of α,β-unsaturated aldehydes. Angew. Chem. Int. Ed. 2007, 46, 4507–4510. [Google Scholar] [CrossRef] [PubMed]
- Maerten, E.; Cabrera, S.; Kjrsgaard, A.; Jorgensen, K.A. Organocatalytic asymmetric direct phosphonylation of alpha, beta-unsaturated aldehydes: Mechanism, scope, and application in synthesis. J. Org. Chem. 2007, 72, 8893–8903. [Google Scholar] [CrossRef] [PubMed]
- Ibrahem, I.; Hammar, P.; Vesely, J.; Rios, R.; Eriksson, L.; Córdov, A. Organocatalytic Asymmetric Hydrophosphination of α,β-Unsaturated Aldehydes: Development, Mechanism and DFT Calculations. Adv. Synth. Catal. 2008, 350, 1875–1884. [Google Scholar] [CrossRef]
- Fu, X.; Jiang, Z.; Tan, C.H. Bicyclic guanidine-catalyzed enantioselective phospha-Michael reaction: Synthesis of chiral β-aminophosphine oxides and β-aminophosphines. J. Chem. Soc. Chem. Commun. 2007, 2007, 5058–5060. [Google Scholar] [CrossRef]
- Terada, M.; Ikehara, T.; Ube, H. Enantioselective 1,4-addition reactions of diphenyl phosphite to nitroalkenes catalyzed by an axially chiral guanidine. J. Am. Chem. Soc. 2007, 129, 14112–14113. [Google Scholar] [CrossRef]
- Wang, J.; Heikkinen, L.D.; Li, H.; Zu, L.; Jiang, W.; Xie, H.; Wang, W. Quinine-catalyzed enantioselective Michael addition of diphenyl phosphite to nitroolefins: Synthesis of chiral precursors of α−substituted β-aminophosphonates. Adv. Synth. Catal. 2007, 349, 1052–1056. [Google Scholar] [CrossRef]
- Zhu, Y.; Malerich, J.P.; Rawal, V.H. Squaramide-catalyzed enantioselective Michael addition of diphenyl phosphite to nitroalkenes. Angew. Chem. 2010, 122, 157–160. [Google Scholar] [CrossRef]
- Tessema, E.; Elakkat, V.; Chiu, C.-F.; Zheng, J.-H.; Chan, K.L.; Shen, C.-R.; Zhang, P.; Lu, N. Recoverable Phospha-Michael Additions Catalyzed by a 4-N,N-Dimethylaminopyridinium Saccharinate Salt or a Fluorous Long-Chained Pyridine: Two Types of Reusable Base Catalysts. Molecules 2021, 26, 1159. [Google Scholar] [CrossRef]
- Sufcer-Mosse, S.; Tissot, M.; Alexakis, A. First Enantioselective Organocatalytic Conjugate Addition of Aldehydes to Vinyl Phosphonates. Org. Lett. 2007, 9, 3749–3752. [Google Scholar] [CrossRef] [PubMed]
- Sulzer-Moss, S.; Alexakis, A.; Mareda, J. Enantioselective Organocatalytic Conjugate Addition of Aldehydes to Vinyl Sulfones and Vinyl Phosphonates as Challenging Michael Acceptors. Chem. Eur. J. 2009, 15, 3204–3220. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.-W.; Liu, Y.-Y.; Ding, W.; Li, T.-R.; Shi, D.Q.; Chen, J.-R.; Xiao, W.-J. Chiral Squaramide Catalyzed Asymmetric Conjugate Additions of 3-Substituted Oxindoles to Vinylphosphonates. Synthesis 2013, 45, 1647–1653. [Google Scholar] [CrossRef]
- Wen, S.; Li, P.; Wu, H.; Yu, F.; Liang, X.; Ye, J. Enantioselective organocatalytic phospha-Michael reaction of α,β-unsaturated ketones. Chem. Commun. 2010, 46, 4806–4808. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Wu, G. Organocatalyzed Enantioselective Michael Addition of 2-Hydroxy-1,4-naphtho-quinone to p,y-Unsaturated Ketophosphonates. J. Org. Chem. 2011, 76, 4119–4124. [Google Scholar] [CrossRef]
- Sohtome, Y.; Horitsugi, N.; Takagi, R.; Nagasawa, K. Enantioselective Phospha-Michael Reaction of Diphenyl Phosphonate with Nitroolefins Utilizing Conformationally Flexible Guanidinium/Bisthiourea Organocatalyst: Assembly-State Tunability in Asymmetric ganocatalysis. Adv. Synth. Cat. 2011, 353, 2631–2636. [Google Scholar] [CrossRef]
- Arai, R.; Hirashima, S.-I.; Nakano, T.; Kawada, M.; Akutsu, H.; Nakashima, K.; Miura, T. Asymmetric Conjugate Addition of Phosphonates to Enones Using Cinchona–Diaminomethylenemalononitrile Organocatalysts. J. Org. Chem. 2020, 85, 3872–3878. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, Z.; Li, X.; Liang, X.; Ye, J. Enantioselective organocatalytic phospha-Michael reaction of α,β-unsaturated aldehydes. RSC Adv. 2011, 1, 698–705. [Google Scholar] [CrossRef]
- Albrecht, L.; Richter, B.; Vila, C.; Krawczyk, H.; Jørgensen, A.K. Organocatalytic Domino Michael-Knoevenagel Condensation Reaction for the Synthesis of Optically Active 3-Diethoxyphosphoryl-2-oxocyclohex-3-enecarboxylates. Chem. Eur. J. 2009, 15, 3093–3102. [Google Scholar] [CrossRef]
- Phillips, A.M.F.; Barros, M.T. Enantioselective organocatalytic synthesis of α-cyclopropylphosphonates through a domino Michael addition/intramolecular alkylation reaction. Eur. J. Org. Chem. 2014, 2014, 152–163. [Google Scholar] [CrossRef]
- Chen, J.; Wen, X.; Wang, Y.; Du, F.; Cai, L.; Peng, Y. Asymmetric Mannich Reaction of Isatin-Based Ketimines with α-Diazomethylphosphonates Catalyzed by Chiral Silver Phosphate. Org. Lett. 2016, 18, 4336–4339. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Kurra, Y.; Liu, W.R. Phospha-Michael Addition as a New Click Reaction for Protein Functionalization. Chembiochem 2016, 17, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Bera, K.; Namboothiri, I.N.N. Quinine-derived thiourea and squaramide catalyzed conjugate addition of nitrophosphonates to enones: Asymmetric synthesis of quaternary aminophosphonates. J. Org. Chem. 2015, 80, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Perera, S.; Zhao, C.-G. Organocatalytic Enantioselective Synthesis of Both Diastereomers of Hydroxyphosphinates. J. Org. Chem. 2010, 75, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Dodda, R.; Zhao, C.G. Organocatalytic Highly Enantioselective Synthesis of Secondary R-Hydroxyphosphonates. Org. Lett. 2006, 8, 4911–4914. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Guo, L.; Xu, L.; Loh, T.-P. Asymmetric P−C Bond Formation: Diastereoselective Synthesis of Adjacent P,C-Stereogenic Allylic Phosphorus Compounds. Chem. Asian J. 2016, 9, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Scriban, C.; Glueck, D.S.; Zakharov, L.N. P-C and C-C Bond Formation by Michael Addition in Platinum-Catalyzed Hydrophosphination and in the Stoichiometric Reactions of Platinum Phosphido Complexes with Activated Alkenes. Organometallics 2006, 25, 5757–5767. [Google Scholar] [CrossRef]
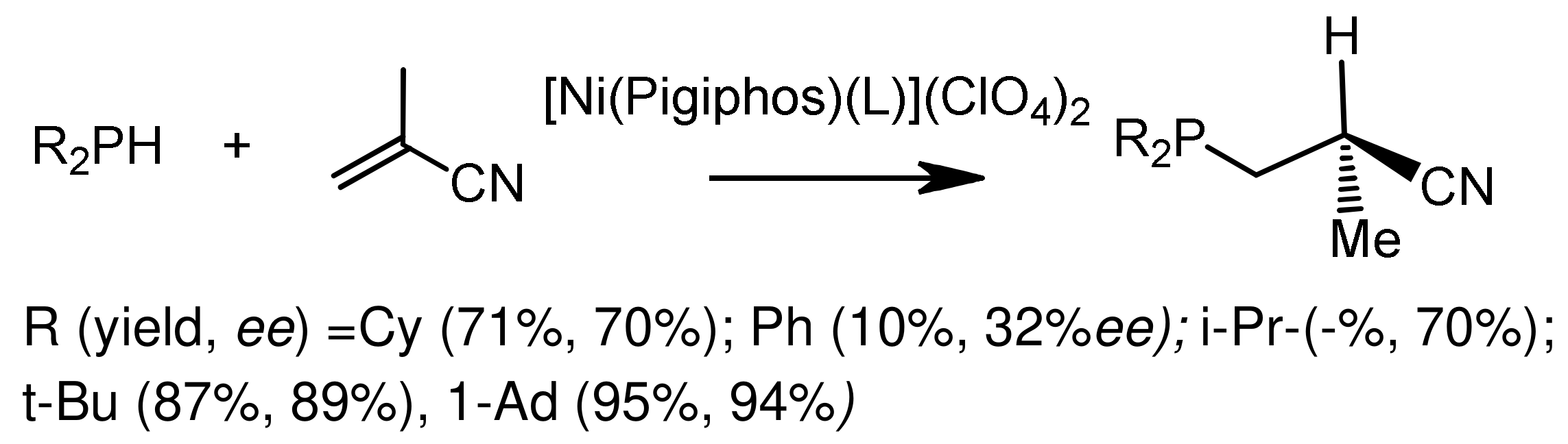
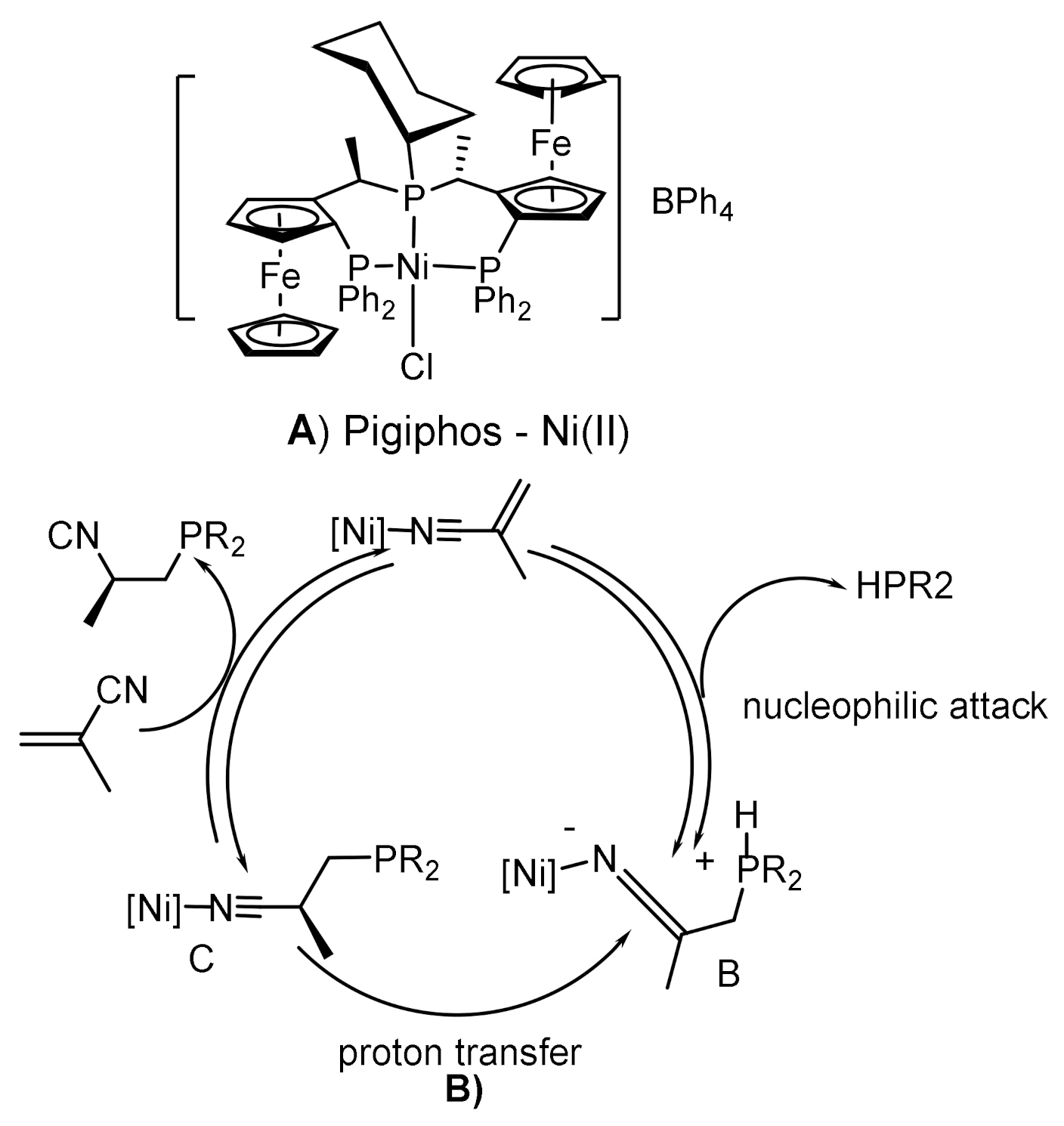
- Sadow, A.D.; Haller, I.; Fadini, L.; Togni, A. Nickel(Il)-Catalyzed Highly Enantioselective Hydrophosphination of Methacrylonitrile. J. Am. Chem. Soc. 2004, 126, 14704–14705. [Google Scholar] [CrossRef]
- Sadow, A.D.; Togni, A. Enantioselective Addition of Secondary Phosphines to Methacrylonitrile: Catalysis and Mechanism. J. Am. Chem. Soc. 2005, 127, 17012–17024. [Google Scholar] [CrossRef]
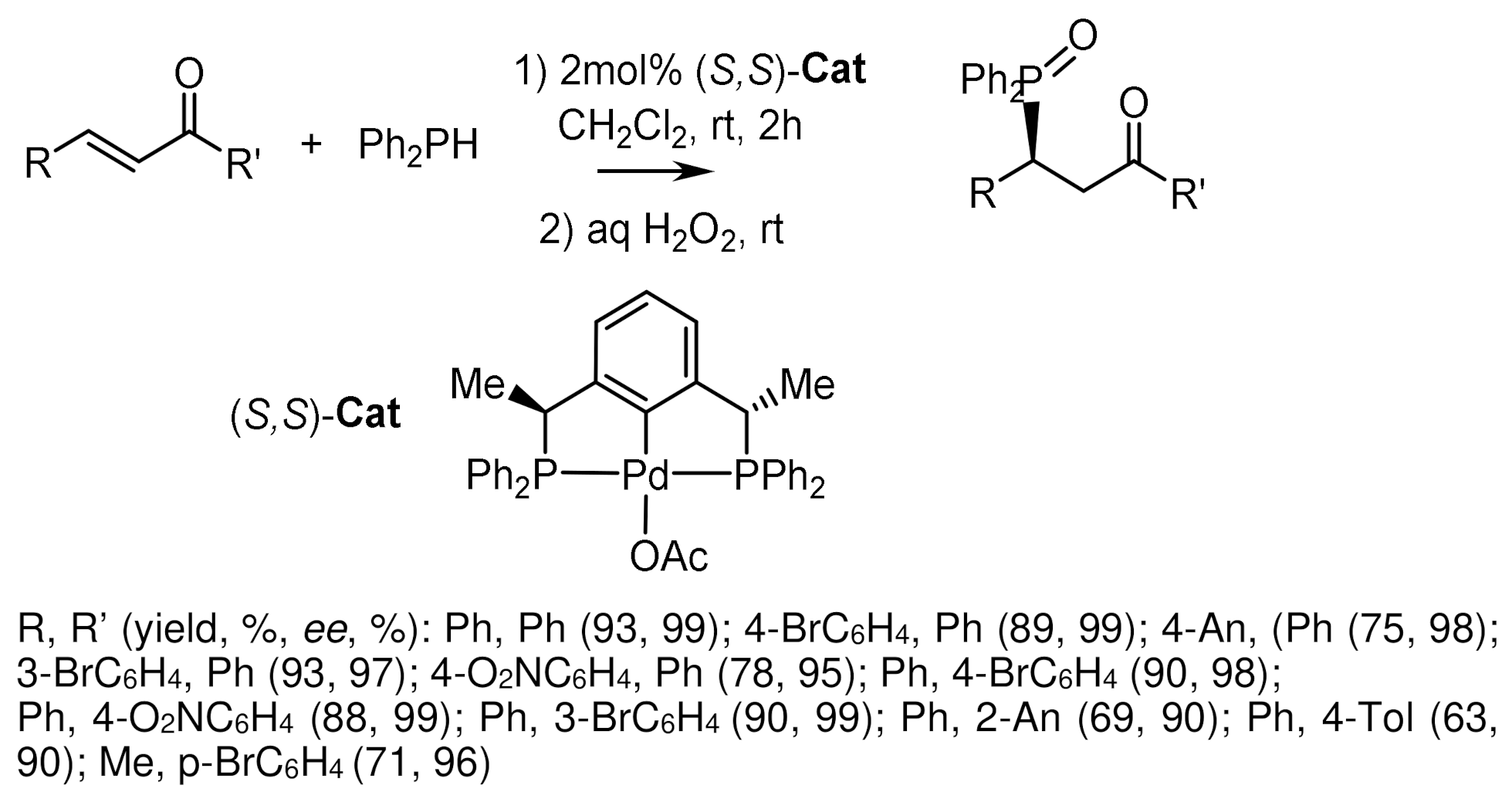
- Feng, J.J.; Chen, X.F.; Shi, M.; Duan, W.L. Palladium-Catalyzed Asymmetric Addition of Diarylphosphines to Enones toward the Synthesis of Chiral Phosphines. J. Am. Chem. Soc. 2010, 132, 5562–5563. [Google Scholar] [CrossRef]
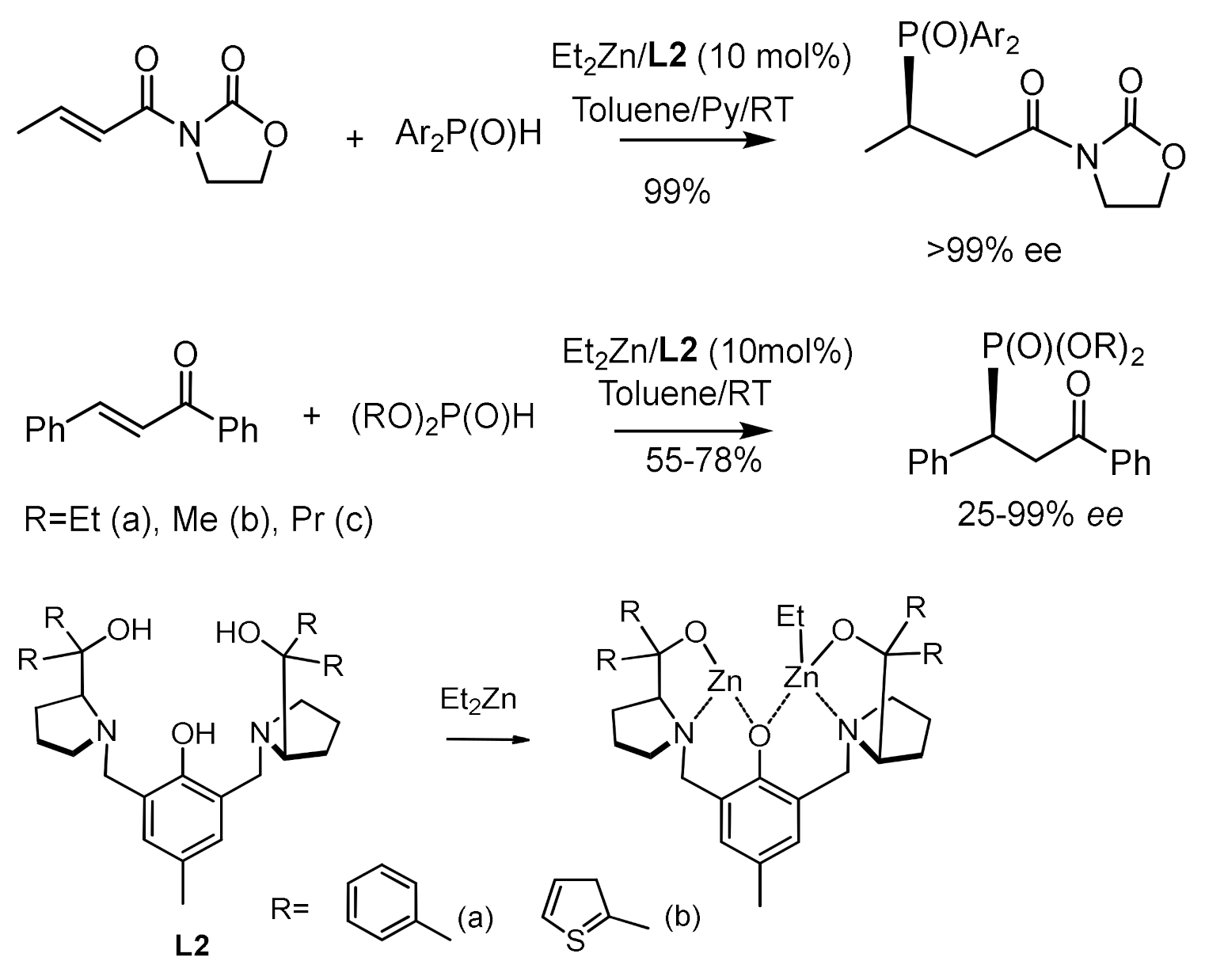
- Zhao, D.; Mao, L.; Wang, L. Catalytic asymmetric construction of tetrasubstituted carbon stereocenters by conjugate addition of dialkyl phosphine oxides to β,β–disubstituted a,b-unsaturated carbonyl compounds. J. Chem. Soc. Chem. Comm. 2012, 48, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Luo, Y.Y.; Lu, H.-J.; Hua, Y.-Z.; Wang, M.-C. Enantioselective phospha-Michael reaction of diethyl phosphonate with exocyclic α,β-unsaturated benzocyclic ketones catalyzed by a dinuclear zinc−AzePhenol catalyst. Tetrahedron 2018, 74, 2130–2142. [Google Scholar] [CrossRef]
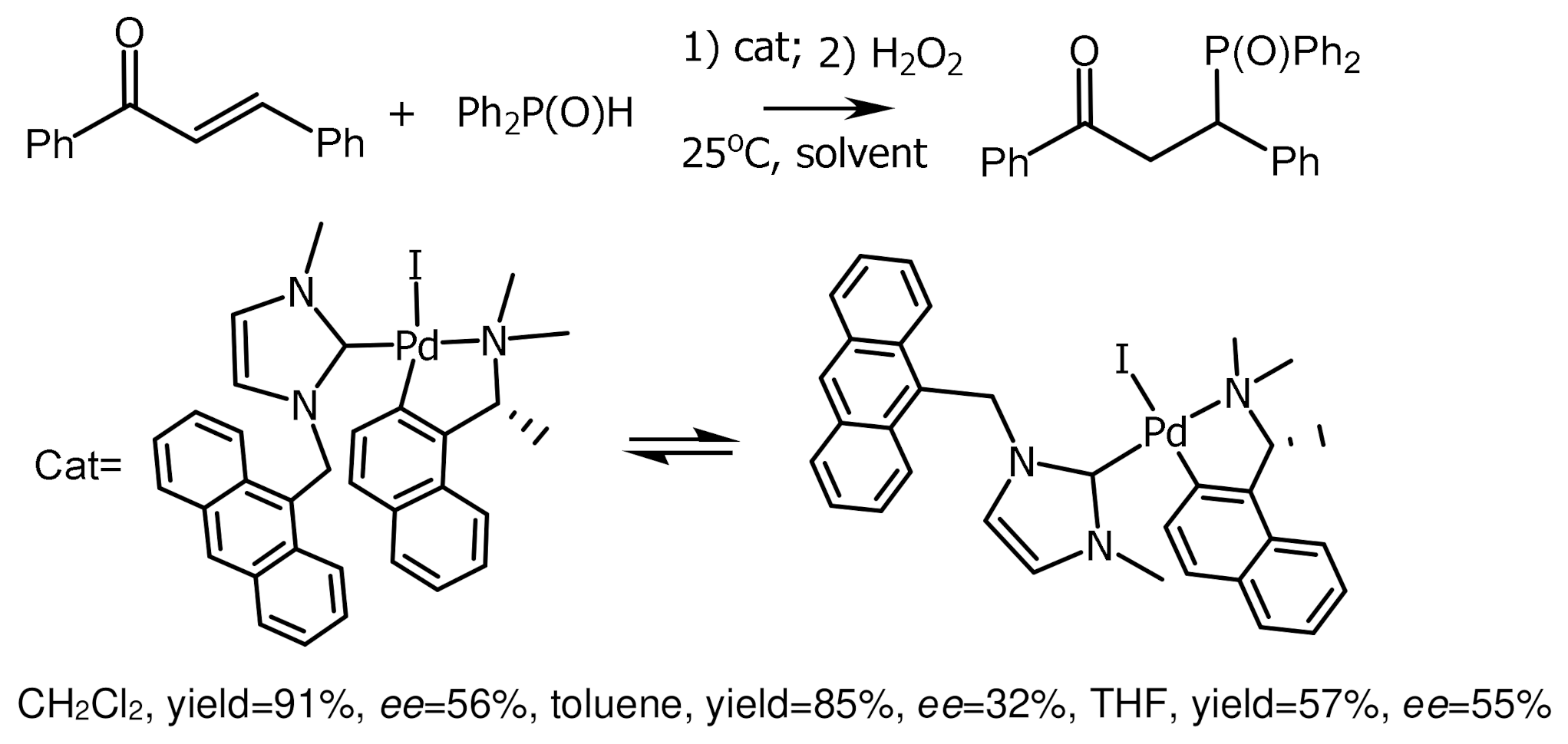
- Sabater, S.; Mata, J.A.; Peris, E. Chiral Palladacycles with N-Heterocyclic Carbene Ligands as Catalysts for Asymmetric Hydrophosphination Chiral Palladacycles with N-Heterocyclic Carbene Ligands as Catalysts for Asymmetric Hydrophosphination. Organometallics 2013, 32, 1112–1120. [Google Scholar] [CrossRef]
- Russo, A.; Lattanzi, A. Asymmetric Organocatalytic Conjugate Addition of Diarylphosphane Oxides to Chalcones. Eur. J. Org. Chem. 2010, 2010, 6736–6739. [Google Scholar] [CrossRef]
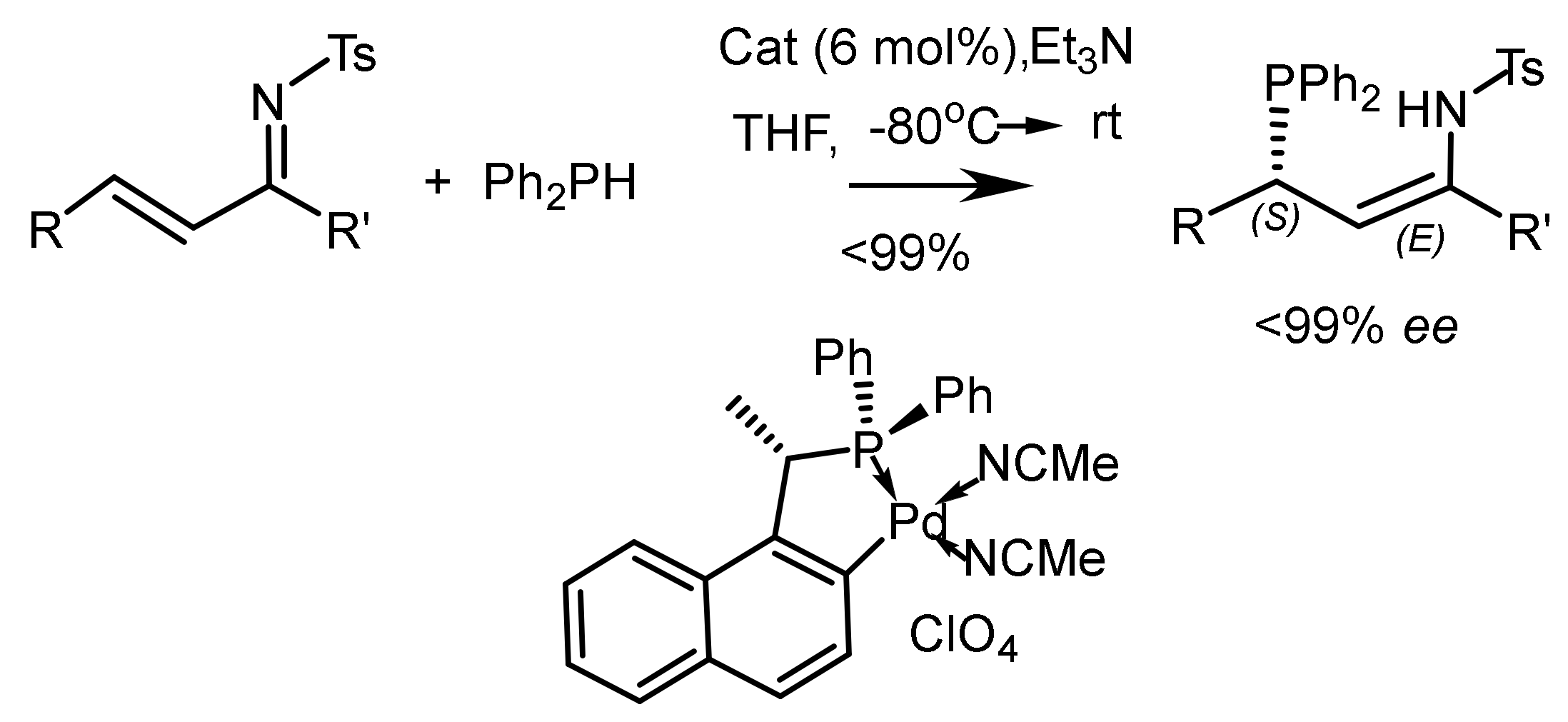
- Huang, Y.R.; Chew, J.; Pullarkat, S.A. Asymmetric Synthesis of Enaminophosphines via Palladacycle-Catalyzed Addition of Ph2PH to α, β-Unsaturated Imines. J. Org. Chem 2012, 77, 6849–6854. [Google Scholar] [CrossRef]
- Nakamura, S.; Hayashi, M.; Hiramatsu, Y.; Shibata, N.; Funahashi, Y.; Toru, T. Catalytic Enantioselective Hydrophosphonylation of Ketimines Using Cinchona Alkaloids. J. Am. Chem. Soc. 2009, 131, 18240–18241. [Google Scholar] [CrossRef]
- Yadavalli, K.P.; Cummines, J.E.; Carlislea, C.J.; Lepore, S.D. Diastereoselective additions of H-phosphinates to alkenyl ketones under phase-transfer conditions. Chem. Commun. 2022, 58, 6441–6444. [Google Scholar] [CrossRef]
- Huang, Y.; Pullarkat, S.A.; Teong, S.; Chew, R.J.; Li, Y.; Leung, P.-H. Palladacycle-Catalyzed Asymmetric Intermolecular Construction of Chiral Tertiary P- Y. Heterocycles by Stepwise Addition of H-P-H Bonds to Bis(enones). Organometallics 2012, 31, 4871–4875. [Google Scholar] [CrossRef]
- Rai, V.; Namboothiri, I.N.N. Enantioselective conjugate addition of dialkyl phosphites to nitroalkenes. Tetrahedron Asymm. 2008, 19, 2335–2338. [Google Scholar] [CrossRef]
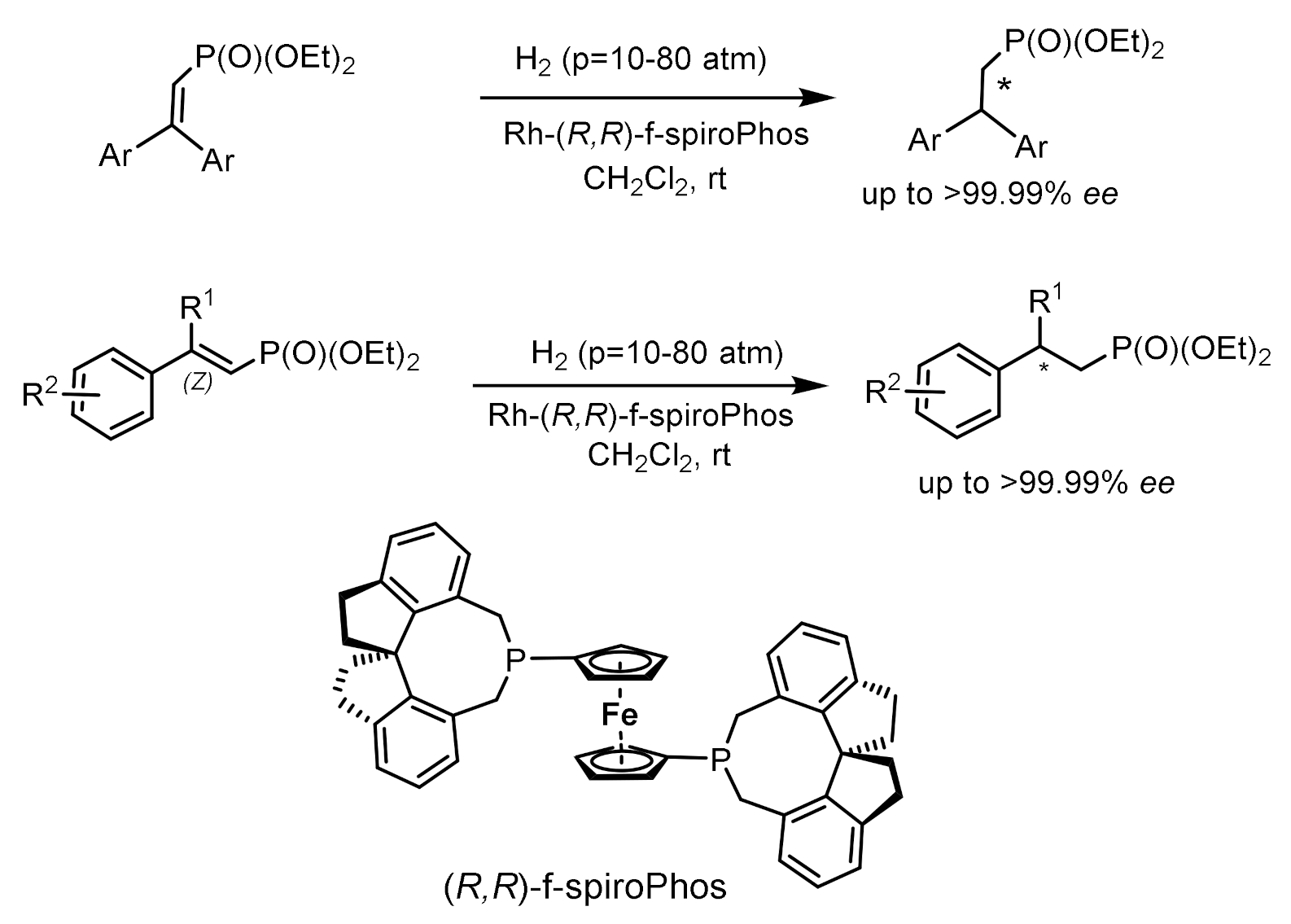
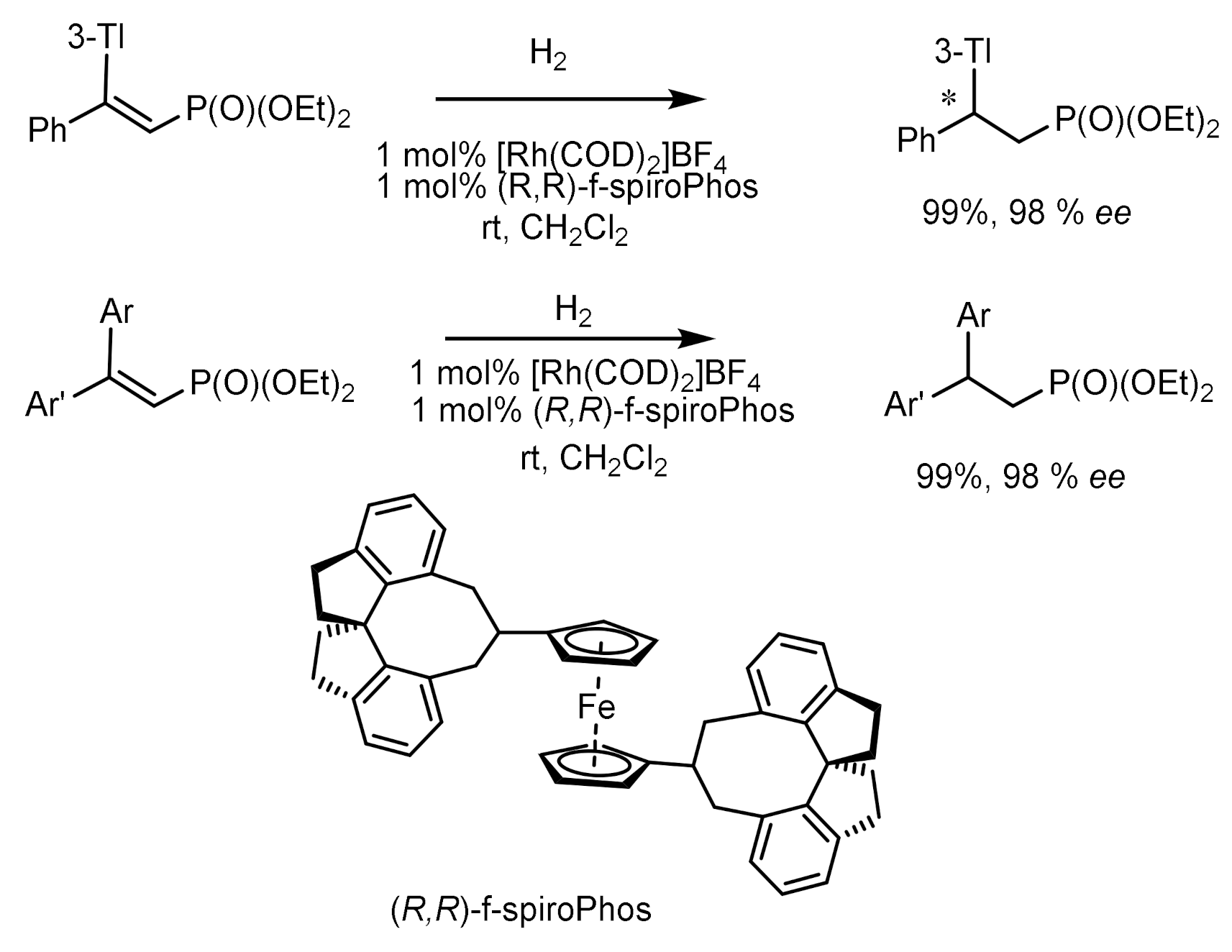
- Wang, C.; Xie, F.; Guo, Q.; Xie, C.; Zi, G.; Ye, W.; Zhou, Z.; Hou, G.; Zhang, Z. Enantioselective Synthesis of Chiral Phosphonates via Rh/f-spiroPhos Catalyzed Asymmetric Hydrogenation of β,β-Disubstituted Unsaturated Phosphonates. J. Org. Chem. 2021, 86, 12034–12045. [Google Scholar] [CrossRef]
- Hou, L.; Kikuchi, J.; Ye, H.; Bao, M.; Terada, M. Chiral Phosphoric Acid-Catalyzed Enantioselective Phospha-Michael-Type Addition Reaction of Diarylphosphine Oxides with Alkenyl Benzimidazoles. J. Org. Chem. 2020, 85, 14802–14809. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A.; del Corte, X.; López-Francés, A.; de Marigorta, E.M.; Palacios, F.; Vicario, J. Asymmetric Synthesis of Tetrasubstituted α-Aminophosphonic Acid Derivatives. Molecules 2021, 26, 3202. [Google Scholar] [CrossRef] [PubMed]
- Vicario, J.; Ortiz, P.; Ezpeleta, J.M.; Palacios, F. Asymmetric synthesis of functionalized tetrasubstituted aminophosphonates through enantioselective aza-Henry reaction of phosphorylated ketimines. J. Org. Chem. 2015, 80, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A.; del Corte, X.; Martinez de Marigorta, E.; Palacios, F.; Vicario, J. Enantioselective synthesis of functionalized -aminophosphonic acid derivatives. Phosph. Sulf. Silicon Relat. Elem. 2019, 194, 287–291. [Google Scholar] [CrossRef]
- Maestro, A.; Martinez de Marigorta, E.; Palacios, F.; Vicario, J. Enantioselective Aza-Reformatsky Reaction with Ketimines. Org. Lett. 2019, 21, 9473–9477. [Google Scholar] [CrossRef]
- Rassukana, Y.V.; Yelenich, I.P.; Vlasenko, Y.G.; Onys’ko, P.P. Asymmetric synthesis of phosphonotrifluoroalanine derivatives via proline-catalyzed direct enantioselective CC bond formation reactions of NH trifluoroacetimidoyl phosphonate. Tetrahedron Asymmetry 2014, 25, 1234–1238. [Google Scholar] [CrossRef]
- Huang, N.; Tong, X.; Zhou, S.; Guo, Q.; Peng, Y. Asymmetric [3 + 2] Cycloaddition Reactions of α-Substituted Diazophosphonates with 3-Acryloyl-2-oxazolidinone to Access Chiral Pyrazoline Derivatives with Phosphonyl at a Tetrasubstituted Stereogenic Center. Adv. Synth. Catal. 2019, 361, 4805–4810. [Google Scholar] [CrossRef]
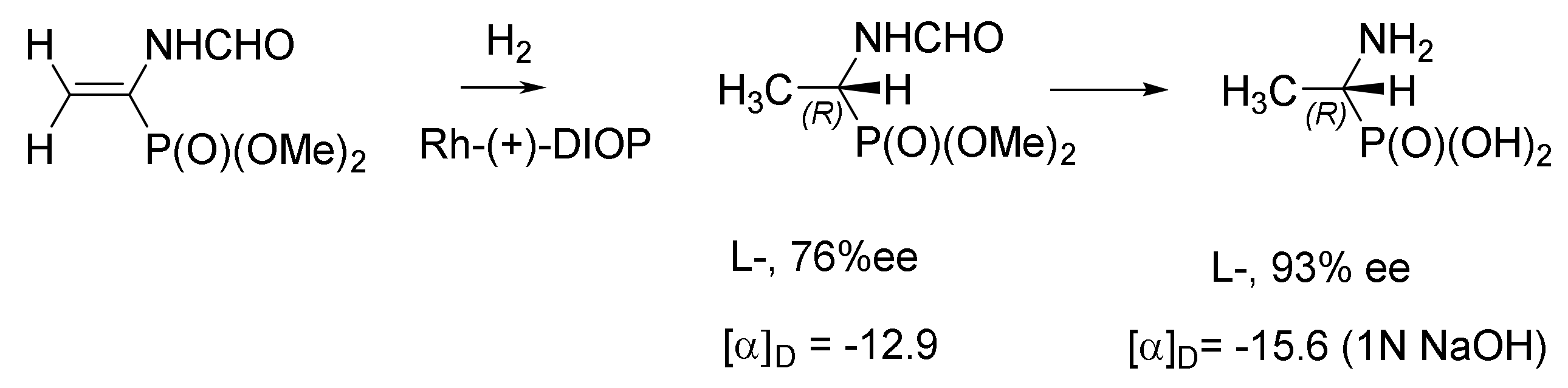
- Kadyrov, R.; Holz, J.; Schaffner, B.; Zayas, O.; Almena, J.; Boerner, A. Synthesis of chiral β-aminophosphonates via Rh-catalyzed asymmetric hydrogenation of β-amido-vinylphosphonates. Tetrahedron Asymmetry 2008, 19, 1189–1192. [Google Scholar] [CrossRef]
- Blaser, H.U. The Chiral Switch of Metolachlor. In Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions; Blaser, H.-U., Schmidt, E., Eds.; Wiley: Basel, Switzerland, 2004; pp. 55–70. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, K.; Wang, Z.; Ding, K. Asymmetric hydrogenation of α- or β-acyloxy α,β-unsaturated phosphonates catalyzed by a Rh(i) complex of monodentate phosphoramidite. Org. Biomol. Chem. 2012, 10, 1598–1601. [Google Scholar] [CrossRef]
- Wang, D.Y.; Hu, X.P.; Huang, J.D.; Deng, J.; Yu, S.B.; Duan, Z.C.; Xu, X.-F.; Zheng, Z. Readily available chiral phosphine−aminophosphine ligands for highly efficient Rh-catalyzed asymmetric hydrogenation of α-enol ester phosphonates and α-enamido phosphonates Z. J. Org. Chem. 2008, 73, 2011–2014. [Google Scholar] [CrossRef]
- Kondoh, A.; Yorimitsu, H.; Oshima, K. Regio- and Stereoselective Hydroamidation of 1-Alkynylphosphine Sulfides Catalyzed by Cesium Base. Org. Lett. 2008, 10, 3093–3095. [Google Scholar] [CrossRef] [PubMed]
- Doherty, S.; Knight, J.G.; Bell, A.L. Rhodium complexes of (R)-Me-CATPHOS and (R)-(S)-JOSIPHOS: Highlyenantioselective catalysts for the asymmetric hydrogenation of (E)- and (Z)- J3-aryl-J3-(enamido) phosphonates. Tetrahedron Asymmetry 2009, 20, 1437–1444. [Google Scholar] [CrossRef]
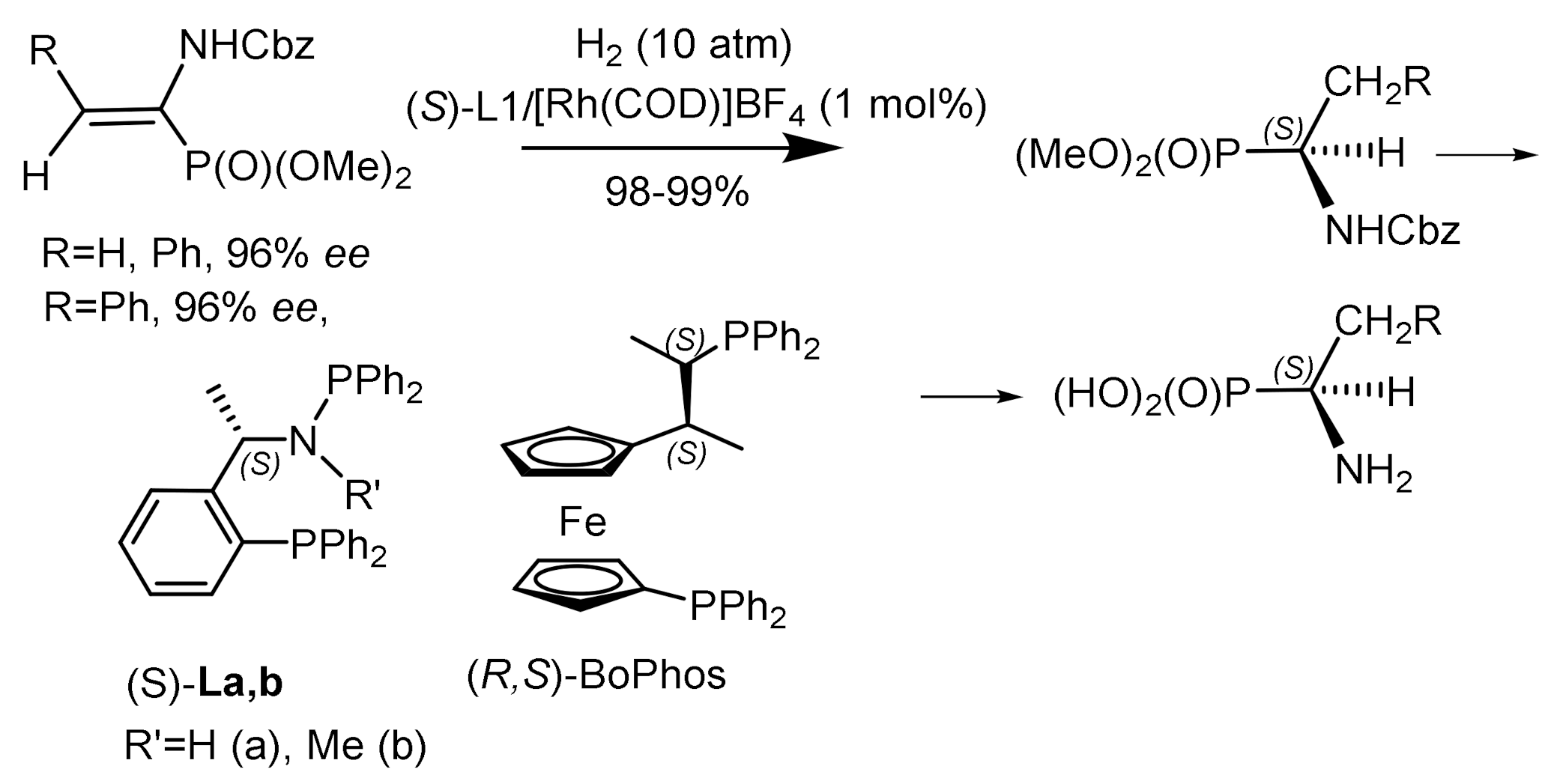
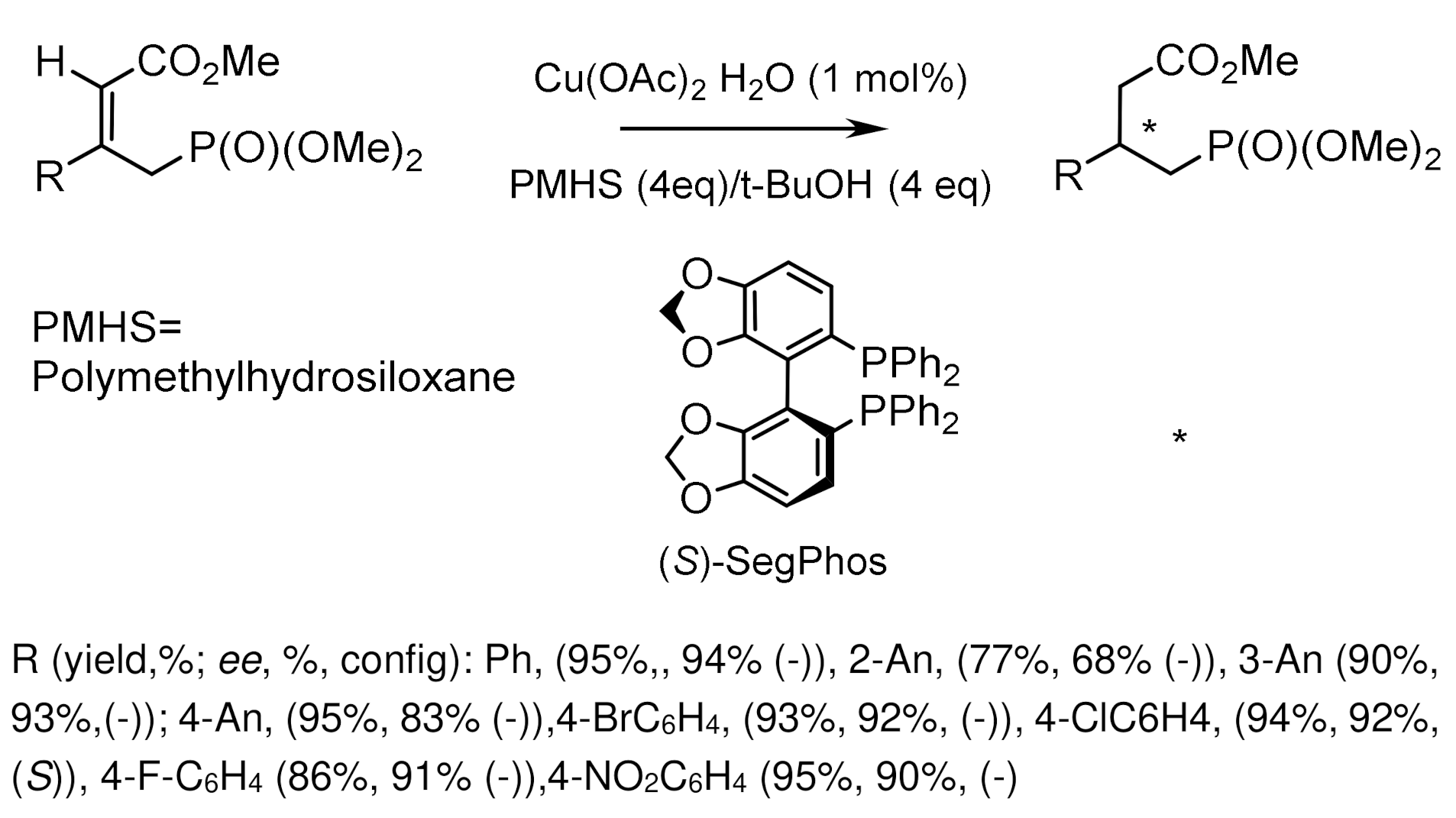
- Duan, Z.-C.; Hu, X.-P.; Wang, D.-Y.; Huang, J.-D.; Yu, S.-B.; Deng, J.; Zheng, Z. Enantioselective Synthesis of Optically Active Alkanephosphonates via Rhodium-Catalyzed Asymmetric Hydrogenation of β-Substituted α,β-Unsaturated Phosphonates with Ferrocene-Based Monophosphoramidite Ligands. Adv. Synth. Cat. 2008, 350, 1979–1983. [Google Scholar] [CrossRef]
- Goulioukina, N.S.; Dolgina, T.M.; Bondarenko, G.N.; Beletskaya, I.P.; MIlyin, M.; Davankov, V.A.; Pfaltz, A. Highly enantioselective hydrogenation of α,β-unsaturated phosphonates with iridium–phosphinooxazoline complex: Synthesis of a phosphorus analogue of naproxen. Tetrahedron Asymmetry 2003, 14, 1397–1401. [Google Scholar] [CrossRef]
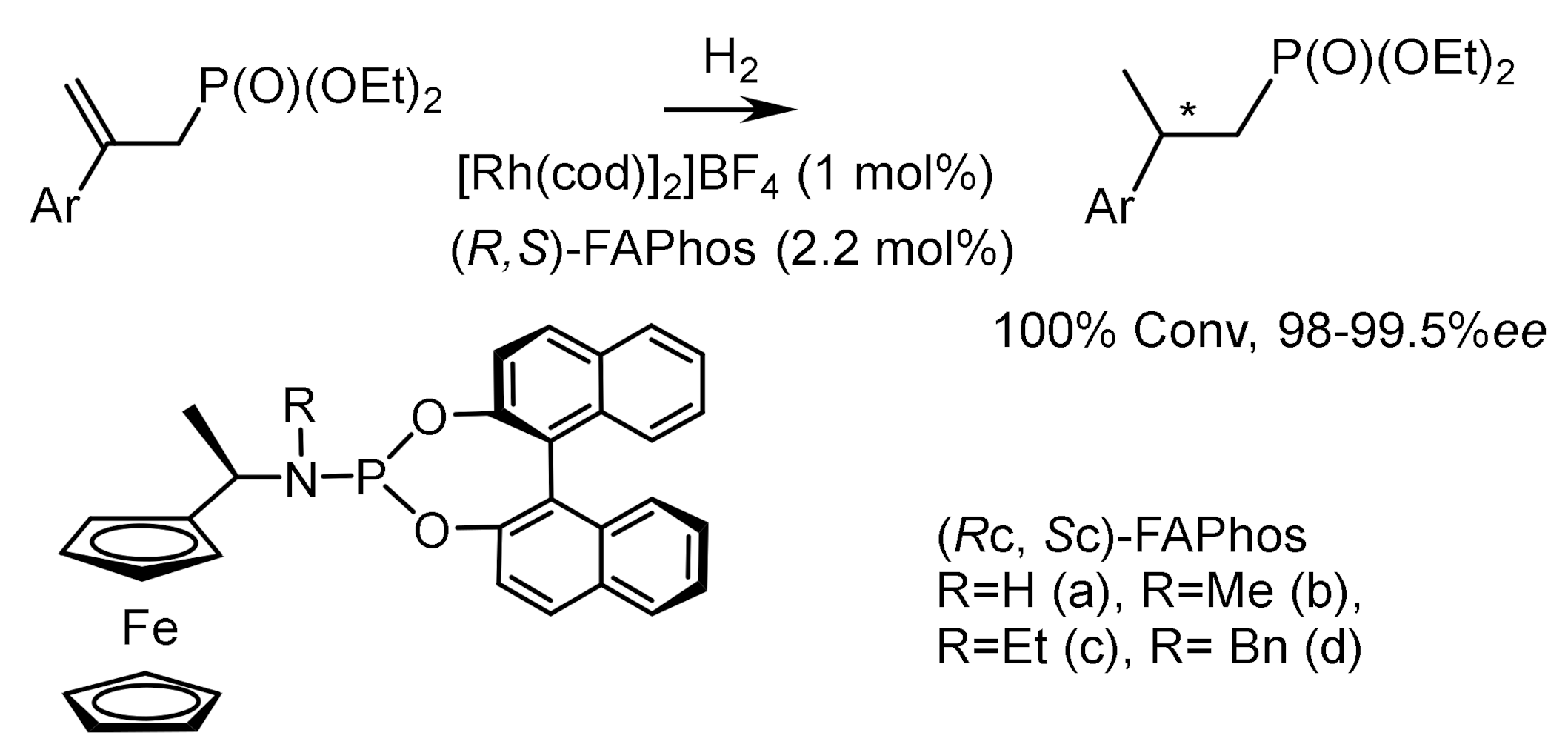
- Duan, Z.C.; Hu, X.P.; Zhang, C.; Wang, D.Y.; Yu, S.B.; Zheng, Z. Highly enantioselective Rh-catalyzed hydrogenation of β,γ-unsaturated phosphonates with chiral ferrocene-based monophosphoramidite ligands. J. Org. Chem. 2009, 74, 9191–9194. [Google Scholar] [CrossRef]
- Oki, H.; Oura, I.; Nakamura, T.; Ogata, K.; Fukuzawa, S.I. Modular synthesis of the ClickFerrophos ligand family and their use in Rhodium- and ruthenium-catalyzed asymmetric hydrogenation. Tetrahedron Asymmetry 2009, 20, 2185–2191. [Google Scholar] [CrossRef]
- Rubio, M.A.; Suárez, A.; Alvarez, E.; Pizzano, A. Highly enantioselective hydrogenation of enol ester phosphonates catalyzed by rhodium phosphine-phosphite complexes. Chem. Commun. 2005, 2005, 628–630. [Google Scholar] [CrossRef]
- Duan, Z.C.; Hu, X.P.; Zhang, C.; Zheng, Z. Enantioselective Rh-Catalyzed Hydrogenation of 3-Aryl-4-phosphonobutenoates with a P-Stereogenic BoPhoz-Type Ligand. J. Org. Chem. 2010, 75, 8319–8321. [Google Scholar] [CrossRef]
- Cheruku, P.; Paptchikhine, A.; Church, T.L.; Andersson, P.G. Iridium-N,P-Ligand-Catalyzed Enantioselective Hydrogenation of Diphenylvinylphosphine Oxides and Vinylphosphonates. J. Am. Chem. Soc. 2009, 131, 8285–8289. [Google Scholar] [CrossRef]
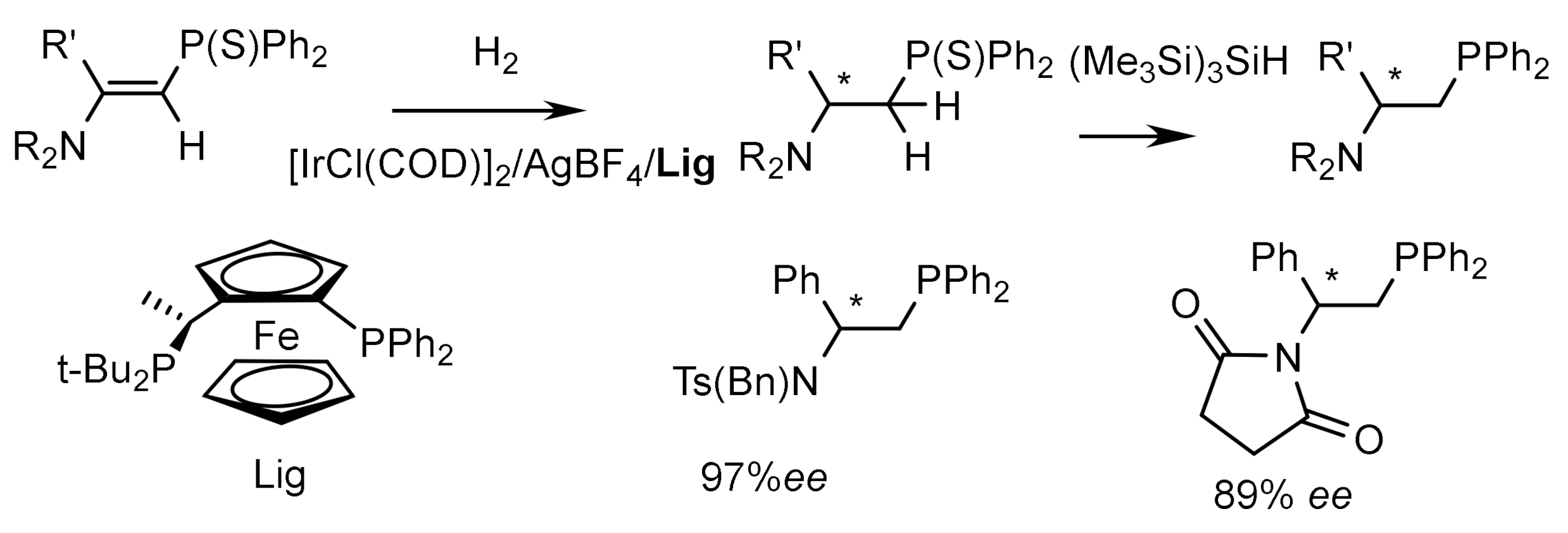
- Fernández-Pérez, H.; Lenartowicz, P.; Carreras, L.; Grabulosa, A.; Kafarski, P.; Vidal-Ferran, A. Access to α-Aminophosphonic Acid Derivatives and Phosphonopeptides by [Rh(P–OP)]-Catalyzed Stereoselective Hydrogenation. J. Org. Chem. 2020, 85, 14779–14784. [Google Scholar] [CrossRef]
- Noyori, R. Asymmetric Catalysis: Science and Opportunities (Nobel Lecture). Angew. Chem. Int. Ed. 2002, 41, 2008–2022. [Google Scholar] [CrossRef]
- Tao, X.; Li, W.; Li, X.; Xie, X.; Zhang, Z. Diastereo- and Enantioselective Asymmetric Hydrogenation of α-Amido-β-keto Phosphonates via Dynamic Kinetic Resolution. Org. Lett. 2013, 15, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-F.; Hou, C.-J.; Wei, D.-Q.; He, X.; Chu, T.-T.; Chen, X.-sh.; Hu, X.-P. Iridium-atalyzed asymmetric hydrogenation of β-ketophosphonates with chiral ferrocenyl P,N,N-ligands. Appl. Organomet. Chem. 2021, 35, e6283. [Google Scholar] [CrossRef]
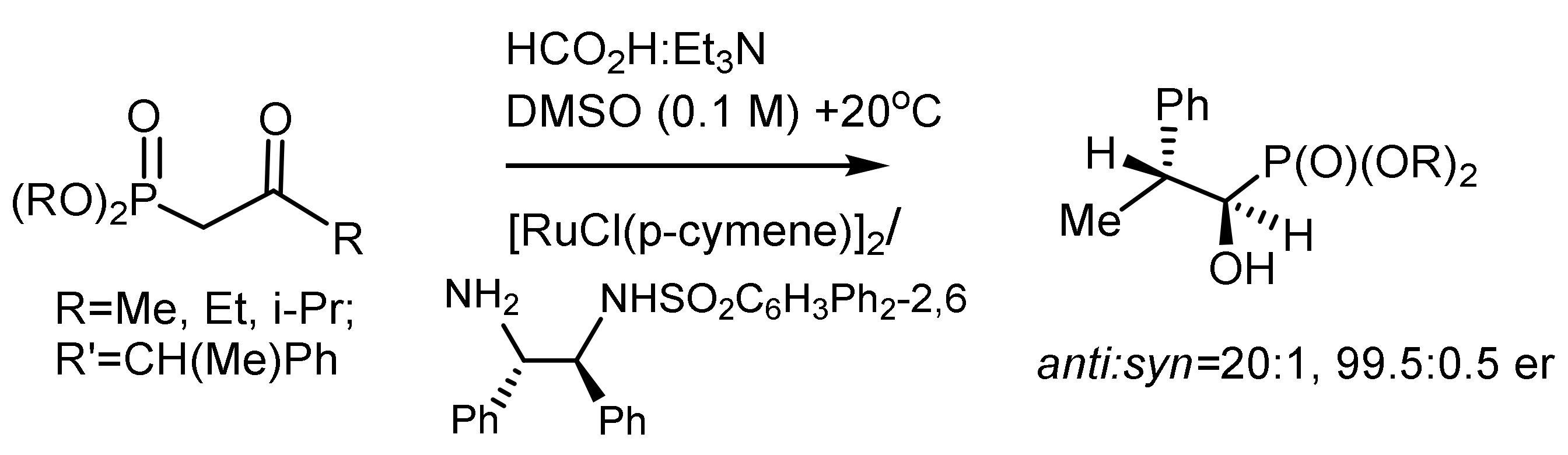
- Tao, X.; Li, W.; Ma, X.; Li, X.; Fan, W.; Zhu, L.; Xie, X.; Zhang, Z. Enantioselective Hydrogenation of β-Ketophosphonates with Chiral Ru(II) Catalysts. J. Org. Chem. 2012, 77, 8401–8409. [Google Scholar] [CrossRef] [PubMed]
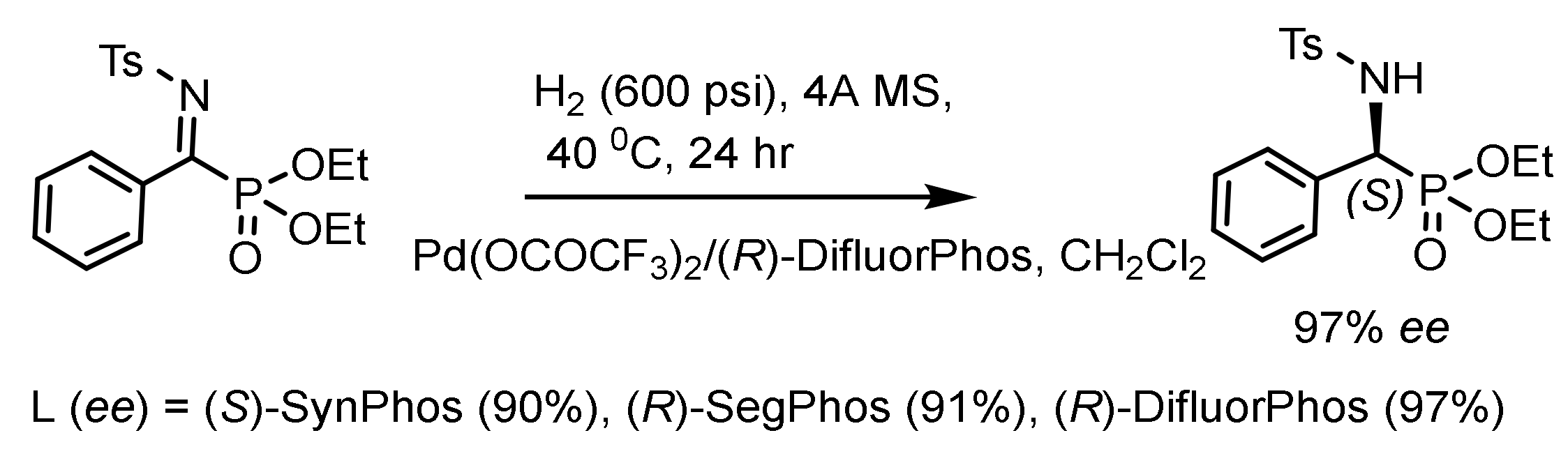
- Yan, Z.; Wu, B.; Gao, X.; Chen, M.-W.; Zhou, Y.-G. Enantioselective Synthesis of α-Amino Phosphonates via Pd-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2016, 18, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Laux, W.H.G.; Bats, J.W. Asymmetric synthesis of chiral, Nonra-cemic dialkyl-α-hydroxyalkylphosphonates via a (−)-Chlorodiisopino-campheylborane (Ipc2B-Cl) reduction. Tetrahedron Asymmetry 1996, 7, 89–94. [Google Scholar] [CrossRef]
- Heravi, M.M.; Asadi, S.; Nazari, N.; Lashkariani, B.M. Application of Corey–Bakshi–Shibata, Corey–Kim, Corey–Seebach, Corey–Winter, Corey–Link, and Corey–Ganem–Gilman in organic and total synthesis. Monatsh. Chem. Chemi. Month. 2016, 147, 961–987. [Google Scholar] [CrossRef]
- Barco, A.; Benetti, S.; Bergamini, P.; de Risi, C.; Marchetti, P.; Pollini, G.P.; Zanirato, V. Diastereoselective synthesis of β-amino-α-hydroxy phosphonates via oxazaborolidine catalyzed reduction of β-phthalimido-α-ketophosphonates. Tetrahedron Lett. 1999, 40, 7705–7708. [Google Scholar] [CrossRef]
- Rassukana, Y.V.; Onys’ko, P.P.; Kolotylo, M.V.; Sinitsa, A.D.; Lyzwa, P.; Mikoiajczyk, M. A new strategy for asymmetric synthesis of aminophosphonic acid derivatives: The first enantioselective catalytic reduction of C-phosphorylated imines. Tetrahedron Lett. 2009, 50, 288–290. [Google Scholar] [CrossRef]
- Onysko, P.P.; Chudakova, T.I.; Pirozhenko, V.V.; Rozhenko, A.B. α-Ketophosphonates in the synthesis of α-iminophosphonates. Curr. Green Chem. 2020, 7, 226–238. [Google Scholar] [CrossRef]
- Palacios, F.; Aparicio, D.; Ochoa de Retana, A.M.; de los Santos, J.M.; Gil, J.I.; Lopez de Munain, R. Asymmetric synthesis of 2H-aziridine phosphonates, and—Or -aminophosphonates from enantiomerically enriched 2H-azirines. Tetrahedron Asymmetry 2003, 14, 689–700. [Google Scholar] [CrossRef]
- Guliaiko, I.; Nesterov, V.; Sheiko, S.; Kolodiazhnyi, O.I.; Freytag, M.; Jones, P.G.; Schmutzler, R. Synthesis of Optically Active Hydroxyphosphonates. Heteroat. Chem. 2008, 19, 133–139. [Google Scholar] [CrossRef]
- Gryshkun, E.V.; Nesterov, V.; Kolodyazhnyi, O.I. Enantioselective reduction of ketophosphonates using adducts of chiral natural acids with sodium bo-rohydride. Arkivoc 2012, 2012, 100–117. [Google Scholar] [CrossRef]
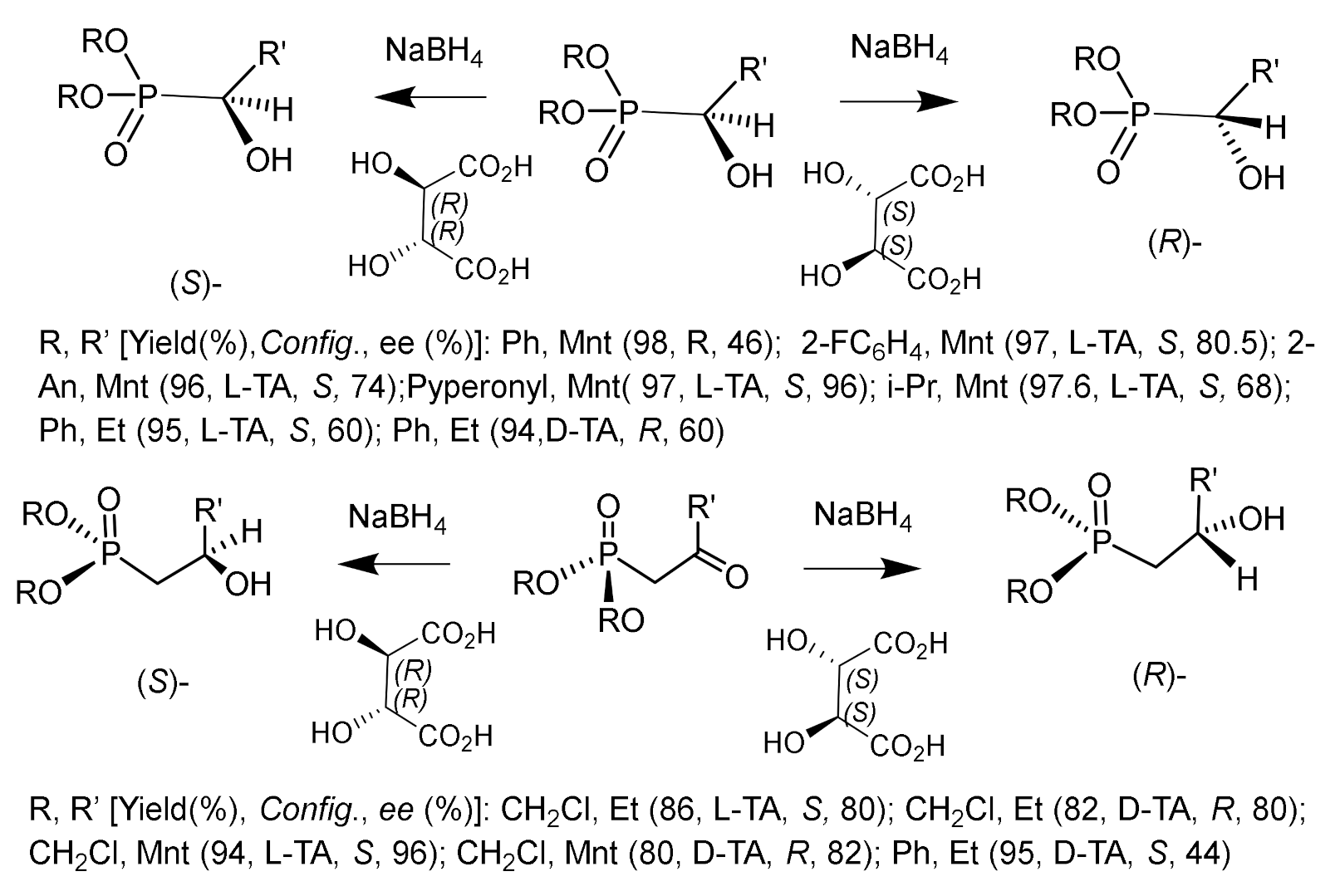
- Nesterov, V.V.; Kolodiazhnyi, O.I. Efficient method for the asymmetric reduction of α– and β–ketophosphonates. Tetrahedron 2007, 63, 6720–6731. [Google Scholar] [CrossRef]
- Nesterov, V.V.; Kolodiazhnyi, O.I. Di(1R,2S,5R)-menthyl 2-hydroxy-3-chloropropyl-phosphonate as useful chiron for the synthesis of α– and β–hydroxyphosphonates. Synlett 2007, 15, 2400–2404. [Google Scholar] [CrossRef]
- Corbett, M.T.; Johnson, J.S. Diametric Stereocontrol in Dynamic Catalytic Reduction of Racemic Acyl Phosphonates: Divergence from α-Keto Ester Congeners. J. Am. Chem. Soc. 2013, 135, 594–597. [Google Scholar] [CrossRef]
- Guo, W.L.; Hou, C.J.; Duan, Z.C.; Hu, X.P. An enantioselective synthesis of 3-aryl-4-phosphonobutyric acid esters via Cu-catalyzed asymmetric conjugate reduction. Tetrahedron Asymmetry 2011, 22, 2161–2164. [Google Scholar] [CrossRef]
- Mhamdi, A.; Touil, S. An operationally simple, fast and green synthesis of novel γ-hydroxyphosphonate and phosphine oxide derivatives. Green Chem. Lett. Rev. 2018, 11, 12–17. [Google Scholar] [CrossRef]
- Chiminazzo, A.; Sperni, L.; Scarso, A.; Strukul, G. Organocatalytic Enantioselective Epoxidation of Some Aryl-Substituted Vinylidenebisphosphonate Esters: On the Way to Chiral Anti-Osteoporosis Drugs. Catalysts 2017, 7, 90. [Google Scholar] [CrossRef]
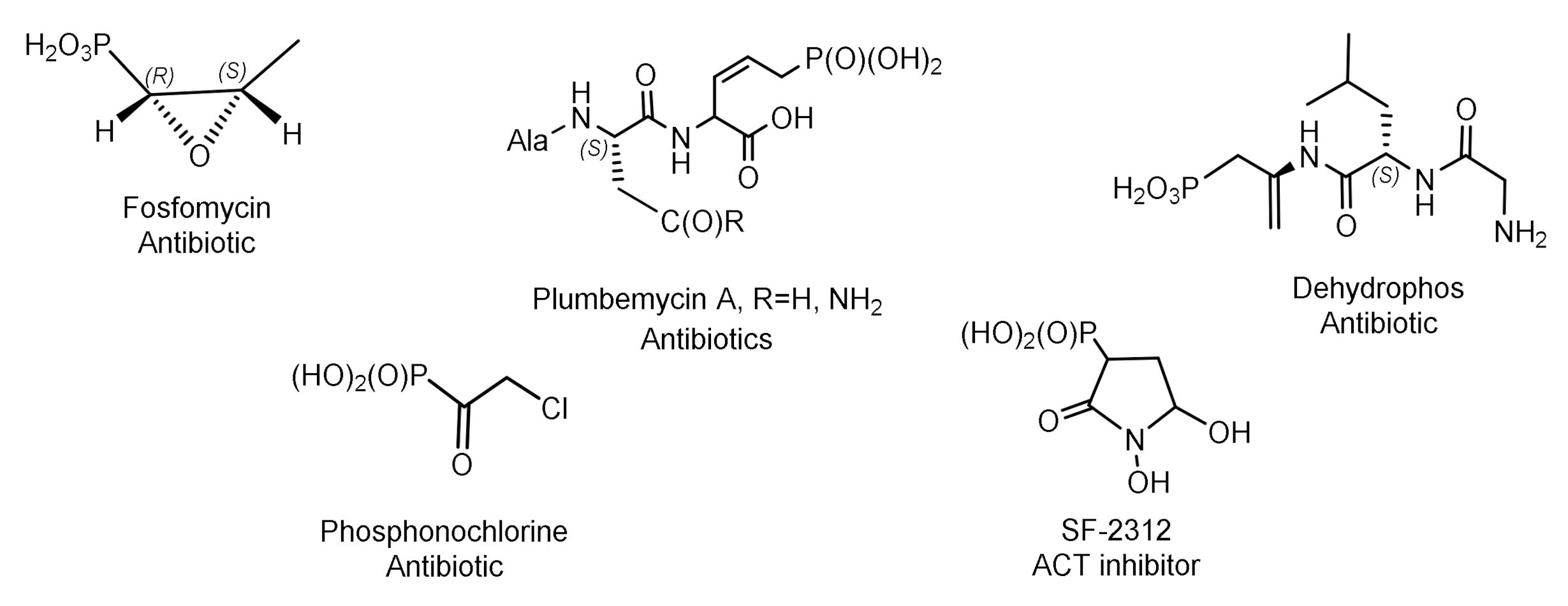
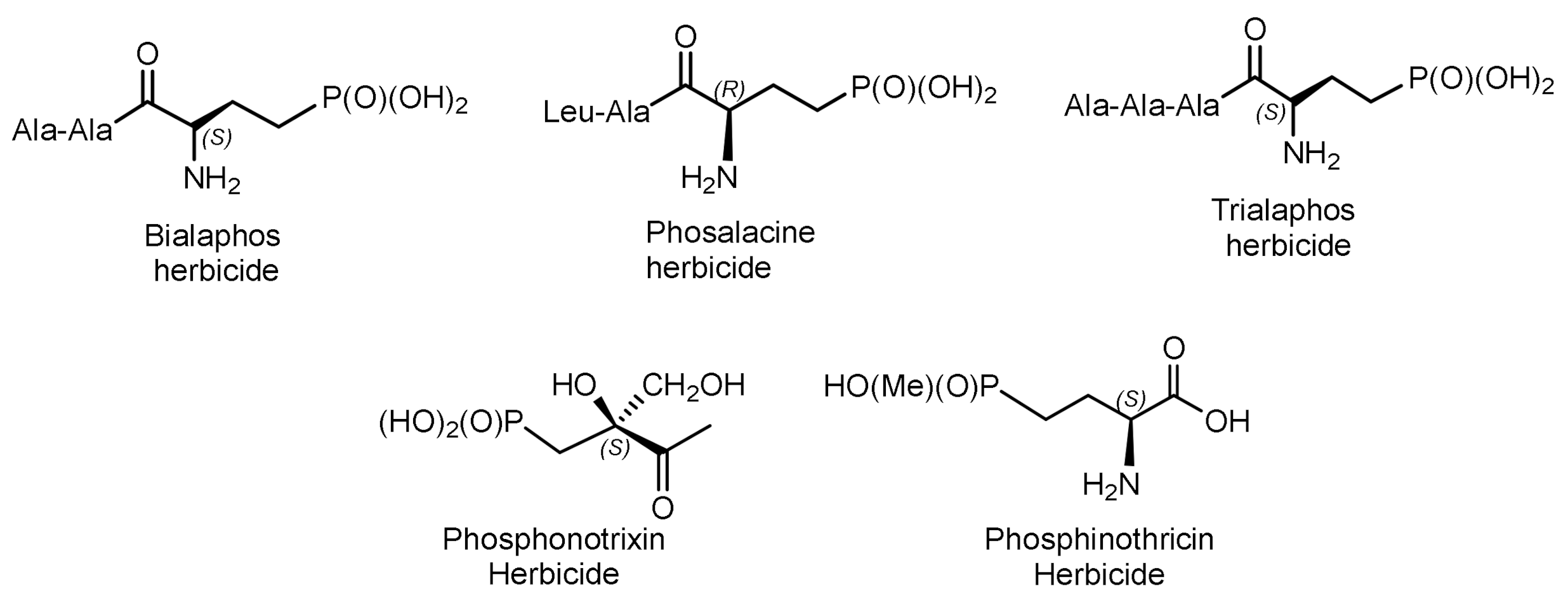

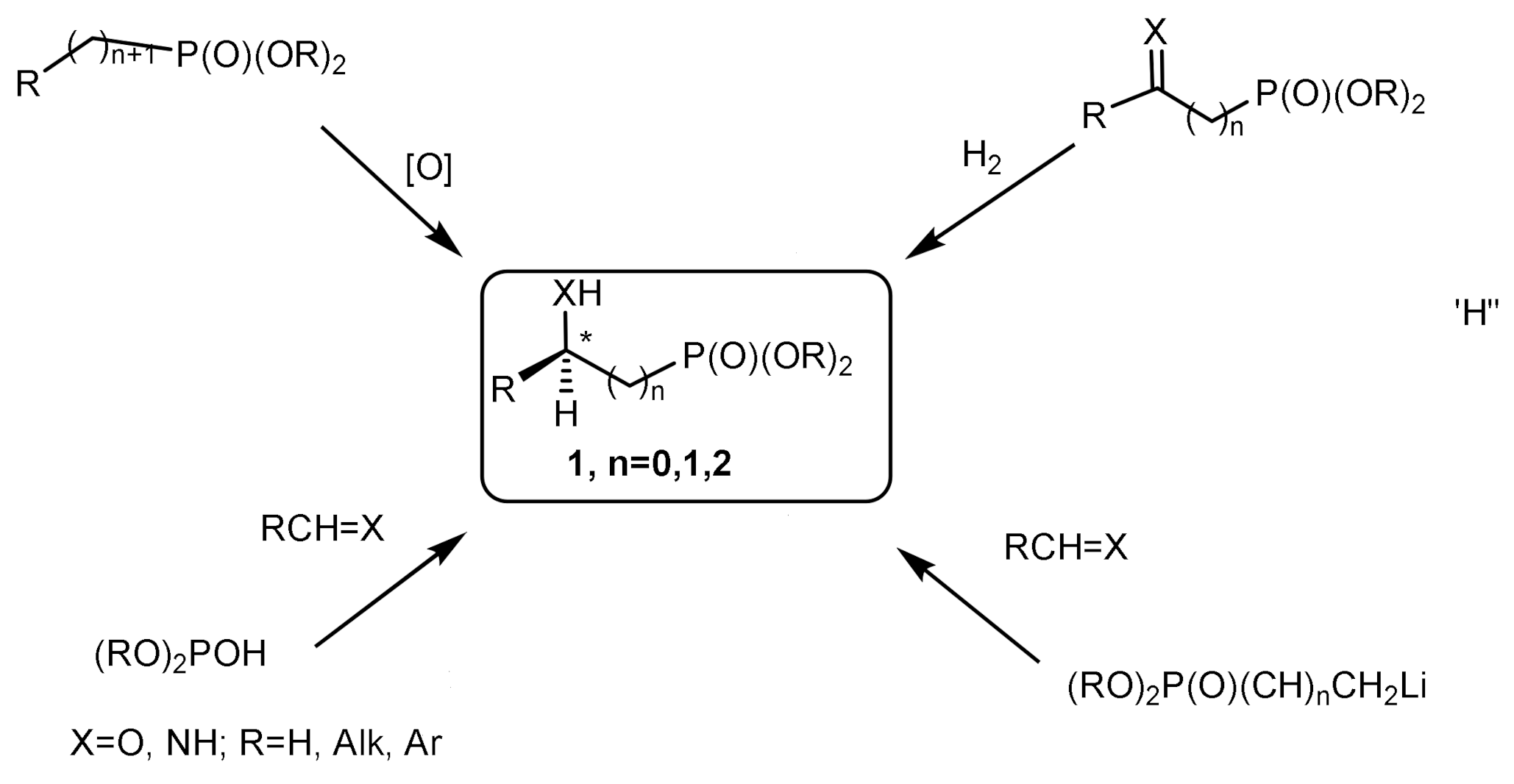
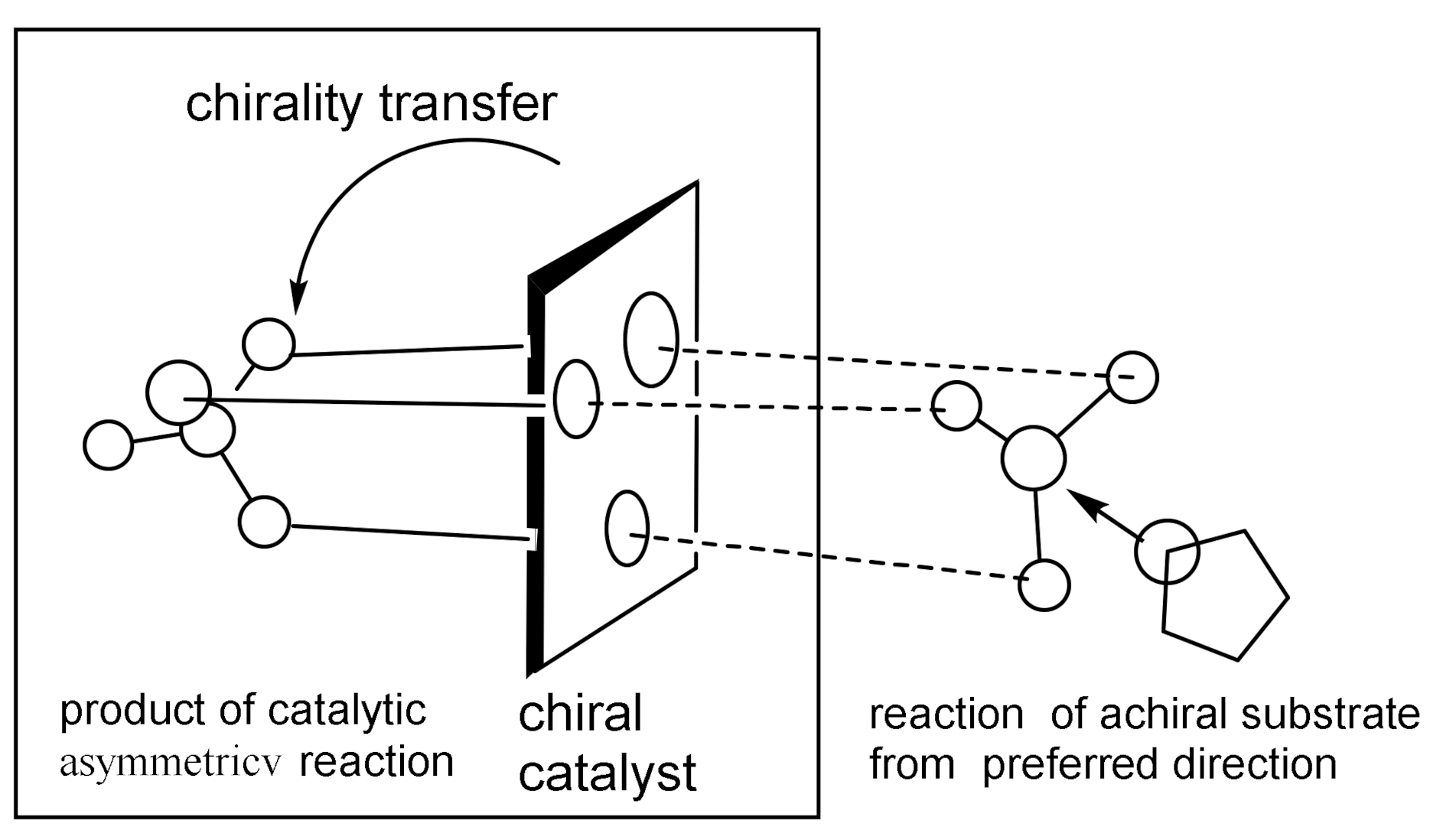





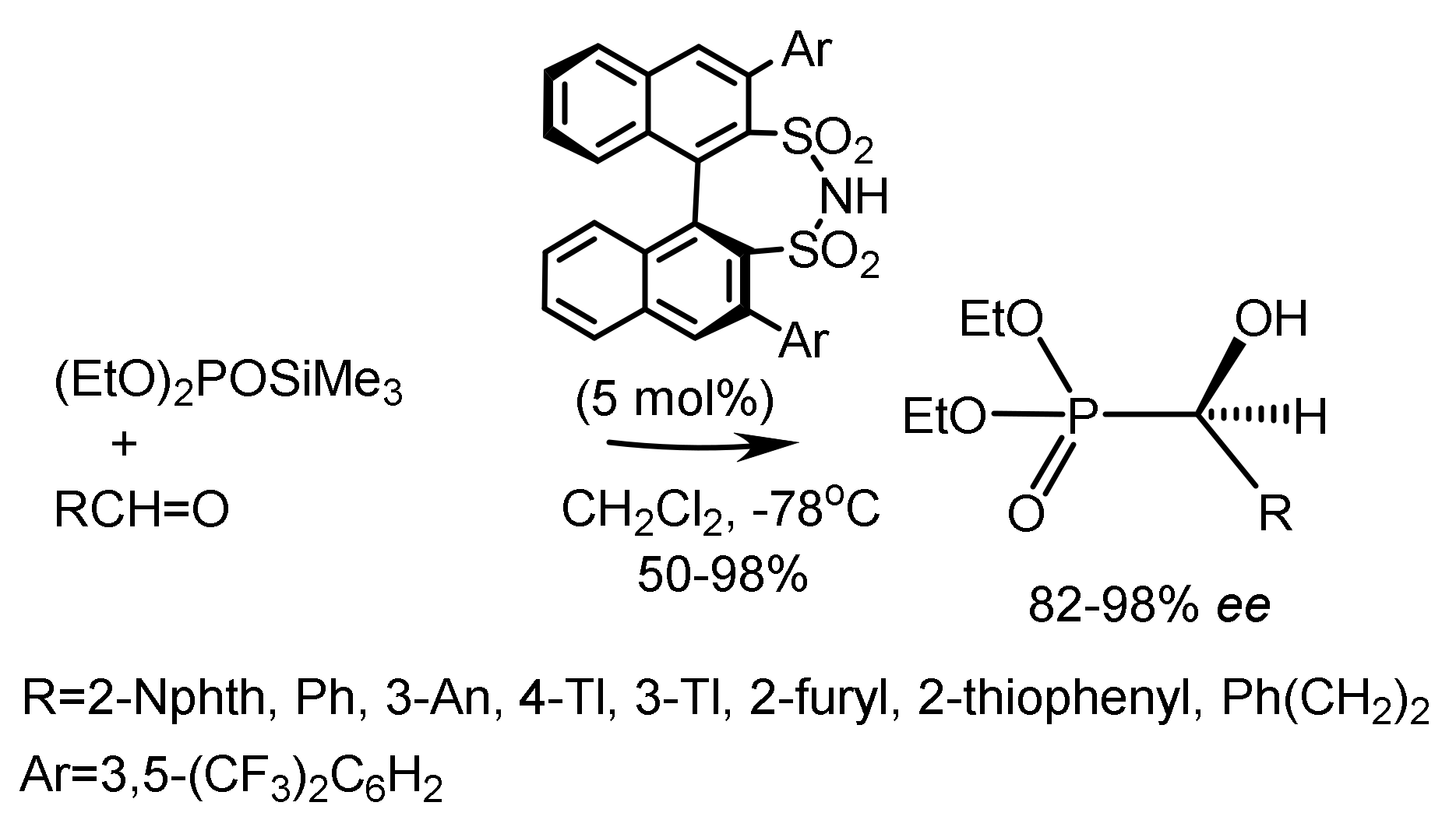
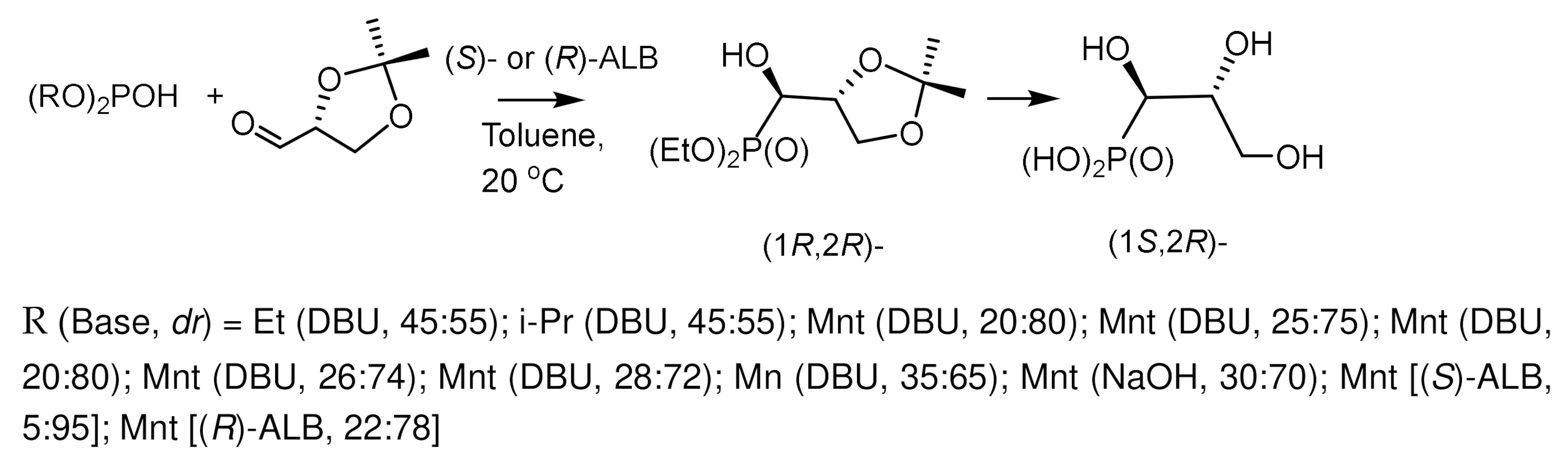
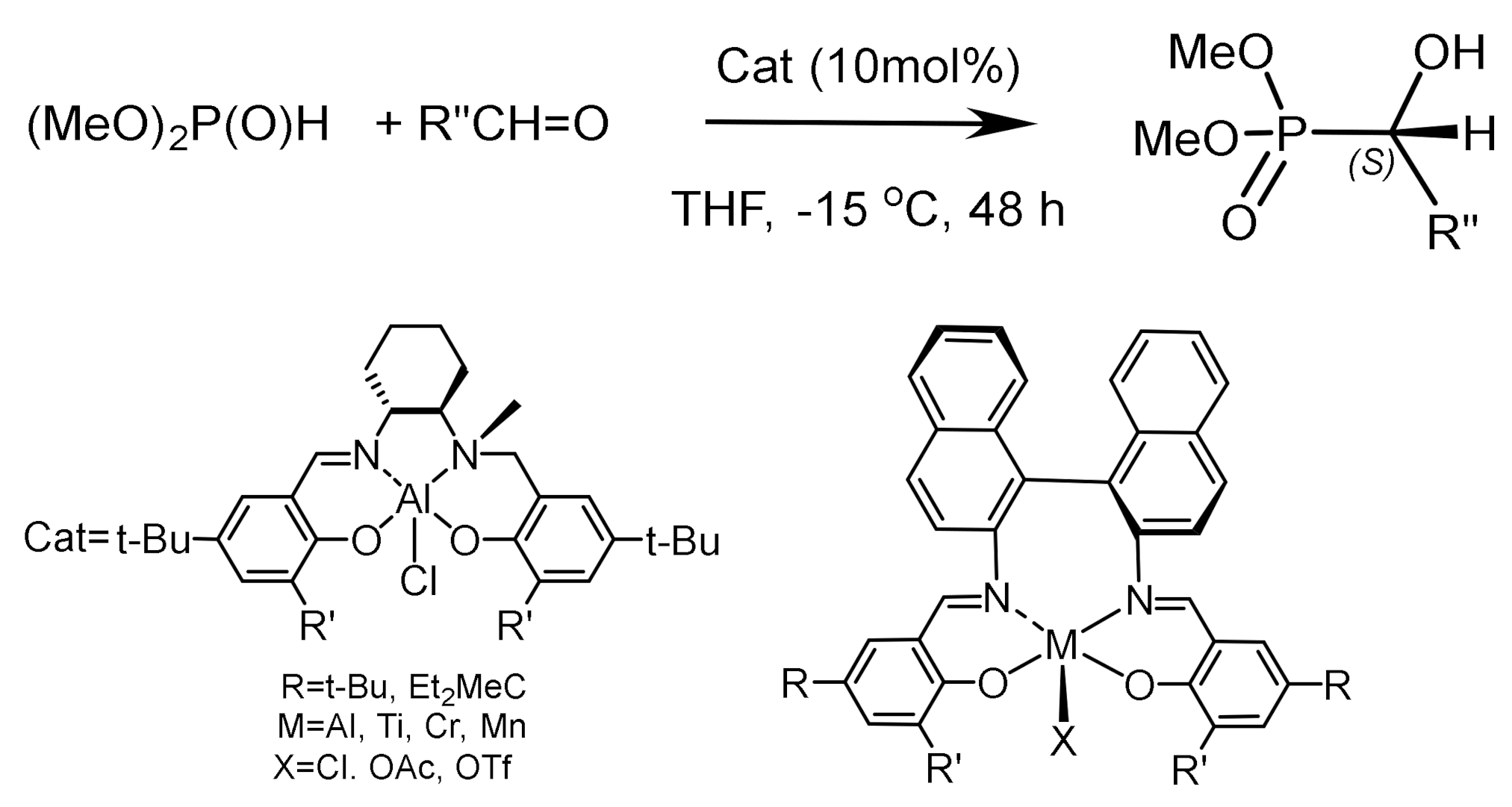

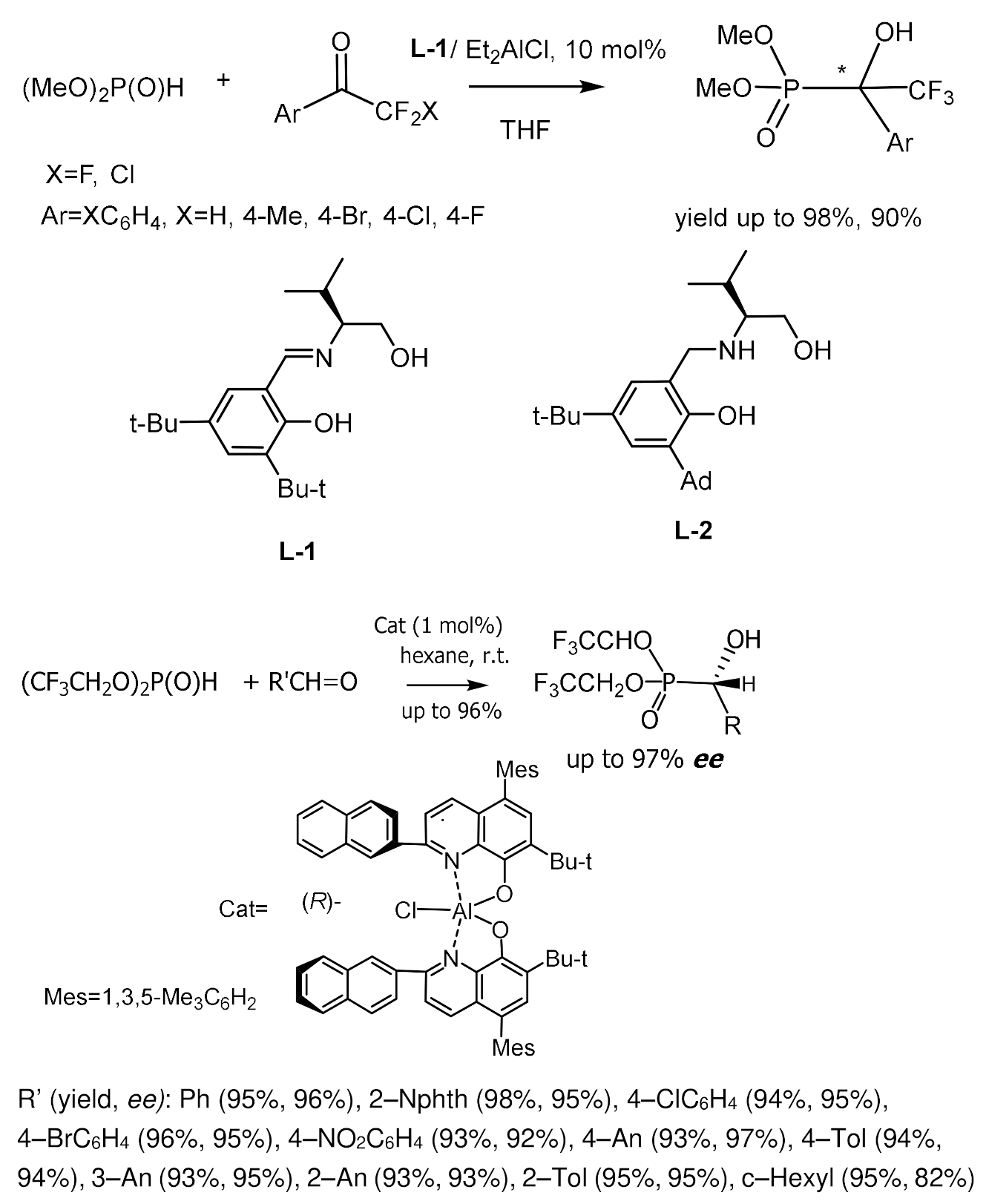




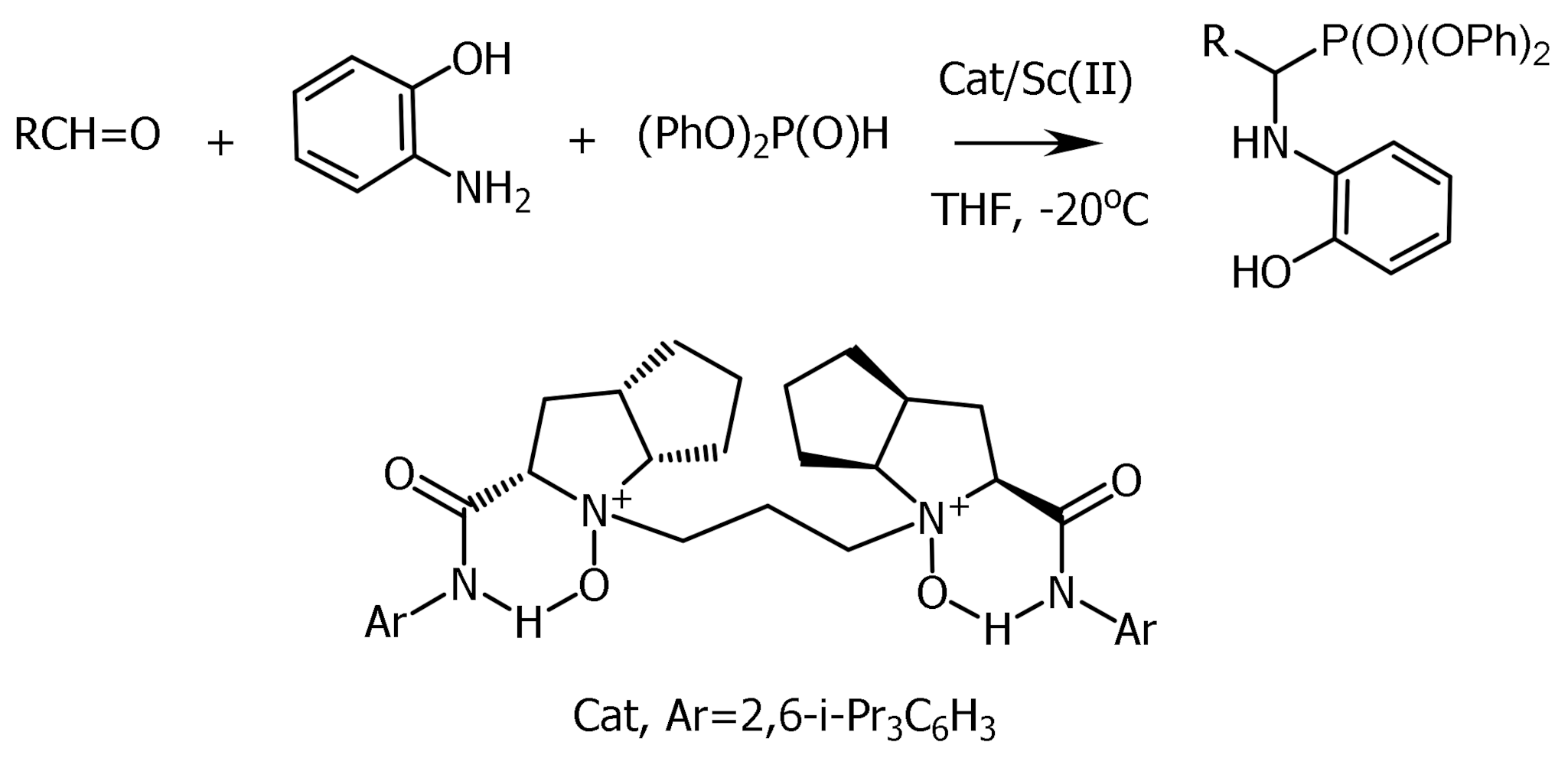
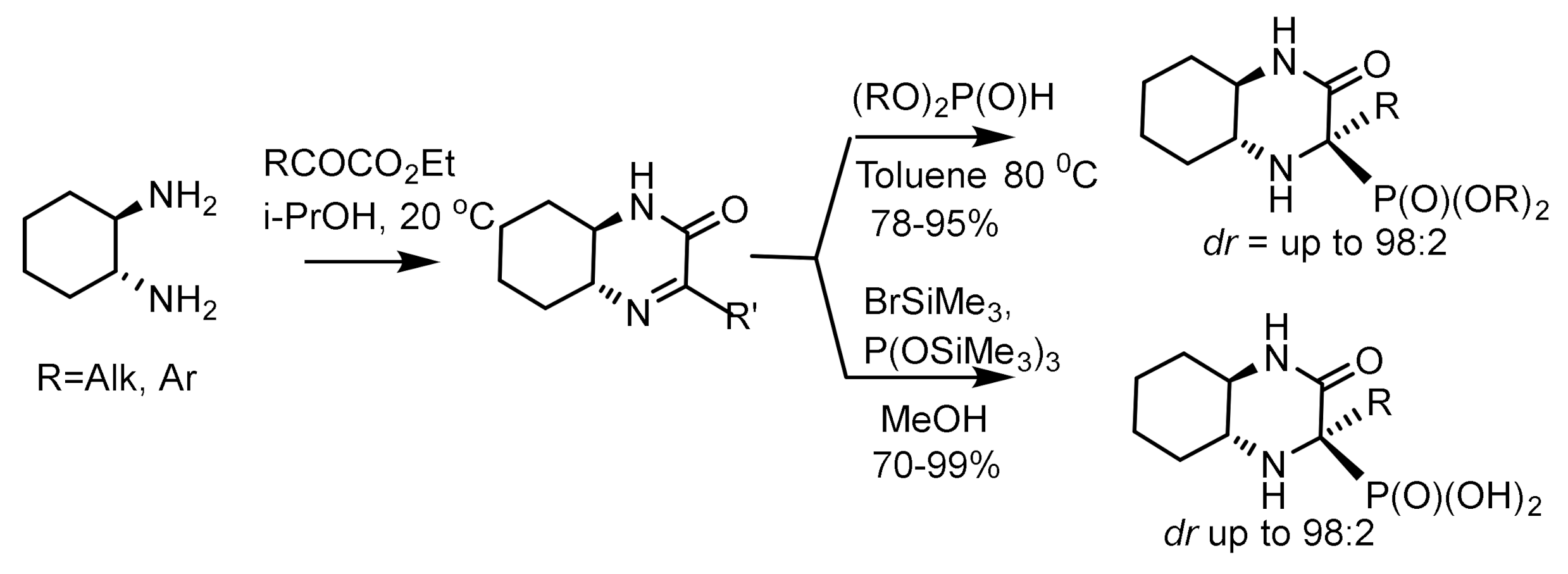
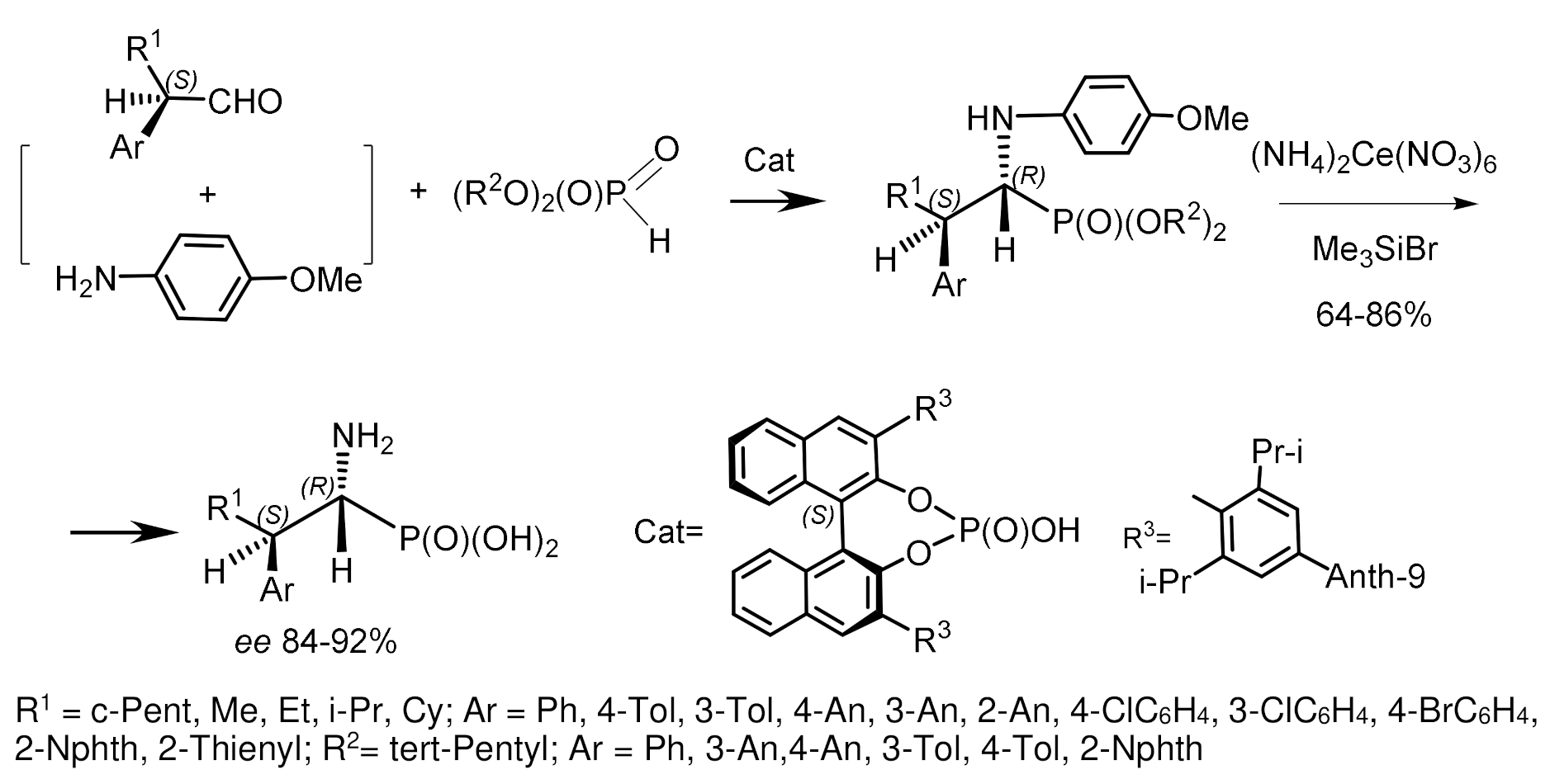

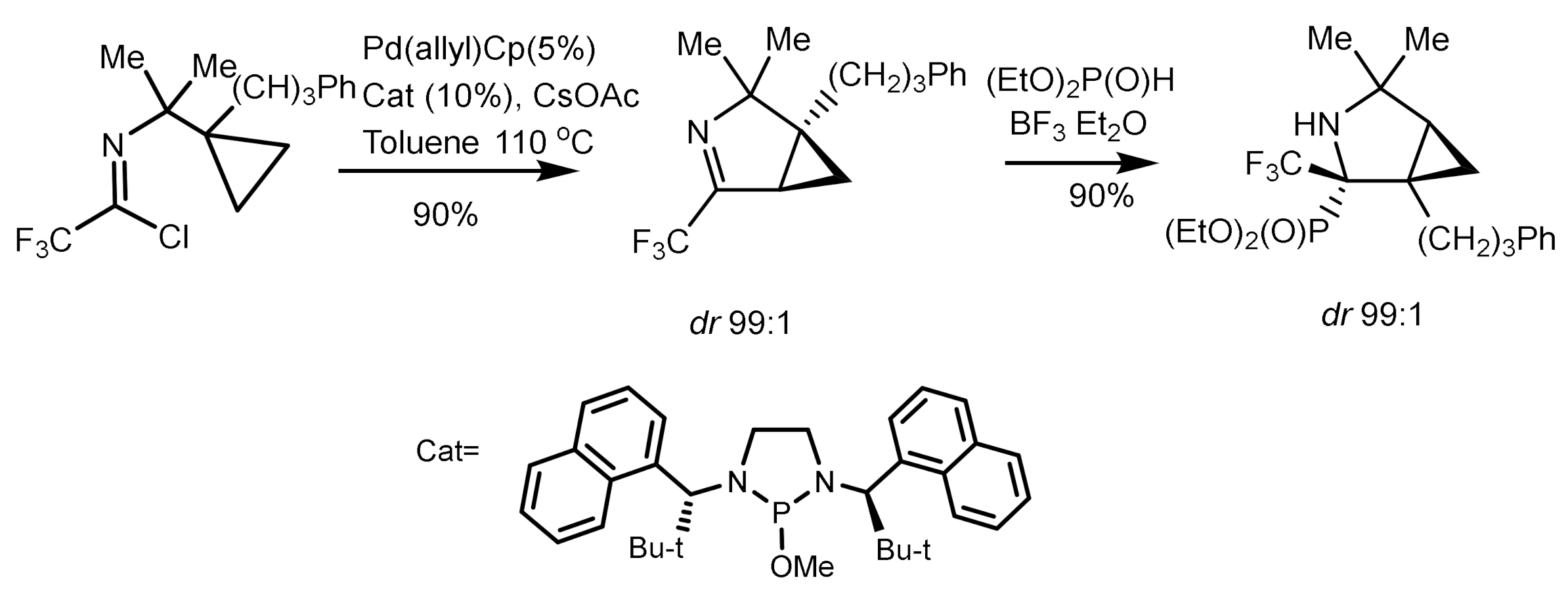






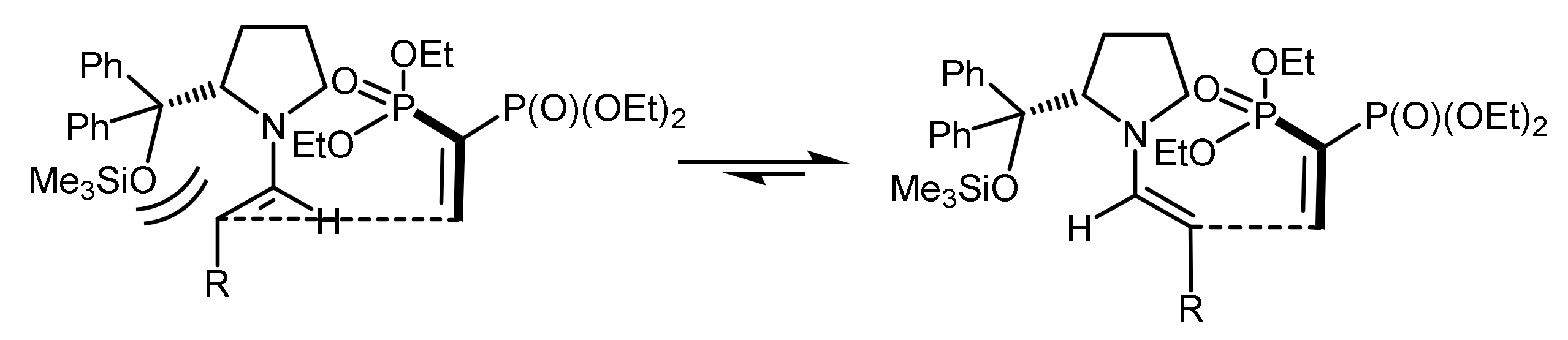
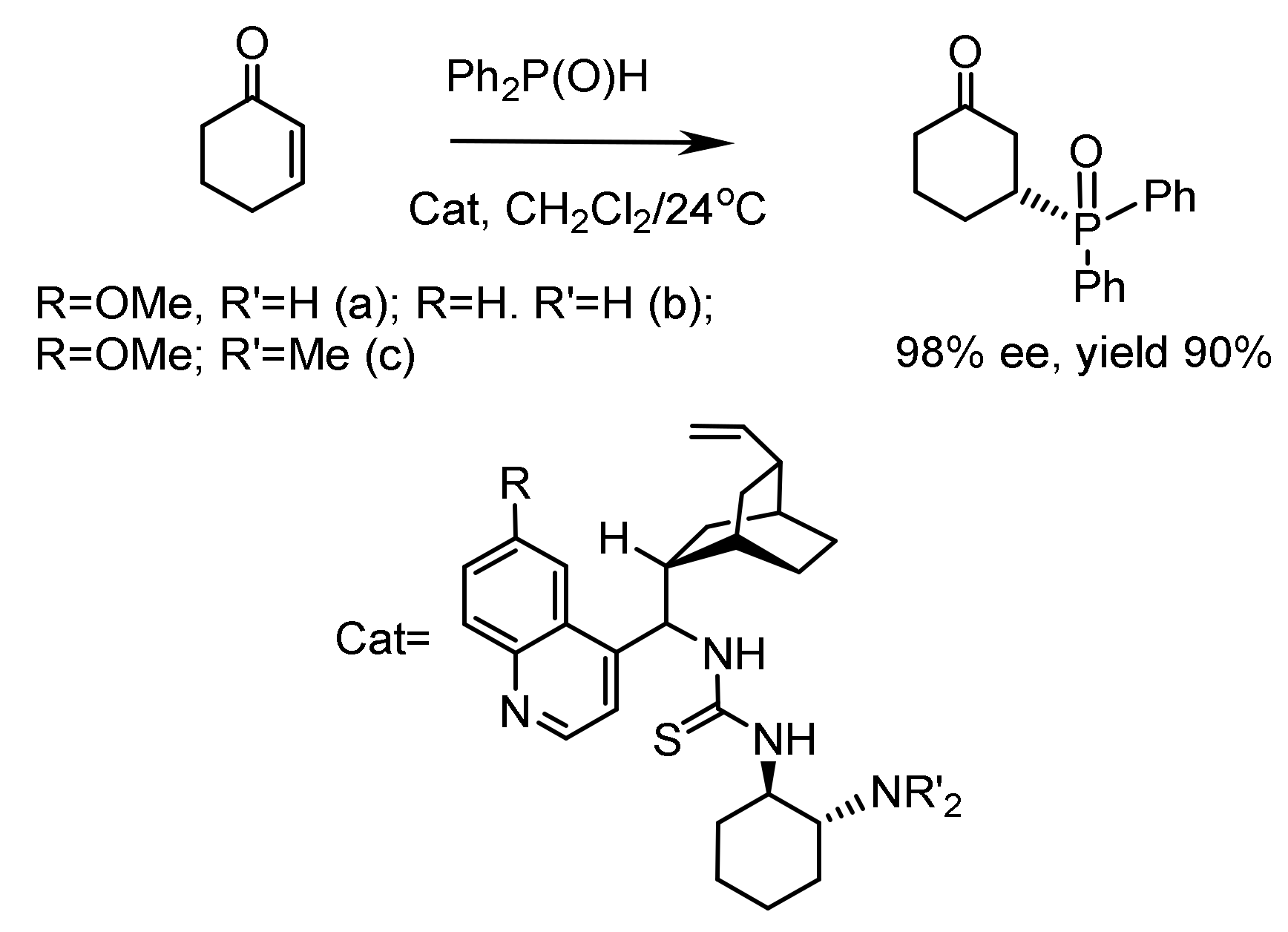
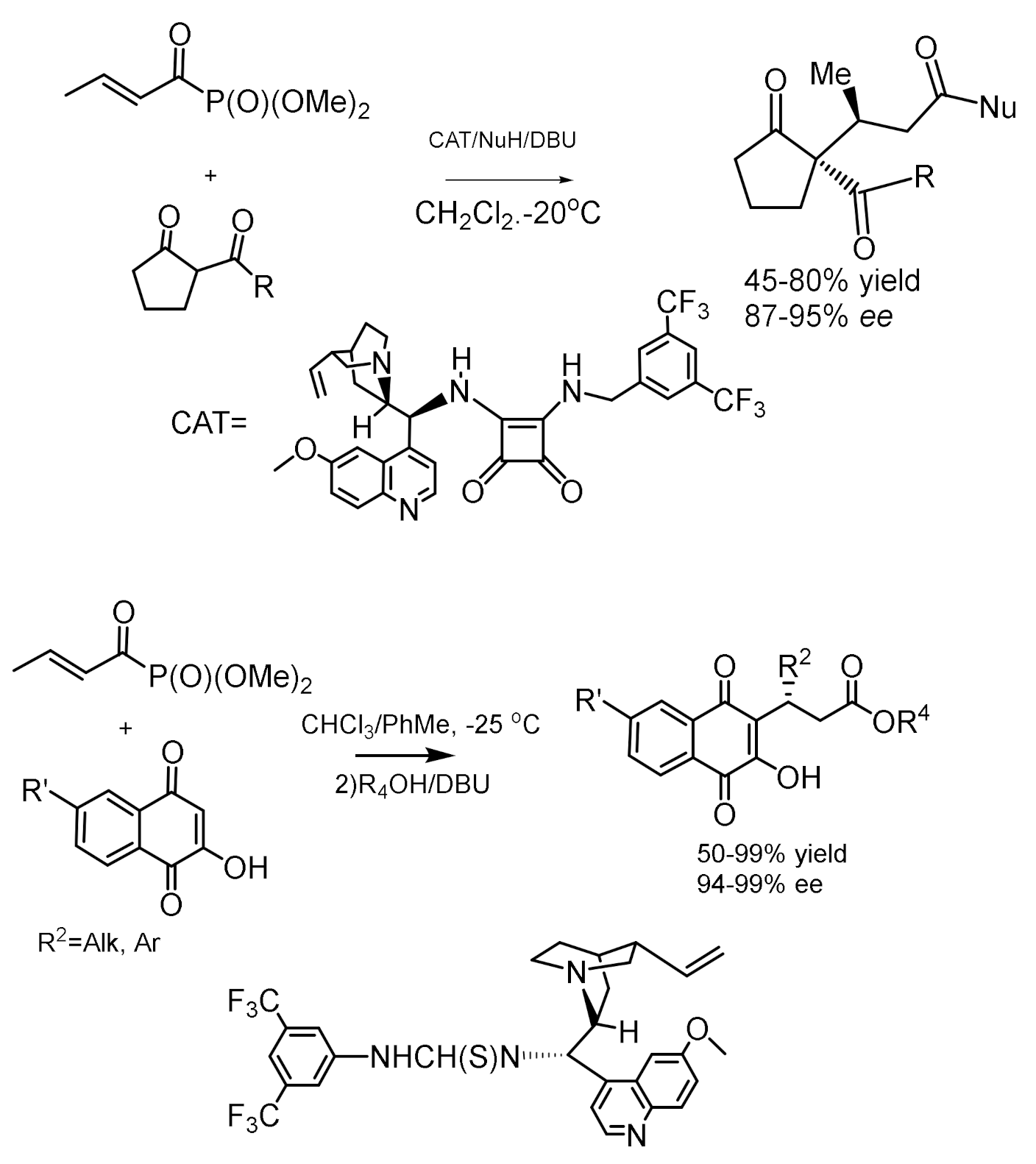
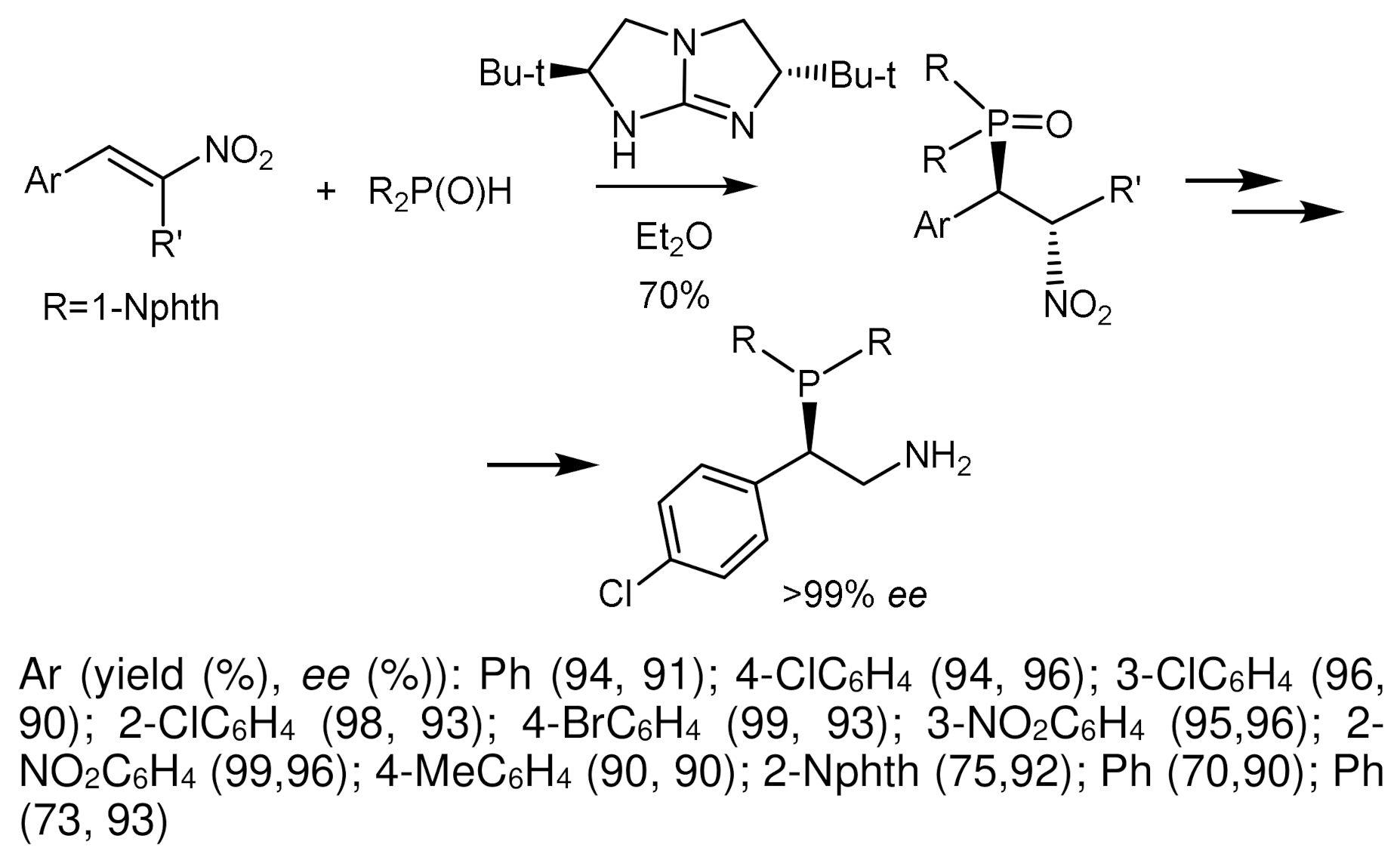




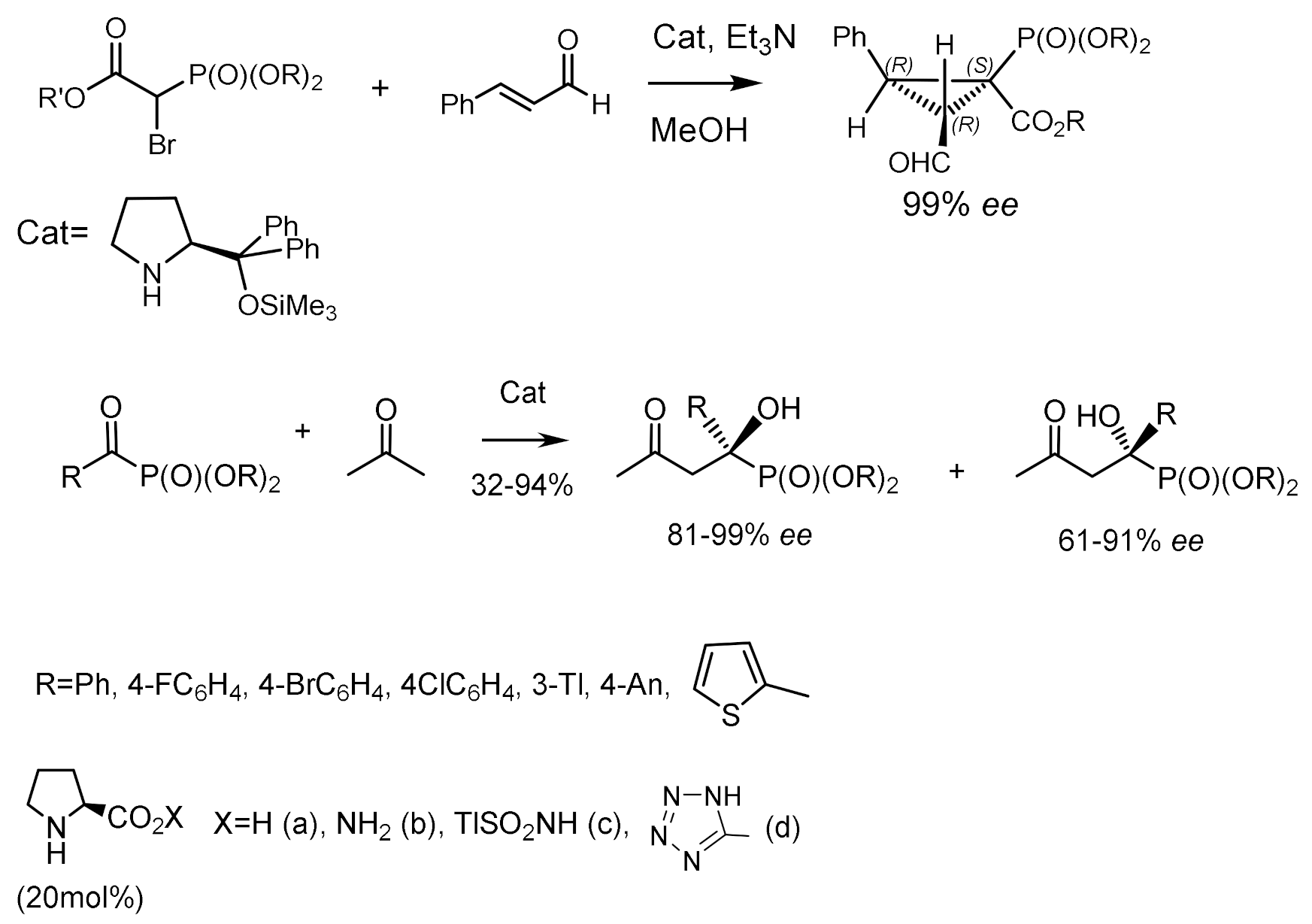
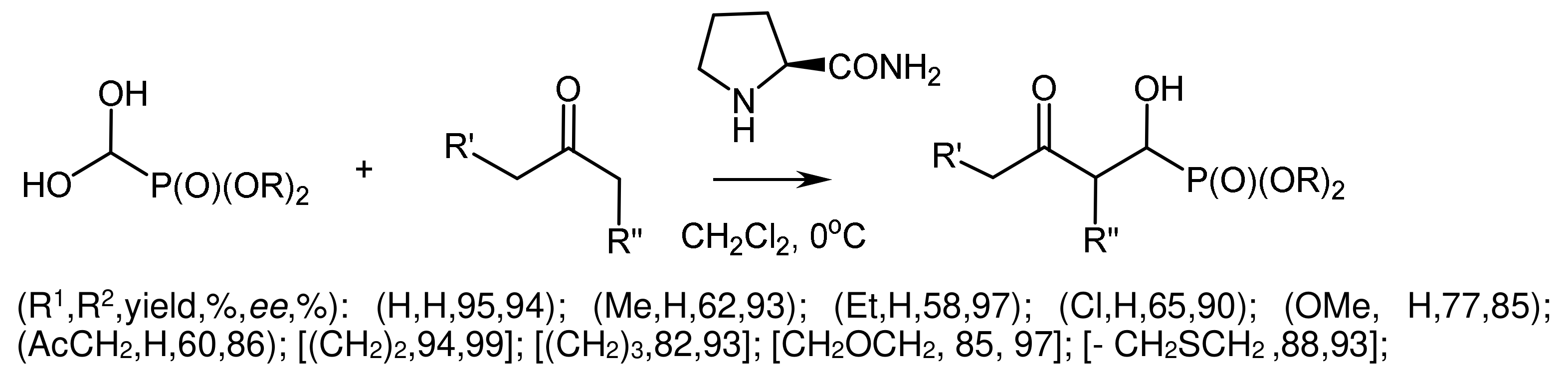
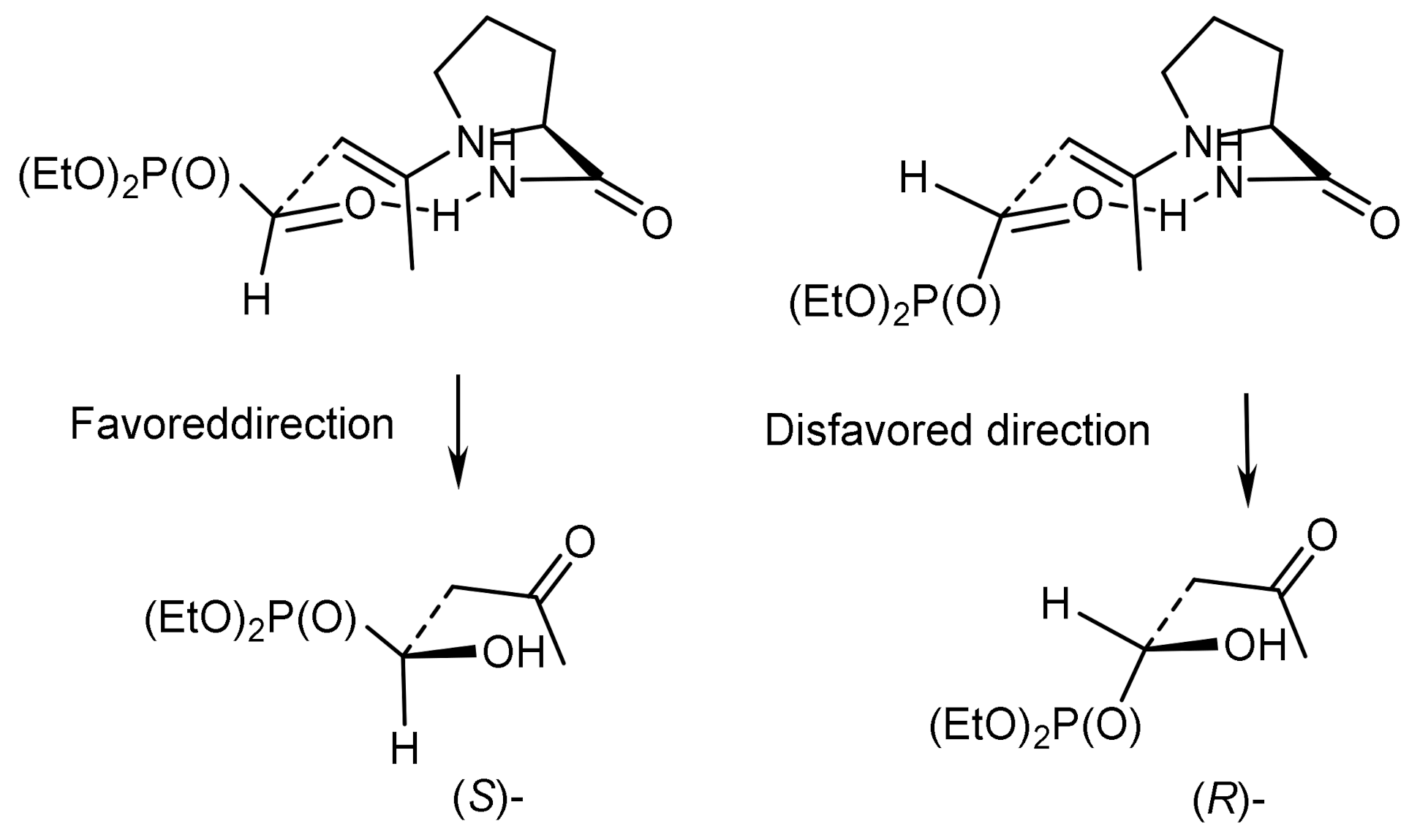
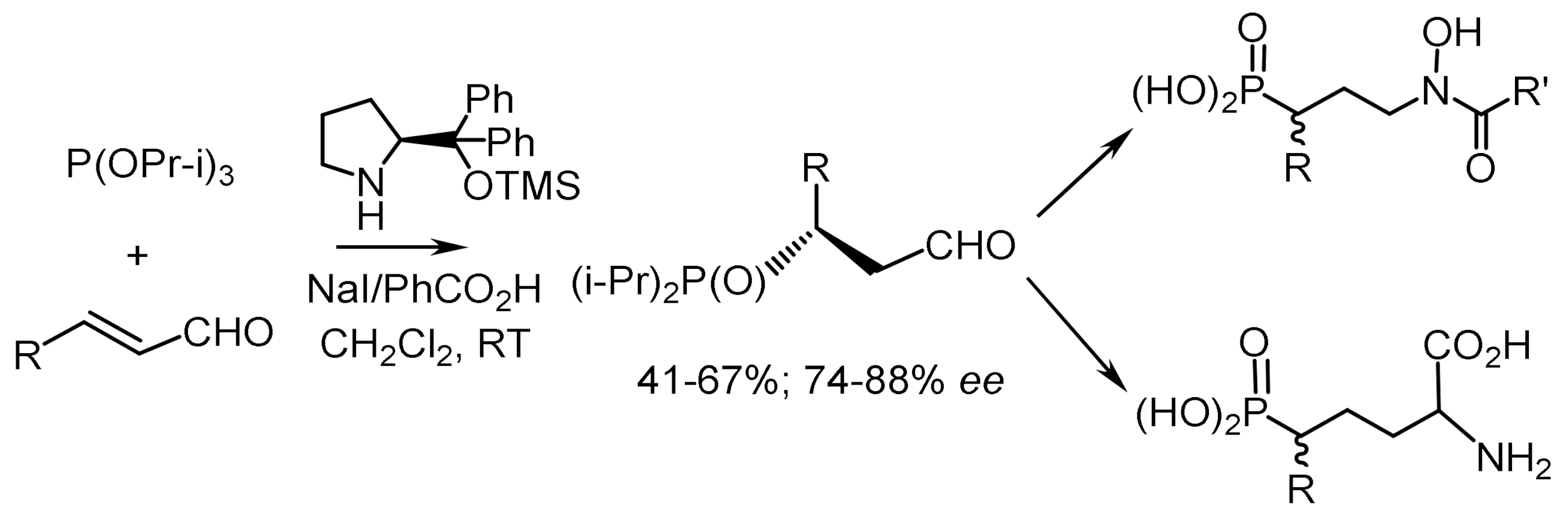


















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolodiazhna, A.O.; Kolodiazhnyi, O.I. Catalytic Asymmetric Synthesis of C-Chiral Phosphonates. Symmetry 2022, 14, 1758. https://doi.org/10.3390/sym14091758
Kolodiazhna AO, Kolodiazhnyi OI. Catalytic Asymmetric Synthesis of C-Chiral Phosphonates. Symmetry. 2022; 14(9):1758. https://doi.org/10.3390/sym14091758
Chicago/Turabian StyleKolodiazhna, Anastasy O., and Oleg I. Kolodiazhnyi. 2022. "Catalytic Asymmetric Synthesis of C-Chiral Phosphonates" Symmetry 14, no. 9: 1758. https://doi.org/10.3390/sym14091758





