Associations between Floral Asymmetry and Individual Genetic Variability Differ among Three Prickly Pear (Opuntia echios) Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species and Study Site
2.2. Individual Genetic Diversity
2.3. FA Measurements
2.4. Relationships between Genetic Diversity and FA (Fluctuating Asymmetry)
3. Results
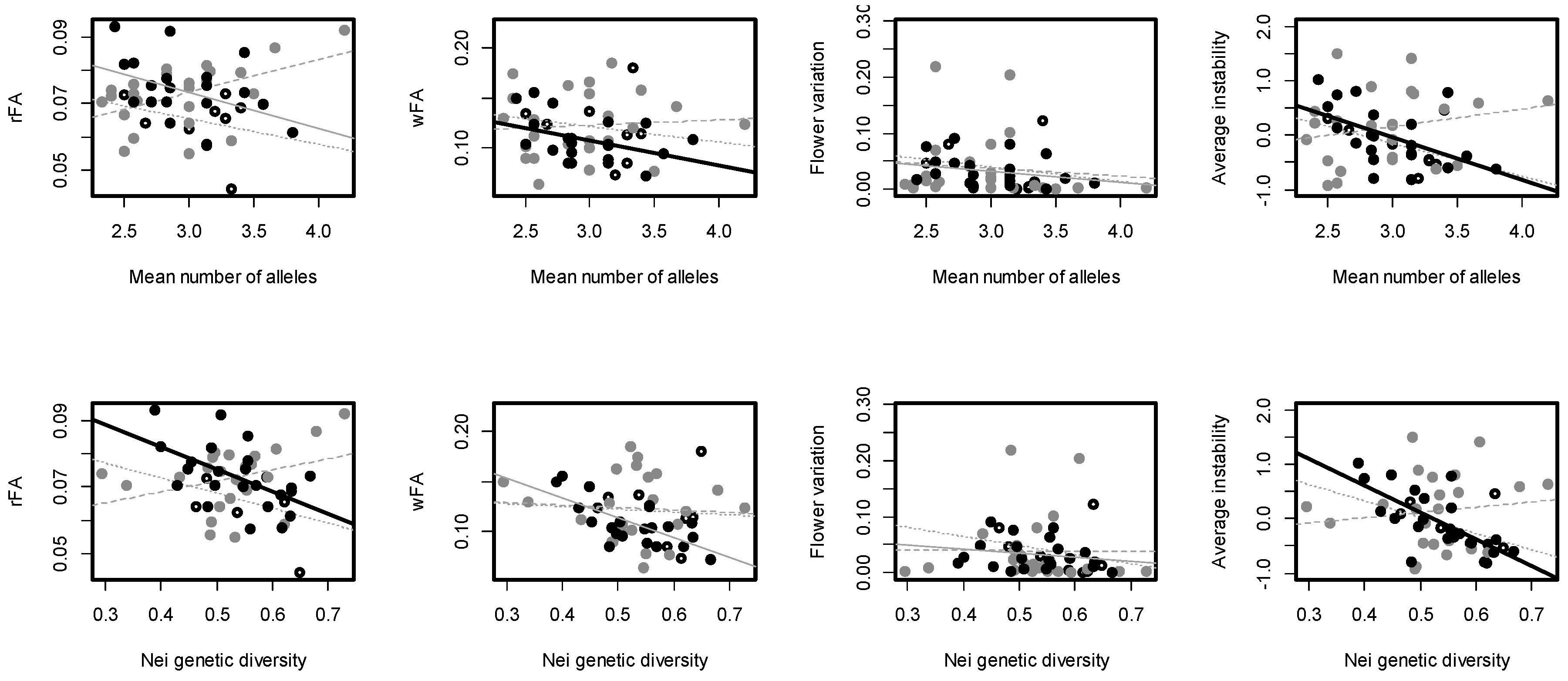
3.1. Genetic Diversity and Fluctuating Asymmetry
3.2. Correlation between Genetic Diversity and FA
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry—measurement, analysis, patterns. Annu. Rev. Ecol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Palmer, A.R. Waltzing with asymmetry. Is fluctuating asymmetry a powerful new tool for biologist or just an alluring new dance step? Bioscience 1996, 46, 518–532. [Google Scholar] [CrossRef]
- Leary, R.F.; Allendorf, F.W.; Knudsen, K.L. Genetics, environmental and developmental causes of meristic variation in rainbow trout. Acta Zool. Fenn. 1992, 191, 79–95. [Google Scholar]
- Lens, L.; Van Dongen, S.; Galbusera, P.; Schenk, T.; Matthysen, E. Developmental instability and inbreeding in natural bird populations exposed to different levels of habitat disturbance. J. Evol. Biol. 2000, 13, 889–896. [Google Scholar] [CrossRef]
- Rivera, G.; Claude, J. Environmental media and shape asymmetry: A case study on turtle shells. Biol. J. Linn. Soc. Lond. 2008, 94, 483–489. [Google Scholar] [CrossRef]
- Whitlock, M. The heritability of fluctuating asymmetry and the genetic control of developmental stability. Proc. Biol. Sci. 1996, 263, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, M. The repeatability of fluctuating asymmetry: A revision and extension. Proc. Biol. Sci. 1998, 265, 1429–1431. [Google Scholar] [CrossRef]
- Van Dongen, S. Fluctuating asymmetry and developmental instability in evolutionary biology: Past, present and future. J. Evol. Biol. 2006, 19, 1727–1743. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, S. How repeatable is the estimation of developmental stability by fluctuating asymmetry? Proc. Biol. Sci. 1998, 265, 1423–1427. [Google Scholar] [CrossRef]
- Parsons, P.A. Fluctuating asymmetry: An epigenetic measure of stress. Biol. Rev. Camb. Philos. Soc. 1990, 65, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Parsons, P.A. Fluctuating asymmetry: A biological monitor of environmental and genomic stress. Heredity 1992, 68, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.M. Fluctuating asymmetry: A technique for measuring developmental stability in natural populations. Acta Zool. Fenn. 1992, 191, 31–36. [Google Scholar]
- Manning, J.T.; Chamberlain, A.T. Fluctuating asymmetry in gorilla canines: A sensitive indicator of environmental stress. Proc. Biol. Sci. 1994, 255, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Söderman, F.; Van Dongen, S.; Pakkasmaa, S.; Merilä, J. Environmental stress increases skeletal fluctuating asymmetry in the moor frog Rana arvalis. Oecologia 2007, 151, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.J.R.; Weier, T.M.; Houle, D. The effect of inbreeding on fluctuating asymmetry of wing veins in two laboratory strains of Drosophila melanogaster. Heredity 2009, 102, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Lens, L.; Eggermont, H. Fluctuating asymmetry as a putative marker of human-induced stress in avian conservation. Bird Conserv. Int. 2008, 18, 125–143. [Google Scholar] [CrossRef]
- Vøllestad, L.A.; Hindar, K.; Møller, A.P. A meta-analysis of fluctuating asymmetry in relation to heterozygosity. Heredity 1999, 83, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Pertoldi, C.; Kristensen, T.N.; Andersen, D.H.; Loeschke, V. Developmental instability as an estimator of genetic stress. Heredity 2006, 96, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, S. Accuracy and power in fluctuation asymmetry studies: Effects of sample size and number of within-individual repeats. J. Evol. Biol. 1999, 12, 547–550. [Google Scholar] [CrossRef]
- Houle, D. A simple model of the relationship between asymmetry and developmental stability. J. Evol. Biol. 2000, 13, 720–730. [Google Scholar] [CrossRef]
- Helsen, P.; Browne, R.A.; Anderson, D.J.; Verdyck, P.; Dongen, S. Galapagos’ Opuntia (prickly pear) cacti: Extensive morphological diversity, low genetic variability. Biol. J. Linn. Soc. 2009, 96, 451–461. [Google Scholar] [CrossRef]
- Helsen, P.; Verdyck, P.; Tye, A.; Van Dongen, S. Low levels of genetic differentiation between Opuntia echios varieties on Santa Cruz (Galápagos). Plant Syst. Evol. 2009, 279, 1–10. [Google Scholar] [CrossRef]
- Helsen, P.; Van Dongen, S. The normal dirstibution as appropriate model of developmental instability in Opuntia cacti flowers. J. Evol. Biol. 2009, 22, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.F.; Walkington, D.L. Cactaceae. In Flora of the Galápagos Islands; Wiggins, I.L., Porter, D.M., Eds.; Stanford University Press: Stanford, CA, USA, 1971; pp. 533–546. [Google Scholar]
- Hicks, D.J.; Mauchamp, A. Evolution and conservation biology of the Galápagos Opuntias (Cactaceae). Haseltonia 1996, 4, 89–102. [Google Scholar]
- Tye, A. Restoration of the vegetation of the dry zone in Galápagos. Lyonia 2006, 9, 1–24. [Google Scholar]
- Jackson, M.H. Galápagos: A Natural History Guide; University of Calgary Press: Calgary, AB, Canada, 1993. [Google Scholar]
- Mauseth, R.A. Collapsible water-storage cells in cacti. Bull. Torrey Bot. Club 1995, 122, 145–151. [Google Scholar] [CrossRef]
- Bruvo, R.; Michiels, N.K.; D’Souza, T.G.; Schulenburg, H. A simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Mol. Ecol. 2004, 13, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Esselink, G.D.; Nybom, H.; Vosman, B. Assignment of allelic configuration in polyploids using the MAC-PR (microsatellite DNA allele counting-peak ratios) method. Theor. Appl. Genet. 2004, 109, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nybom, H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol. Ecol. 2004, 13, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Nybom, H.; Esselink, G.D.; Werlemark, G.; Leus, L.; Vosman, B. Unique genomic configuration revealed by microsatellite DNA in polyploid dogroses, Rosa sect. Can. J. Evol. Biol. 2006, 19, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image processing with IMAGEJ. Biophoton. Int. 2004, 11, 36–42. [Google Scholar]
- Graham, J.H.; Shimizu, K.; Emlen, J.M.; Freeman, D.C.; Merkel, J. Growth models and the expected distribution of fluctuating asymmetry. Biol. J. Linn. Soc. 2003, 80, 57–65. [Google Scholar] [CrossRef]
- Graham, J.H.; Raz, S.; Hel-Or, H.; Nevo, E. Fluctuating asymmetry: Methods, theory, and applications. Symmetry 2010, 2, 466–540. [Google Scholar] [CrossRef]
- Van Dongen, S.; Møller, A.P. On the distribution of developmental errors: Comparing the normal, gamma, and log-normal distribution. Biol. J. Linn. Soc. 2007, 92, 197–210. [Google Scholar] [CrossRef]
- Lerner, I.M. Genetic Homeostasis; Wiley: New York, NY, USA, 2004. [Google Scholar]
- Freeman, D.C.; Graham, J.H.; Emlen, J.M.; Tracy, M.; Hough, R.A.; Alados, C.L.; Escós, J. Plant developmental instability: New measures, applications, and regulation. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA, 2003; pp. 367–386. [Google Scholar]
- De Coster, G.; Van Dongen, S.; Malaki, P.; Muchane, M.; Alcantara-Exposito, A.; Matheve, H.; Lens, L. Fluctuating asymmetry and environmental stress: Understanding the role of trait history. PLoS ONE 2013, 8, e57966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arp, G.K. The Galápagos opuntias: Another interpretation. Noticias de Galápagos 1972, 21, 33–37. [Google Scholar]
- Racine, C.H.; Downhower, J.F. Vegetative and reproductive strategies of Opuntia (Cactaceae) in the Galapagos Islands. Biotropica 1974, 6, 175–186. [Google Scholar] [CrossRef]
- Burger, J.C.; Louda, S.M. Interaction of diffuse competition and insect herbivory in limiting brittle prickly pear cactus, Opuntia fragilis (Cactaceae). Am. J. Bot. 1999, 160, S135–S146. [Google Scholar] [CrossRef]
- Marchand, H.L.; Paillat, G.; Montuire, S.; Butet, A. Fluctuating asymmetry in bank vole populations (Rodentia, Arvicolinae) reflects stress caused by landscape fragmentation in the Mont-Saint-Michel Bay. Biol. J. Linn. Soc. Lond. 2003, 80, 37–44. [Google Scholar] [CrossRef]

| (a) | ||||
| Location | Genetics | |||
| n | na | Nei’s GD | mNA | |
| Santa Cruz | 21 | 68 | 0.528 ± 0.078 | 2.98 ± 0.37 |
| Baltra | 24 | 75 | 0.528 ± 0.092 | 2.94 ± 0.46 |
| Plaza Sur | 8 | 57 | 0.574 ± 0.071 | 3.08 ± 0.33 |
| (b) | ||||
| Location | Asymmetry | |||
| rFA | wFA | Flower Variation | Average Instability | |
| Santa Cruz | 0.074 ± 0.010 | 0.085 ± 0.018 | 0.045 ± 0.014 | 0.10 ± 0.13 |
| Baltra | 0.073 ± 0.010 | 0.091 ± 0.022 | 0.027 ± 0.007 | −0.03 ± 0.10 |
| Plaza Sur | 0.065 ± 0.010 | 0.075 ± 0.024 | 0.038 ± 0.014 | −0.20 ± 0.14 |
| Significance Tests for the ANCOVA Models | |||||
|---|---|---|---|---|---|
| Genetic Diversity Index | Model Factor | rFA | wFA | Flower variation | Average instability |
| Mean number of alleles | Pop: | F2,47 = 3.10 | F2,47 = 1.50 | F2,47 = 0.11 | F2,47 = 1.13 |
| GD: | F1,47 = 0.31 | F1,47 = 0.42 | F1,47 = 1.14 | F1,47 = 0.36 | |
| Pop×GD: | F2,47 = 5.33 ** | F2,47 = 0.99 | F2,47 = 0.02 | F2,47 = 3.46 * | |
| Nei GD | Pop: | F2,47 = 3.08 | F2,47 = 1.16 | F2,47 = 0.11 | F2,47 = 1.21 |
| GD: | F1,47 = 0.53 | F1,47 = 3.20 | F1,47 = 0.28 | F1,47 = 2.45 | |
| Pop×GD: | F2,47 = 5.13 ** | F2,47 = 1.50 | F2,47 = 0.20 | F2,47 = 4.31 * | |
| Correlation coefficients for association between measures of DI and genetic diversity in each population, for the mean number of alleles and Nei GD respectively | |||||
| Population: | rFA | wFA | Flower variation | Average instability | |
| Plaza sur | −0.28/−0.36 | −0.06/−0.16 | −0.19/−0.26 | −0.47/−0.49 | |
| Baltra | 0.46/0.33 | −0.07/0.07 | −0.12/−0.00 | 0.22/0.13 | |
| Santa Cruz—CDRS | −0.40/−0.53 * | −0.70 ***/−0.42 | −0.26/−0.21 | −0.52 */−0.70 *** | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helsen, P.; Van Dongen, S. Associations between Floral Asymmetry and Individual Genetic Variability Differ among Three Prickly Pear (Opuntia echios) Populations. Symmetry 2016, 8, 116. https://doi.org/10.3390/sym8110116
Helsen P, Van Dongen S. Associations between Floral Asymmetry and Individual Genetic Variability Differ among Three Prickly Pear (Opuntia echios) Populations. Symmetry. 2016; 8(11):116. https://doi.org/10.3390/sym8110116
Chicago/Turabian StyleHelsen, Philippe, and Stefan Van Dongen. 2016. "Associations between Floral Asymmetry and Individual Genetic Variability Differ among Three Prickly Pear (Opuntia echios) Populations" Symmetry 8, no. 11: 116. https://doi.org/10.3390/sym8110116






