1. Introduction
Scientists have collected a large amount of evidence supporting behavioural bias spread in vertebrates, and even invertebrates [
1,
2,
3]. This is even clearer if we consider that being lateralized could bring benefits, hence, affecting the fitness of individuals that present it [
4,
5,
6].
Brain asymmetries can be manifested and studied as behavioural visual asymmetries, or the preferential use of a specific eye for looking at a type of stimulus, with the latter being especially easily evident in animals with laterally-placed eyes [
7,
8]. We know that different reactions to right-and left-placed stimuli have been ascertained in several species, verifying the specialization of the brain to perceive information with the left or right eye and in elaborating it with the contralateral hemisphere, according to the nature of the cue (for review, see [
1,
4,
8]).
Being so lateralized could be advantageous by allowing better processing of two tasks at the same time, each one perceived with an eye, and then elaborated by the contralateral hemisphere [
4,
8,
9]. This can enhance a lateralized individual’s cognition to simultaneously attend to multiple cues [
4,
10,
11]. As the behaviours involved, i.e., lateralized, are generally usual and important for survival, such as feeding and vigilance, it could be extremely advantageous that they can be performed simultaneously [
12,
13,
14].
Recently, the scientific interest about lateralized ectotherms has increased for mammals, as well as for birds [
15,
16,
17,
18]. Lizards are an interesting model for studying visual lateralization because they have almost complete decussation of the optic chiasma and they lack the larger number of interhemispheric connections present in mammals, allowing cues perceived with one eye to elaborate almost entirely with the contralateral half of the brain [
19,
20]. Each visual system could then work largely independently [
21]. Some previous works focused on
Podarcis muralis, a lizard species widespread in Europe, highlighting that this species is lateralized for some crucial daily behaviours [
22]. According to the bibliography, this species shows preferences in using the left eye in predatory tasks (detailed observation of stimuli), and the right eye in vigilance and exploratory tasks (global attention and spatial processing of stimuli), also in the wild [
23,
24,
25,
26,
27]. Speculations hypothesized that the lateralization present in this species could be related to its strong territoriality [
28,
29] and consequent habit of exploring with a high vigilance level during its activities; hence, laterality in this lizard could be evolved as an adaptive character in response to specific environmental needs [
22,
30]. In this work, we attempted to investigate an eventual form of lateralization in a non-territorial and elusive lizard species,
Zootoca vivipara, with different life habits [
31], environment, and needs than to
P. muralis, so as to compare results for both species, and attempt to understand the importance/weight of ecological conditions on the manifestation of behavioural and cerebral biases. Starting from the study conducted by Csermely et al. [
32], we focused on exploration, an activity closely related to life in the natural environment and biology.
2. Materials and Methods
2.1. Subjects and Housing
From June to July we collected 10 wild adult Zootoca vivipara lizards, five males and five females, from Ampola Lake, a biotope in the southwestern area of Trentino, near the town Tiano di Sopra (TN). We obtained the required administrative permit for capturing the lizards from the wild from Comunità Alto Garda e Ledro (prot. 11083/11.4, 25 May 2012). Captures were made by noosing or hands; the lizards were put in cloth bags immediately after and carried to the terraria. Behavioural observations were carried out in the research and the didactical station SperimentArea, situated in Rovereto (Trento, Italy). Here the lizards were housed in 80 × 50 × 40 cm glass terraria or 40 × 40 × 30 cm plastic cages, under the natural Italian summer photoperiod (16:8 h light/dark cycle) and temperature (25–35 °C) regulated with artificial lighting, if necessary. Each terrarium had a floor covered with a sand substratum with the addition of soil, bark, and musk, other than rocks and bricks for refuge and/or basking. The lizards were fed daily with multivitamin powder-dusted mealworm larvae (Tenebrio molitor) and crickets; water was provided ad libitum. In order to maintain the correct substratum humidity, the terrain was adjusted daily with water vaporization, if necessary. Once entering the terrarium, the lizards were allowed to accustom themselves to the new environment for seven days before the tests started. At the end of the experiment lizards were released at the same site of capture; none of them was harmed by the experiment, which was carried out under license from Italian authorities.
2.2. Apparatus
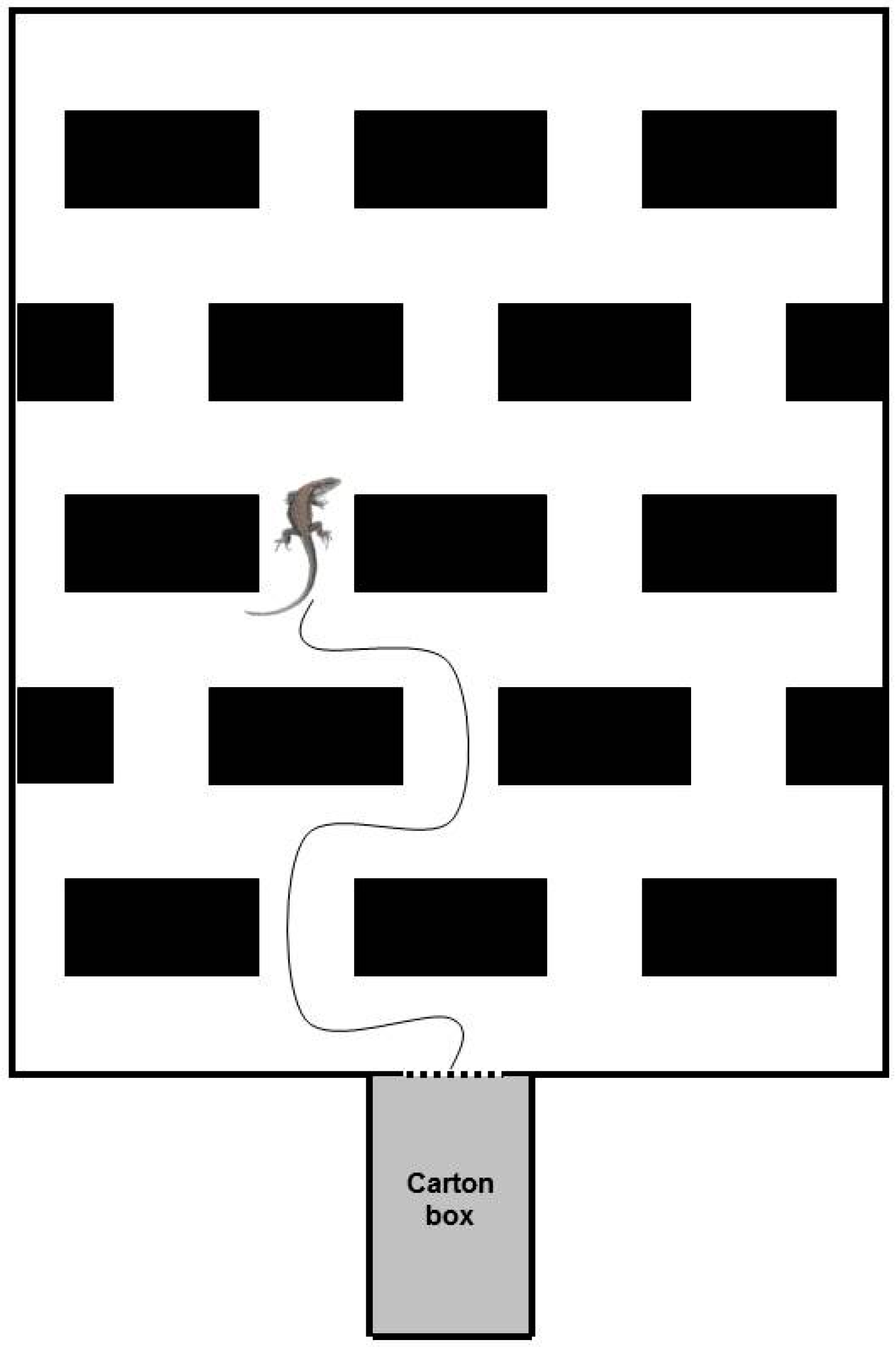
In order to compare the explorative behaviour of
Zootoca vivipara with that of
Podarcis muralis, we employed the same experimental apparatus previously used in Csermely et al. [
32] (
Figure 1), modified, consisting in a 54 × 66 cm PVC base maze with 10 cm high sides. Thirteen 12 × 10 × 6 cm blocks were scattered regularly on the base at the distance of 6 cm each other; four additional 6 × 10 × 6 cm blocks were located against two sides of the base; their length was limited to one-half of that of the others so as to maintain the regular reciprocal distance among the blocks. The blocks’ presence had to induce the exploring lizard to continuously change direction when it arrived at the T-crossroads, then forcing it to decide to go either to the left or to the right. The blocks were attached to the base with adhesive tape. They were made of a series of commercial Duplo
® bricks (Lego A/S, Billund, Denmark) and covered with plastic adhesive paper with marble coloration to prevent the lizards from climbing them. Experiments were conducted without transparent cover, for possible interferences of the reflectance of neon light placed on the top of the maze. The apparatus was located in a circular tub (110 cm of diameter, 50 cm high), necessary to contain animals in case of escape and to avoid possible surrounding influences on the individual behaviour. In addition, four black corrugated honeycomb panels were collocated all around the apparatus, working as screens for the operator.
2.3. Procedure
Before the beginning of the tests, we allowed lizards to thermoregulate at least 30 min under the light of a 50-W halogen lamp, allowing them to reach the temperature for maximal locomotor performance, necessary to express correct exploring behaviour. Experiments were conducted in the same place as the lizard housing, hence, with the same light and temperature conditions. Afterwards, a lizard was gently removed from the terrarium and placed in a 15 × 9 × 6.5 cm carton box external to the maze, but attached to it. The lizard remained in the box for 5 min to acclimatize; thereafter, the operator, located behind a black Poliplak® screen (RÖHM GmbH, Sontheim/Brenz, Germany), using a thin cable, lifted up the PVC gateway that had prevented the lizard from entering the maze through the opening before the beginning of the test. The test started when the lizard entered the maze. After 20 min, if the lizard did not spontaneously enter it, the operator beat, with a small stick, the distal part of the box containing the lizard to encourage it to move out. The lizard could move freely within the maze for 20 min. During the experiment, the gateway remained open; hence, the lizard could come back to the box. At the end, it was returned to its terrarium and the maze floor and walls were cleaned with ethyl alcohol to prevent any possible effect of chemical cues on subsequent individuals.
The tests were carried out when the air temperature was within the 27–35 °C range. Light was homogeneous and both natural and artificial by a neon lamp placed on the experimental apparatus. We considered the following behaviour parameters: when the subject entered the maze, we assessed (1) the rotation of the head to the left or the right, and (2) the frequency of direction of the turn (leftward or rightward). Afterward, while the lizard was exploring the maze, we observed (3) the delay time (duration of hesitation) at each T-crossroads, and (4) the duration of each turning, (5) the total frequency of direction of turning; and (6) when passing a T-crossroad for the first time (excluding any possible olfactory influence).
All tests were recorded with a digital mini DV colour video camera Sony ‘‘Handycam” DCR-SR58 (Sony, Tokyo, Japan) 17.0 × 9.0 × 8.0 cm placed above the maze. Frame by frame analysis of the footage was possible by the Windows Live Movie Maker 6.0 video software (Microsoft Corporation, Redmond, WA, USA).
2.4. Statistical Analyses
We used the binomial test to compare the number of turns to the left or to the right performed by each lizard and the individual preference in turning the head. The Wilcoxon matched-pairs signed ranks test (T+) was used to compare the number of turns to the left or to the right and the preference in turning the head in the group as a whole. For comparing durations, we used the Mann–Whitney U test (U). Calculations have been performed using the SPSS 18.0 for Windows software [
33]. Means are ± Standard Error (SE) and the probability, set at α = 0.05, was two-tailed throughout, unless otherwise stated.
3. Results
Although all lizards hesitated in entering the maze, they all moved out of the box and concluded the experiment. Some lizards, before exiting, required a stimulation by gently tapping on the starting box with a stick. During experiments lizards did not appear frightened but explored the environment, walking inside it and turning around the blocks. They walked both in the central and lateral routes of the maze; sometimes they tried to climb the blocks or the maze walls. During the exploration some individuals arrived near the entrance box and entered it, but they shortly moved back out to the maze. During the experiment lizards moved for 619.15 ± 71.45 s and froze for 580.85 ± 57.82 s, without any difference inside the group and between sexes for both the time of movement and immobility.
Immediately after entering the maze six lizards out of 10 (four males and two females) rotated the head to the right (binomial test; p = 0.289), two lizards (both females) to the left, and two lizards (one male and one female) did not rotate the head before entering.
Three lizards (one male and two females) out of 10 performed the first turn immediately after entering the maze on the left and seven lizards (four males and three females) out of 10 on the right (binomial test; p = 0.3438).
The subsequent movements of the lizards were in various directions, moving progressively further from the entering point. The delay time for turning at each T-crossroad showed similar results for both the left and right directions (2.53 ± 0.90 s and 2.00 ± 0.72 s, respectively; z = −0.227; p = 0.821), and also between sexes (males left: 3.96 ± 1.12 s; females left: 1.10 ± 0.24 s; U = −0.522; p = 0.690; males right: 3.23 ± 0.89 s; females right: 0.78 ± 0.09 s; U = −1.567; p = 0.117). During each T-crossroad turning, lizards kept the head right-turned for 4.62 ± 0.88 s and left-turned for 6.19 ± 0.23 s (U = −0.076; p = 0.940); no differences emerged between sexes in keeping the head right-turned (males: 4.74 ± 0.97 s; females: 4.51 ± 0.90; U = −0.522; p = 0.6) and left-turned (males: 8.66 ± 3.26 s; females: 3.73 ± 0.52 s; U = −0.83; p = 0.4).
The average number of turns per lizard per test was 27.70 ± 3.19, with no significant differences between sexes (males: 25.80 ± 2.75, females: 29.60 ± 4.01; U = 11.500;
p = 0.834). Statistical analyses did not reveal any population-level bias for turning to the left or to the right among the lizards (13.2 ± 2.30 and 14.5 ± 1.68, respectively; T+ = −0.153;
p = 0.878) and between sexes for the right turning (T+ = −0.674;
p = 0.500), but females showed a bias in turning left compared with males (T+ = −2.032;
p = 0.042). Males performed 9.80 ± 1.40 left-turns and 16.0 ± 2.00 right-turns (T+ = −1.826,
p = 0.068) and females performed 16.60 ± 2.67 left-turns vs. 13.0 ± 1.342 right-turns (T+ = −1.214,
p = 0.225). If we consider the average number of turns that lizards performed when encountering a T-crossroad for the first time (i.e., without any olfactory influence) there emerged a preference in turning right (8.5 ± 1.02) compared with the left direction (5.9 ± 0.78; T+ = −1.963,
p = 0.050) in the population. This result is due to the males’ choice (right: 8.5 ± 1.02; left: 4.4 ± 0.52) more than the females’ choice (right: 8.0 ± 0.72; left: 7.4 ± 0.72;
Table 1). Moreover, females turned more frequently than males to the left (T+ = −2.032;
p = 0.042) than to the right (T+ = −0.412;
p = 0.680).
Considering the total number of turns per lizard to the left or to the right, 2 individuals of the 10 tested showed a preference for turning right (Binomial test;
p = 0.029 and
p = 0.035), both males (
Table 2).
By the number of first turn performed by each lizard it emerged that only one individual (a male) showed a preference, in particular in turning rightward (binomial test; p = 0.0192).
4. Discussion
Overall, our lizards resulted in not showing any evident bias or side preference in exploring a novel environment. Hence, the explorative behaviour of
Zootoca vivipara lizards does not seem to be controlled by a form of lateralization. This is interesting as this result is in strong contrast with what was found by Csermely et al. [
32] in
Podarcis muralis. In fact, although experiments were conducted in the same way, and with the same experimental apparatus,
P. muralis evidenced a strong bias in turning left, that the authors associated to a visual guided bias during exploration, i.e., a visual lateralization [
32]. As such, these results suggest that differences emerging between these species are probably due to their remarkably different ecology, although, at present, there is no evidence of a clear explanation for the differential lateralization of the two species.
As a first point, we observed that although during the experiment almost all
Z. vivipara individuals gave good clear signals of exploration, they all showed hesitations in entering the maze and the time they spent in exploring was similar to the time they spent in freezing. This poor activity and the overall low level of confidence in the maze could be related to the secretive behaviour of
Z. vivipara and, in particular, likened to the thermally-heterogeneous habitats where this species is commonly found, allowing less active movements in general and, at the same time, more time spent in thermoregulation [
34,
35].
Results on behavioural lateralization highlighted in
P. muralis have been explained by the authors with its strong territoriality and its consequent natural high predisposition to explore [
32]. A support of this is the fact that, in Csermely et al. [
32], an evident higher frequency of turning emerged, especially in males, mainly motivated by the need to defend their own area. In contrast, our
Z. vivipara lived in a wild, cold-climate environment, characterized by the presence of unique ecological factors potentially influencing the explorative behaviour (therefore, the
Z. vivipara lateralization). For example, the human impact and presence in such areas is generally low, in contrast with the high anthropic level locations, where
P. muralis was studied by Csermely et al. [
32] lived. This could force individuals to maintain a high level of attention and vigilance, pushing towards a stronger lateralization in the explorative behaviour.
Nevertheless, an overall female bias in turning left emerged here, referring also to the first and more spontaneous encounter with a T-crossroad. Whereas the lack of turning preferences in the overall T-maze could be due to the low sample used in these experiments, the turning bias in the first T-crossroad could be explained either as a visual lateralization or a motor lateralization. As a visual lateralization, the direction of choice is consistent with that found in
P. muralis, and with previous studies, which appointed to the right hemisphere the capability of processing global aspects of the environment [
14,
32,
36]. However, it is in strong contrast with the evidence that, in
P. muralis, the turning bias is found in males, not in females [
32]. As this previous result could be linked with males’ territorial attitude, we suggest that
Z. vivipara females’ visual preference could be related to the viviparous nature of several populations of this species, which constrains female individuals in having a longer reproductive period in respect to oviparous females, and a consequently higher level of attention compared with male individuals [
34]. During the pregnant period, females must be more vigilant to guarantee the offspring’s survival, hence, to increase their fitness. However, gestation incurs some costs, such as a shift in thermoregulatory needs and locomotor impairment [
34,
35,
37,
38,
39]. Pregnant individuals are physically limited by their body increase which may affect and reduce their fleetness and speed, thus, with locomotor costs. Being lateralized, in particular for the same direction in the same population, could be advantageous for the possibility of coordination in behaviour between individuals, in particular for anti-predatory tasks [
4]. This is especially true for social/gregarious species [
4,
40,
41]. Although there is no evidence of gregarious habits in
Zootoca vivipara, the absence of territoriality allows tolerance between individuals, and a coordination in moving may become, for these lizards, one of the evolutive strategies for contrasting costs of viviparity. This could become a hypothesis of explanation of the necessity for female lizards to be specialized in vigilance as a group, especially in moving.
Movement, particularly in exploratory behaviour, is preceded by a high-level observation that probably guides the subsequent direction choice. There are several indications of left-eye processing in using the environmental layout to guide locomotion to a target site using spatial information [
42]. However, we emphasize that the female leftward preference in turning, which emerged at T-crossroads is not supported by the head rotation durations we measured during each T-crossroad turning. These comparisons, easily indicative of visual system involvement, did not show any significance, not sustaining a visual influence in the direction choice. It is therefore possible to advance the hypothesis in this context, that the left-turning females’ bias highlighted by this work may be evidence of footedness, hence, a motor bias more than a visual one.
Very differently to
P. muralis, closely related to dry and bare environments,
Z. vivipara is strictly dependent on habitats, as wetlands, where the vegetation cover is prominent and could become a visual impediment between individuals. Thus, it could be difficult for these individuals to maintain a visual link in groups of conspecifics. This is also true during thermoregulatory exposure. In fact, because of their viviparity, female lizards preferentially used the half-basking behaviour (partially hidden), although basking in the open is more efficient [
34]. This allowed these lizards to significantly reduce the risk of facing exposure to predators, optimizing the trade-off between predation risks and basking efficiency [
34,
43,
44]. In this context, it could be disadvantageous or simply necessary to visually coordinate the behaviour, but it may help to synchronise a motor response.
All this contributes to explaining the different response Z. vivipara provide compared with P. muralis, i.e., the main absence of lateralization and the different sex evidence, also underlining the importance and close relationship between the living environment and conditions and the evolution of biases.
In conclusion, our results show, that in general Z. vivipara is not lateralized in exploring a new environment. However, females showed a bias for turning left during exploration, possibly more easily explained as a motor bias. As these results are in contrast with what emerged in P. muralis individuals in previous studies, which showed a visual lateralization especially in males, we propose it could be related to the different ecology of the species, in particular, with differences of the territorial and viviparous natures. Moreover, this is a confirmation of the crucial role of the real-life environment and habits in the emergence and evolution of cerebral lateralization, supporting its advantageous nature, which contributes in its manifestation in different contexts.