Mineralogical and Geochemical Characteristics of Triassic Lithium-Rich K-Bentonite Deposits in Xiejiacao Section, South China
Abstract
:1. Introduction
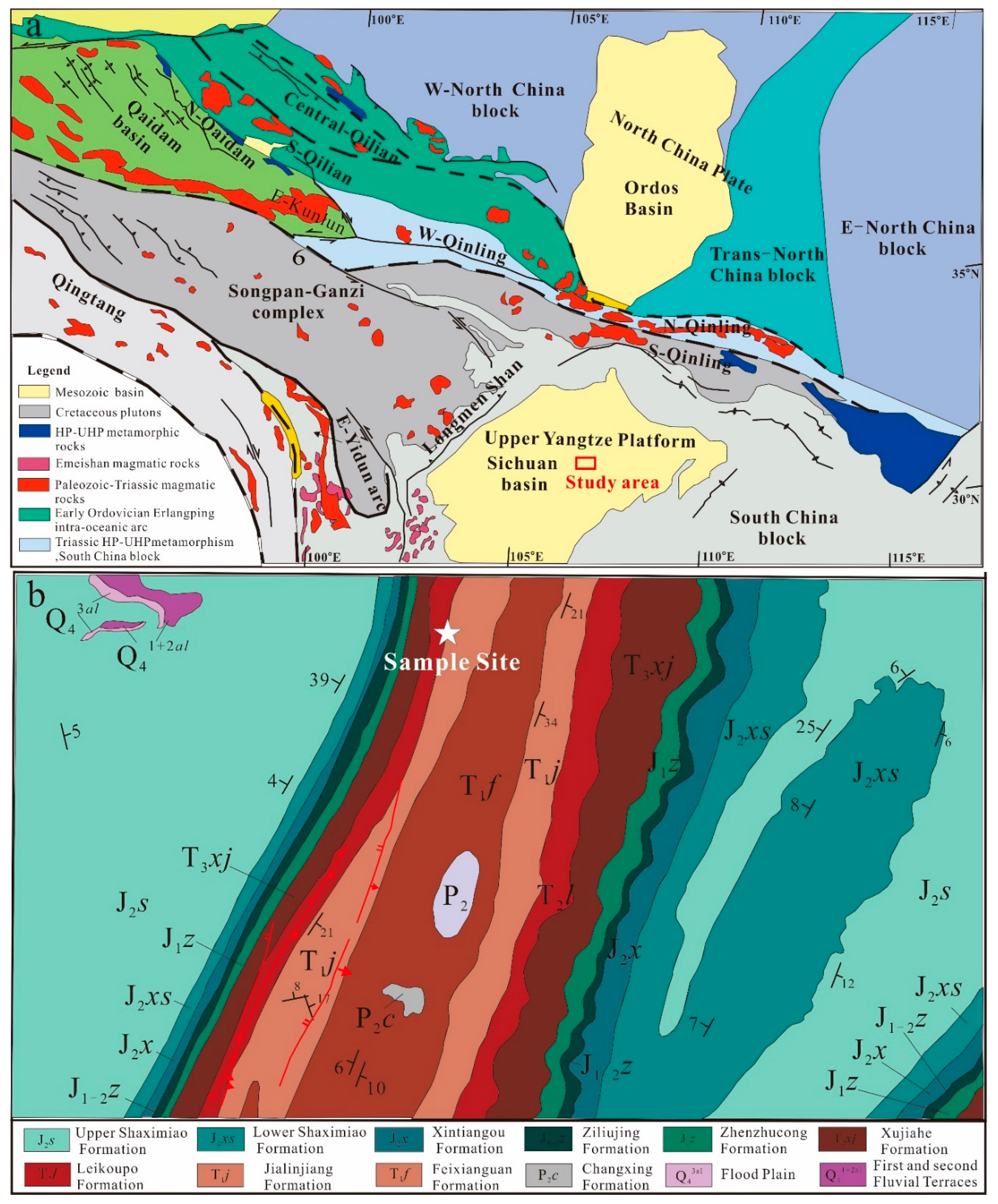
2. Geological Setting
3. Samples and Methods
4. Results
4.1. Bulk and Clay Mineralogy
4.2. Elemental Geochemistry
4.3. Sr Isotopic Compositions
5. Discussions
5.1. Source Magmas and Tectonic Settings
5.2. Formation Mechanism of Li-Rich Clay
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benson, T.R.; Coble, M.A.; Rytuba, J.J.; Mahood, G.A. Lithium enrichment in intracontinental rhyolite magmas leads to Li deposits in caldera basins. Nat. Commun. 2017, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, D.; Gao, Y.; Han, J.; Ma, S.; Fan, X.; Gu, W.; Zhang, L. Geochemical characteristics of lithium- rich mung bean rocks in Tongliang County, Chongqing. Acta Petrol. Mineral. 2018, 37, 395–403, (In Chinese with English Abstract). [Google Scholar]
- Ju, P.; Wang, X.; Wang, Z.; Liu, X.; Zhong, J.; Zhang, Z. Characteristics and geological significance of the Triassic mungbean rocks the Wenquan Town area, northern Chongqing. Geoscience 2019. accepted. [Google Scholar]
- Zhu, Z.; Wang, G. Paleogeography of before and after deposition of greeb-bean rock(altered tuff) between the early and middle Triassic in the Uper Yangtze platform and its adjacent areas. Oil Gas Geol. 1986, 7, 344–355, (In Chinese with English Abstract). [Google Scholar]
- Chen, Z.; Shen, M.; Zhao, J.; Tang, H. New Analysis of ‘Mungbean Rock’ Composition. J. Southwest Pet. Inst. 1999, 21, 39–42. (In Chinese) [Google Scholar]
- Zhu, L. The Genesis and Characteristics of clay minerals of Green-bean rock in Guizhou Province. Acta Mineral. Sin. 1995, 15, 75–81, (In Chinese with English Abstract). [Google Scholar]
- Ma, S.; Wang, D.; Sun, Y.; Li, C.; Zhong, H. Geochronology and Geochemical Characteristics of Lower-Middle Triassic Clay Rock and Their Significances for Prospecting Clay-Type Lithium Deposit. Earth Sci. 2019, 44, 2–14, (In Chinese with English Abstract). [Google Scholar]
- Sun, Y.; Gao, Y.; Wang, D.; Dai, H.; Gu, W.; Li, J.; Zhang, L. Zircon U-Pb Dating of ‘Mung Bean Rock’ in the Tongliang Area, Chongqing and Its Geological Significance. Rock Miner. Anal. 2017, 36, 649–658, (In Chinese with English Abstract). [Google Scholar]
- Yin, A.; Nie, S. An indentation model for the North and South China collision and the development of the Tan-Lu and Honam Fault Systems, eastern Asia. Tectonics 1993, 12, 801–813. [Google Scholar] [CrossRef]
- Ratschbacher, L.; Hacker, B.R.; Calvert, A.; Webb, L.E.; Grimmer, J.C.; McWilliams, M.O.; Ireland, T.; Dong, S.; Hu, J. Tectonics of the Qinling (Central China): Tectonostratigraphy, geochronology, and deformation history. Tectonophysics 2003, 366, 1–53. [Google Scholar] [CrossRef]
- Zhong, Y.-J.; Chen, A.-Q.; Huang, K.-K.; Yang, S.; Xu, S.-L. Hydrothermal activity in the fourth member of the Triassic Leikoupo Formation in the Yuanba area, northeast Sichuan, SW China. Geol. J. 2019, 54, 266–277. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Li, L.; Liu, H.; Cao, J.; Wu, X.; Zhou, S.; Shi, X. Mega-shoaling in carbonate platform of the Middle Triassic Leikoupo Formation, Sichuan Basin, southwest China. Sci. China Earth Sci. 2014, 57, 465–479. [Google Scholar] [CrossRef]
- Zhu, J. Characteristics and origin of polycrystalline dolomite needles in the Triassic Jialingjiang Formation, Upper Yangtze Platform, southwest China. Sediment. Geol. 1998, 118, 119–126. [Google Scholar]
- Enkelmann, E.; Weislogel, A.; Ratschbacher, L.; Eide, E.; Renno, A.; Wooden, J. How was the Triassic Songpan-Ganzi basin filled? A provenance study. Tectonics 2007, 26, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Liang, Z. Geological Survey Report of the Dianjiang Area, Sichuang Province (Scale 1:200000 Pieces); 107 Geological Brigade of Sichuan Bureau of Geology & Mineral Resources: Chongqing, China, 1980; pp. 1–91. (In Chinese)
- Zhou, Z.; Luo, H.; Zhu, Y.; Cai, H.; Xu, B.; Chen, J.; Zhao, Y. Early Trassic Anachronistic Sediments at the Xiejiacao Section of Guangan, Sichuan Province and Their paleoecological Significances: A restudy of a Classical Section. J. Stratigr. 2013, 37, 73–80, (In Chinese with English Abstract). [Google Scholar]
- Yang, H.; Luo, H.; Zhu, Y.; Cai, H.; Xu, B.; Zhou, Z. Early and middle Triassic foraminifera from the Xiejiacao section Guangan, Sichuan Province, China. Acta Micropalaeontol. Sin. 2017, 34, 390–405, (In Chinese with English Abstract). [Google Scholar]
- Jackson, K.; Jonasson, I.; Skippen, G. The nature of metals—Sediment—Water interactions in freshwater bodies, with emphasis on the role of organic matter. Earth Sci. Rev. 1978, 14, 97–146. [Google Scholar] [CrossRef]
- Wang, C.; Li, M.; Fang, X.; Liu, Y.; Yan, M. Structural characteristic of mixed-layer illite/Smectite clay minerals of the SG-1 core in the western Qaidam basin and its environmental significance. Quat. Sci. 2016, 36, 917–925, (In Chinese with English Abstract). [Google Scholar]
- Yang, X.; Zhu, B.; White, P.D. Provenance of aeolian sediment in the Taklamakan Desert of western China, inferred from REE and major-elemental data. Quat. Int. 2007, 175, 71–85. [Google Scholar] [CrossRef]
- Li, Y.; Xing, Y.; Wang, S. Part 14: Determination of ferrous oxide content. In Methods for Chemical Analysis of Silicate Rocks; Standards Press of China: Beijing, China, 2010; pp. 1–3. (In Chinese) [Google Scholar]
- Wang, S.; Yan, M. Part 28: Determination of 16 major and minor elements content. In Methods for Chemical Analysis of Silicate Rocks; Standards Press of China: Beijing, China, 2010; pp. 1–6. (In Chinese) [Google Scholar]
- Ling, H.-F.; Feng, H.-Z.; Pan, J.-Y.; Jiang, S.-Y.; Chen, Y.-Q.; Chen, X. Carbon isotope variation through the Neoproterozoic Doushantuo and Dengying Formations, South China: Implications for chemostratigraphy and paleoenvironmental change. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 254, 158–174. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, W.; Wang, X.; Lu, Y.; Wang, L.; Liao, Z.; Li, W. Resetting of Mg isotopes between calcite and dolomite during burial metamorphism: Outlook of Mg isotopes as geothermometer and seawater proxy. Geochim. Cosmochim. Acta 2017, 208, 24–40. [Google Scholar] [CrossRef] [Green Version]
- Lin, X. X-Ray Diffraction Analysis Technology and Its Geological Application; Petroleum Industry Press: Beijing, China, 1990. [Google Scholar]
- Środoń, J. X-ray Powder Diffraction Identification of Illitic Materials. Clays Clay Miner. 1984, 32, 337–349. [Google Scholar] [CrossRef]
- Inoue, A.; Lanson, B.; Marques-Fernandes, M.; Sakharov, B.A.; Murakami, T.; Meunier, A.; Beaufort, D. Illite-smectite mixed-layer minerals in the hydrothermal alteration of volcanic rocks: I. One-dimensional XRD structure analysis and characterization of component layers. Clays Clay Miner. 2005, 53, 423–439. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution. An Examination of the Geochemical Record Preserved in Sedimentary Rocks; Blackwell Science: Oxford, UK, 1985. [Google Scholar]
- Hong, H.; Algeo, T.J.; Fang, Q.; Zhao, L.; Ji, K.; Yin, K.; Wang, C.; Cheng, S. Facies dependence of the mineralogy and geochemistry of altered volcanic ash beds: An example from Permian-Triassic transition strata in southwestern China. Earth Sci. Rev. 2019, 190, 58–88. [Google Scholar] [CrossRef]
- Yard, W.N. Mineralogy and petrology of some ordovician K-bentonites and related limestones. Geol. Soc. Am. Bull. 1953, 64, 921–943. [Google Scholar]
- Altaner, S.P.; Hower, J.; Whitney, G.; Aronson, J.L. Model for K-bentonite formation: Evidence from zoned K-bentonites in the disturbed belt, Montana. Geology 1984, 12, 412–415. [Google Scholar] [CrossRef]
- Huff, D.W. K-bentonites: A review. Am. Mineral. 2016, 101, 43–70. [Google Scholar] [CrossRef]
- Sun, S.; McDonough, W.F. Chemical and isotopic systematics of ocean basalts: Implications for mantle composition and processes. In Magmatism in the Ocean Basins; Geological Socoety of London Special Publication: London, UK, 1989; pp. 1–345. [Google Scholar]
- Jiang, L.; Cai, C.F.; Worden, R.H.; Li, K.-K.; Xiang, L. Reflux dolomitization of the Upper Permian Changxing Formation and the Lower Triassic Feixianguan Formation, NE Sichuan Basin, China. Geofluids 2013, 13, 232–245. [Google Scholar] [CrossRef]
- Davis, A.C.; Bickle, M.J.; Teagle, D.A.H. Imbalance in the oceanic strontium budget. Earth Planet. Sci. Lett. 2003, 211, 173–187. [Google Scholar] [CrossRef]
- Yang, S.; Hu, W.; Wang, X.; Jiang, B.; Yao, S.; Sun, F.; Huang, Z.; Zhu, F. Duration, evolution, and implications of volcanic activity across the Ordovician–Silurian transition in the Lower Yangtze region, South China. Earth Planet. Sci. Lett. 2019, 518, 13–25. [Google Scholar] [CrossRef]
- Ramkumar, M.; Stüben, D.; Berner, Z.; Schneider, J. 87Sr/86Sr anomalies in Late Cretaceous-Early Tertiary strata of the Cauvery basin, south India: Constraints on nature and rate of environmental changes across K-T boundary. J. Earth Syst. Sci. 2010, 119, 1–17. [Google Scholar] [CrossRef]
- Gong, N.; Hong, H.; Huff, W.D.; Fang, Q.; Bae, C.J.; Wang, C.; Yin, K.; Chen, S. Influences of Sedimentary Environments and Volcanic Sources on Diagenetic Alteration of Volcanic Tuffs in South China. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Mou, C.; Wang, C.; Men, X.; Chen, C.; Hou, Q. Mineralogical and geochemical characteristics of K-bentonites from the Late Ordovician to the Early Silurian in South China and their geological significance. Geol. J. 2019, 54, 514–528. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Zhong, Y.-T.; Xu, Y.-G.; Li, X.-H. Triggers of Permo-Triassic boundary mass extinction in South China: The Siberian Traps or Paleo-Tethys ignimbrite flare-up? Lithos 2014, 204, 258–267. [Google Scholar] [CrossRef]
- Berry, R.W. Eocene and Oligocene Otay-Type Waxy Bentonites of San Diego County and Baja California: Chemistry, Mineralogy, Petrology and Plate Tectonic Implications. Clays Clay Miner. 1999, 47, 70–83. [Google Scholar] [CrossRef]
- Sugitani, K. Anomalously low Al2O3/TiO2 values for Archean cherts from the Pilbara Block, Western Australia—Possible evidence for extensive chemical weathering on the early earth. Precambrian Res. 1996, 80, 49–76. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Winchester, J.A.; Floyd, P.A. Geochemical discrimination of different magma series and their differentiation products using immobile elements. Chem. Geol. 1977, 20, 325–343. [Google Scholar] [CrossRef] [Green Version]
- Pearce, J.A.; Harris, N.B.W.; Tindle, A.G. Trace Element Discrimination Diagrams for the Tectonic Interpretation of Granitic Rocks. J. Petrol. 1984, 25, 956–983. [Google Scholar] [CrossRef] [Green Version]
- Watson, E.B.; Harrison, T.M. Zircon saturation revisited: Temperature and composition effects in a variety of crustal magma types. Earth Planet. Sci. Lett. 1983, 64, 295–304. [Google Scholar] [CrossRef]
- Ducea, M.N.; Barton, M.D. Igniting flare-up events in Cordilleran arcs. Geology 2007, 35, 1047–1050. [Google Scholar] [CrossRef]
- Su, W.; Huff, W.D.; Ettensohn, F.R.; Liu, X.; Zhang, J.; Li, Z. K-bentonite, black-shale and flysch successions at the Ordovician–Silurian transition, South China: Possible sedimentary responses to the accretion of Cathaysia to the Yangtze Block and its implications for the evolution of Gondwana. Gondwana Res. 2009, 15, 111–130. [Google Scholar] [CrossRef]
- Huff, W.D.; Bermöm, S.M.; Kolata, D.R. Silurian K-bentonites of the Dnestr Basin, Podolia, Ukraine. J. Geol. Soc. 2000, 157, 493–504. [Google Scholar] [CrossRef]
- Mizota, C.; Faure, K. Hydrothermal origin of smectite in volvanic ash. Clays Clay Miner. 1998, 46, 178–182. [Google Scholar] [CrossRef]
- Bohor, T.B.; Triplehorn, M.D. Tonsteins: Altered Volcanic-Ash Layers in Coal-Bearing Sequences; Geological Society of America: Boulder, CO, USA, 1993; Volume 285, ISBN 0-8137-2285-3. [Google Scholar]
- Fisher, R.V.; Schmincke, H.-U. Pyroclastic Rocks; Springer: Berlin/Heidelberg, Germany, 1984; ISBN 978-3-540-51341-4. [Google Scholar]
- De la Fuente, S.; Cuadros, J.; Fiore, S.; Linares, J. Electron Microscopy Study of Volcanic Tuff Alteration to Illite-Smectite under Hydrothermal Conditions. Clays Clay Miner. 2000, 48, 339–350. [Google Scholar] [CrossRef]
- Lanson, B.; Sakharov, B.A.; Claret, F.; Drits, V.A. Diagenetic smectite-to-illite transition in clay-rich sediments: A reappraisal of X-ray diffraction results using the multi-specimen method. Am. J. Sci. 2009, 309, 476–516. [Google Scholar] [CrossRef] [Green Version]
- Środoń, J.; Clauer, N.; Huff, W.; Dudek, T.; Banaś, M. K-Ar dating of the Lower Palaeozoic K-bentonites from the Baltic Basin and the Baltic Shield: Implications for the role of temperature and time in the illitization of smectite. Clay Miner. 2009, 44, 361–387. [Google Scholar] [CrossRef]
- Lanson, B. Late-Stage Diagenesis of Illitic Clay Minerals as Seen by Decomposition of X-ray Diffraction Patterns: Contrasted Behaviors of Sedimentary Basins with Different Burial Histories. Clays Clay Miner. 1998, 46, 69–78. [Google Scholar] [CrossRef]
- Nadeau, P.H. Burial and Contact Metamorphism in the Mancos Shale. Clays Clay Miner. 1981, 29, 249–259. [Google Scholar] [CrossRef]
- Deconinck, J.F.; Crasquin, S.; Bruneau, L.; Pellenard, P.; Baudin, F.; Feng, Q. Diagenesis of clay minerals and K-bentonites in Late Permian/Early Triassic sediments of the Sichuan Basin (Chaotian section, Central China). J. Asian Earth Sci. 2014, 81, 28–37. [Google Scholar] [CrossRef]
- McCarty, D.K.; Sakharov, B.A.; Drits, V.A. New insights into smectite illitization: A zoned K-bentonite revisited. Am. Mineral. 2009, 94, 1653–1671. [Google Scholar] [CrossRef]
- Bozkaya, Ö.; Yalçin, H. Geochemistry of mixed-layer illite-smectites from an extensional basin, Antalya unit, southwestern Turkey. Clays Clay Miner. 2010, 58, 644–666. [Google Scholar] [CrossRef]
- Hints, R.; Kirsimäe, K.; Somelar, P.; Kallaste, T.; Kiipli, T. Multiphase Silurian bentonites in the Baltic Palaeobasin. Sediment. Geol. 2008, 209, 69–79. [Google Scholar] [CrossRef]
- Šucha, V.; Kraust, I.; Gerthofferová, H.; Peteš, J.; Sereková, M. Smectite to Illite Conversion in Bentonites and Shales of the East Slovak Basin. Clay Miner. 1993, 28, 243–253. [Google Scholar] [CrossRef]
- Zielinski, R.A. The mobility of uranium and other elements during alteration of rhyolite ash to montmorillonite: A case study in the Troublesome Formation, Colorado, U.S.A. Chem. Geol. 1982, 35, 185–204. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Europium redox equilibria in aqueous solution. Earth Planet. Sci. Lett. 1984, 67, 70–78. [Google Scholar] [CrossRef]
- Püspöki, Z.; Kozák, M.; Kovács-Pálffy, P.; Szepesi, J.; McIntosh, R.; Kónya, P.; Vincze, L.; Gyula, G. Geochemical Records of a Bentonitic Acid-Tuff Succession Related to a Transgressive Systems Tract—Indication of Changes in the Volcanic Sedimentation Rate. Clays Clay Miner. 2008, 56, 23–38. [Google Scholar] [CrossRef]
- Kramer, W.; Weatherall, G.; Offler, R. Origin and correlation of tuffs in the Permian Newcastle and Wollombi Coal Measures, NSW, Australia, using chemical fingerprinting. Int. J. Coal Geol. 2001, 47, 115–135. [Google Scholar] [CrossRef]
- Siir, S.; Kallaste, T.; Kiipli, T.; Hints, R. Internal stratification of two thick Ordovician bentonites of Estonia: Deciphering primary magmatic, sedimentary, environmental and diagenetic signatures. Est. J. Earth Sci. 2015, 64, 140–158. [Google Scholar] [CrossRef]
- Le Maitre, R.W. The Chemical Variability of some Common Igneous Rocks. J. Petrol. 1976, 17, 589–598. [Google Scholar] [CrossRef]
- Huff, W.D.; Bergström, S.M.; Kolata, D.R.; Cingolani, C.A.; Astini, R.A. Ordovician K-bentonites in the Argentine Precordillera: Relations to Gondwana margin evolution. Geol. Soc. Lond. Spec. Publ. 1998, 142, 107–126. [Google Scholar] [CrossRef]
- Arslan, M.; Abdioğlu, E.; Kadir, S. Mineralogy, Geochemistry, and Origin of Bentonite in Upper Cretaceous Pyroclastic Units of the Tirebolu Area, Giresun, Northeast Turkey. Clays Clay Miner. 2010, 58, 120–141. [Google Scholar] [CrossRef]
- Zielinski, R.A. Element mobility during alteration of silicic ash to kaolinite-a study of tonstein. Sedimentology 1985, 32, 567–579. [Google Scholar] [CrossRef]
- Schiffman, P.; Staudigel, H. The smectite to chlorite transition in a fossil seamount hydrothermal system: The Basement Complex of La Palma, Canary Islands. J. Metamorph. Geol. 1995, 13, 487–498. [Google Scholar] [CrossRef]
- Bettison-Varga, L. The Role of Randomly Mixed-Layered Chlorite/Smectite in the Transformation of Smectite to Chlorite. Clays Clay Miner. 1997, 45, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Jin, P.; Ou, C.; Ma, Z.; Li, D.; Ren, Y.; Zhao, Y. Evolution of montmorillonite and its related clay minerals and their effects on shale gas development. Geophys. Prospect. Pet. 2018, 57, 344–355, (In Chinese with English Abstract). [Google Scholar]




| NO. | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | MnO | TiO2 | P2O5 | LOI | FeO | F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-1 | 67.73 | 12.41 | 0.944 | 4.18 | 0.418 | 0.015 | 10.04 | 0.009 | 0.238 | 0.029 | 3.74 | 0.21 | 0.261 |
| S-2 | 0.696 | 0.154 | 0.075 | 0.373 | 54.77 | 0.089 | 0.082 | 0.005 | 0.006 | 0.014 | 43.46 | <0.10 | 0.044 |
| S-3 | 9.93 | 1.82 | 1.06 | 1.97 | 45.68 | 0.023 | 0.824 | 0.041 | 0.109 | 0.043 | 38.47 | 0.19 | 0.09 |
| S-4 | 1.6 | 0.402 | 0.357 | 7.2 | 46.13 | <0.010 | 0.281 | 0.015 | 0.032 | 0.031 | 43.92 | 0.19 | 0.045 |
| XJC-1-R-1 | 66.96 | 14.18 | 0.60 | 3.84 | 0.37 | 0.06 | 11.04 | 0.01 | 0.23 | 0.05 | 3.36 | - | - |
| TL-1 | 58.52 | 16.00 | 0.66 | 8.11 | 0.42 | <0.01 | 9.02 | <0.01 | 0.24 | 0.12 | - | - | - |
| TL-2 | 62.02 | 15.00 | 2.96 | 3.35 | 1.36 | <0.01 | 8.44 | 0.03 | 0.84 | 0.38 | - | - | - |
| TL-3 | 58.24 | 16.74 | 0.80 | 7.29 | 0.69 | <0.01 | 8.84 | <0.01 | 0.27 | 0.33 | - | - | - |
| NO. | Cs | Rb | Pb | Ba | Th | U | B | Ta | Hf | Nb | Zr | Sr | Li | Ga | Zn | Cu | V | Sc | Co | Cr | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-1 | 1.52 | 122 | 2.69 | 65.4 | 19.8 | 4.47 | 867 | 1.14 | 4.1 | 9.63 | 102 | 22.4 | 321 | 13 | 7.08 | 4.1 | 17.3 | 2.12 | 0.97 | 2.37 | 2.02 |
| S-2 | 0.04 | 1.12 | 0.89 | 3.31 | 0.12 | 0.9 | 6 | 0.01 | 0.54 | 0.13 | 0.9 | 1227 | 1.96 | 0.22 | 1.75 | 4.36 | 15.5 | 0.31 | 1.67 | 2.18 | 39.7 |
| S-3 | 0.91 | 16.3 | 2.23 | 62 | 1.8 | 2.91 | 47.2 | 0.16 | 0.11 | 2.09 | 16.3 | 92.2 | 50.1 | 2.67 | 17 | 7.09 | 23.1 | 1.99 | 3.17 | 11.1 | 35.9 |
| S-4 | 0.06 | 2.34 | 1.85 | 6.83 | 0.33 | 2.23 | 25.5 | 0.03 | 0.08 | 0.68 | 5.2 | 60.7 | 3.68 | 0.53 | 2.94 | 4.4 | 21 | 0.72 | 2.35 | 6.7 | 35.7 |
| XJC-1-R-1 | 1.96 | 140.52 | 2.86 | 95.04 | 31 | 5.74 | - | 1.33 | 5.43 | 11.94 | 137.67 | 101.14 | 380.02 | 17.38 | 2.64 | 4.09 | 11.16 | 3.37 | 1.38 | 3.01 | 1.87 |
| TL-1 | 11.8 | 291 | 5.28 | 120 | 29.5 | 8.02 | - | 1.55 | 5.39 | 12.5 | 140 | 26.5 | 663 | 22.1 | 49.1 | 4.23 | 4.59 | 4.03 | 0.12 | - | - |
| TL-2 | 12.5 | 157 | 34.5 | 287 | 21.5 | 9.81 | - | 1.5 | 6.03 | 17.8 | 205 | 50.4 | 257 | 0.81 | 123 | 35.9 | 95.3 | 10.7 | 8.86 | - | - |
| TL-3 | 16.8 | 301 | 14.2 | 55.1 | 16.8 | 13.3 | - | 1.59 | 5.60 | 13.3 | 142 | 21.4 | 594 | 0.49 | 60.7 | 5.09 | 4.94 | 4.4 | 1.94 | - | - |
| NO. | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-1 | 16.3 | 27.3 | 2.99 | 10.3 | 2.6 | 0.176 | 2.75 | 0.599 | 3.83 | 22.3 | 0.807 | 2.32 | 0.461 | 2.96 | 0.386 |
| S-2 | 4.36 | 6.95 | 1.01 | 3.45 | 0.762 | 0.114 | 0.641 | 0.098 | 0.553 | 2.8 | 0.098 | 0.309 | 0.054 | 0.21 | 0.067 |
| S-3 | 0.729 | 1.3 | 0.176 | 0.527 | 0.155 | 0.02 | 0.11 | 0.02 | 0.097 | 0.476 | 0.012 | 0.044 | 0.009 | 0.046 | 0.009 |
| S-4 | 1.02 | 2.06 | 0.26 | 1.01 | 0.11 | 0.03 | 0.131 | 0.016 | 0.208 | 0.998 | 0.042 | 0.182 | 0.023 | 0.079 | 0.022 |
| XJC-1-R-1 | 45.599 | 99.297 | 11.286 | 40.372 | 6.312 | 0.28 | 3.752 | 0.696 | 4.506 | 23.174 | 0.819 | 2.555 | 0.373 | 2.349 | 0.348 |
| TL-1 | 15.9 | 37.3 | 4.64 | 18.5 | 4.38 | 0.24 | 4.61 | 0.89 | 6.34 | 31.2 | 1.29 | 3.92 | 0.62 | 3.99 | 0.55 |
| TL-2 | 79.3 | 172 | 20.80 | 79.3 | 16.6 | 1.3 | 15.4 | 2.5 | 15.5 | 76.8 | 2.95 | 8.34 | 1.23 | 7.89 | 1.08 |
| TL-3 | 26.9 | 63.6 | 8.13 | 33.7 | 8.98 | 0.48 | 10.2 | 1.86 | 12.2 | 61.6 | 2.39 | 6.82 | 1.03 | 6.38 | 0.9 |
| Sample No. | Sample Type | 87Sr/86Sr | Std. Error |
|---|---|---|---|
| S-1 | Li-rich clays | 0.75975786 | 0.00000577 |
| S-2 | limestones | 0.70821791 | 0.00000562 |
| S-3 | limestones | 0.71073714 | 0.00000575 |
| S-4 | limestones | 0.70876008 | 0.00000603 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Zheng, M.; Zhang, Y.; Xing, E.; Redfern, S.A.T.; Xu, J.; Zhong, J.; Niu, X. Mineralogical and Geochemical Characteristics of Triassic Lithium-Rich K-Bentonite Deposits in Xiejiacao Section, South China. Minerals 2020, 10, 69. https://doi.org/10.3390/min10010069
Lin Y, Zheng M, Zhang Y, Xing E, Redfern SAT, Xu J, Zhong J, Niu X. Mineralogical and Geochemical Characteristics of Triassic Lithium-Rich K-Bentonite Deposits in Xiejiacao Section, South China. Minerals. 2020; 10(1):69. https://doi.org/10.3390/min10010069
Chicago/Turabian StyleLin, Yongjie, Mianping Zheng, Yongsheng Zhang, Enyuan Xing, Simon A. T. Redfern, Jianming Xu, Jiaai Zhong, and Xinsheng Niu. 2020. "Mineralogical and Geochemical Characteristics of Triassic Lithium-Rich K-Bentonite Deposits in Xiejiacao Section, South China" Minerals 10, no. 1: 69. https://doi.org/10.3390/min10010069





