Significance and Distribution of Apatite in the Triassic Doig Phosphate Zone, Western Canada Sedimentary Basin
Abstract
:1. Introduction
Geology
2. Materials and Methods
2.1. X-ray Diffraction
2.2. Geochemistry
Control Samples
2.3. Petrography
2.4. Scanning Electron Microscopy
3. Results
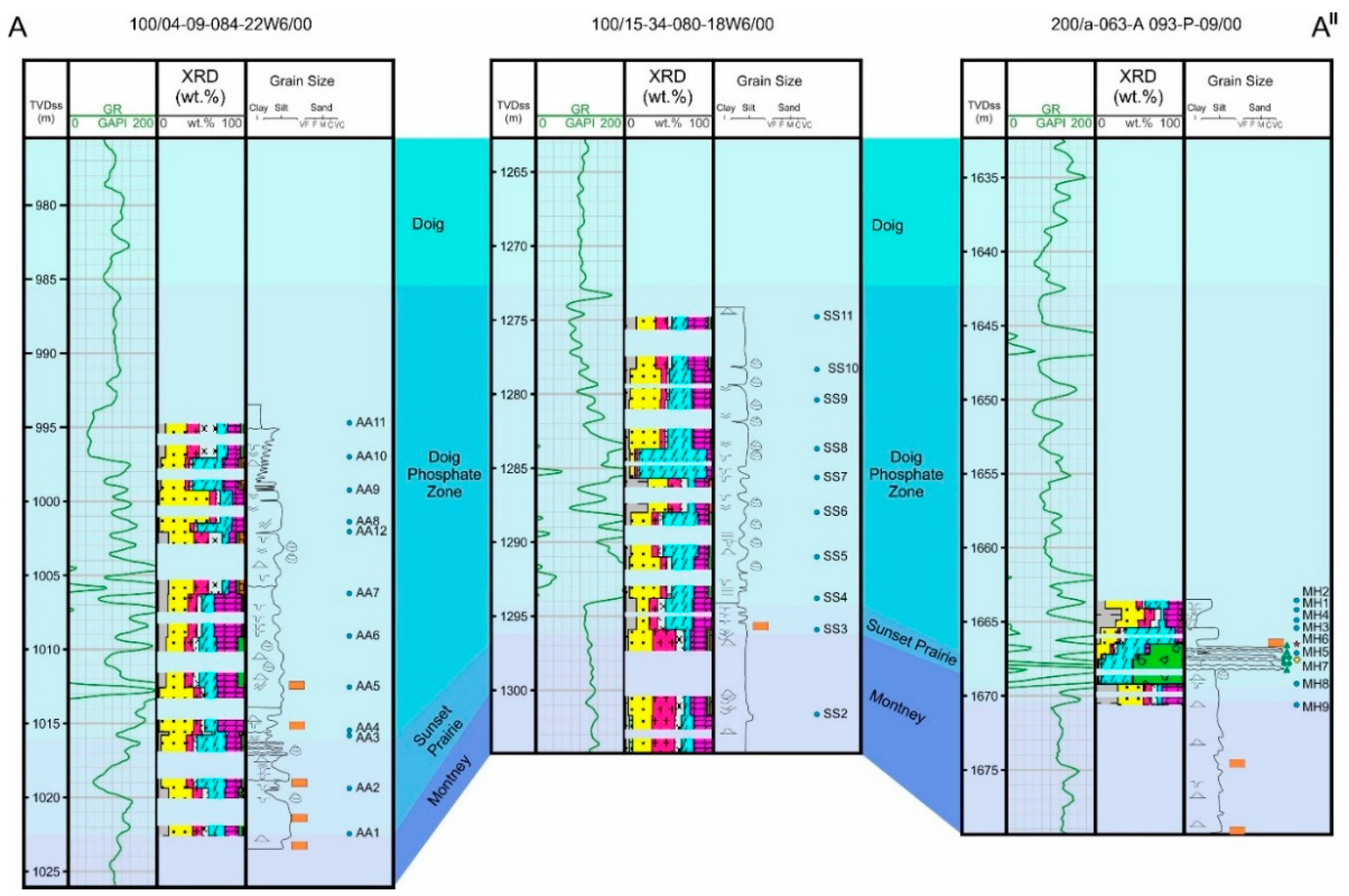
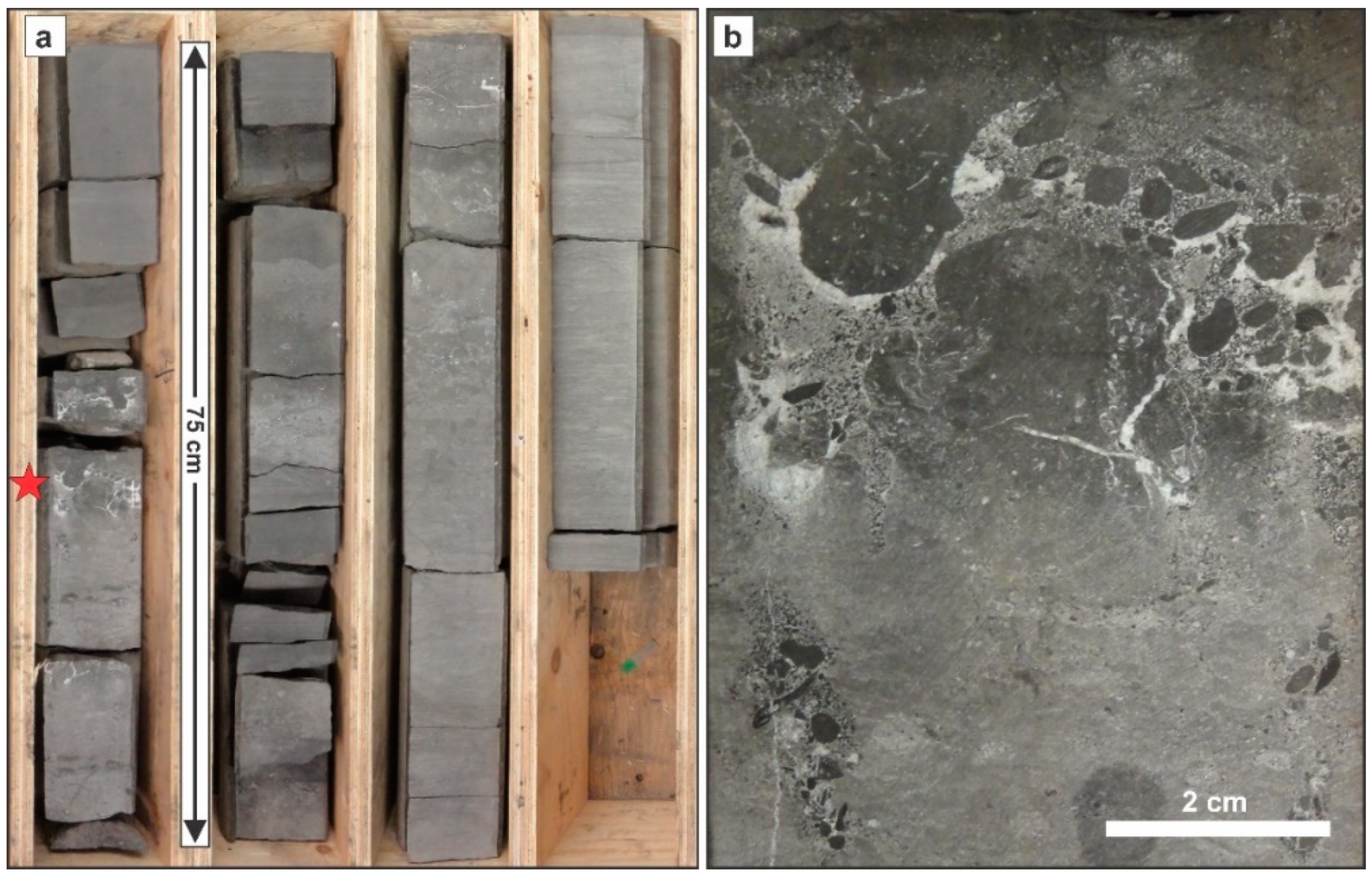
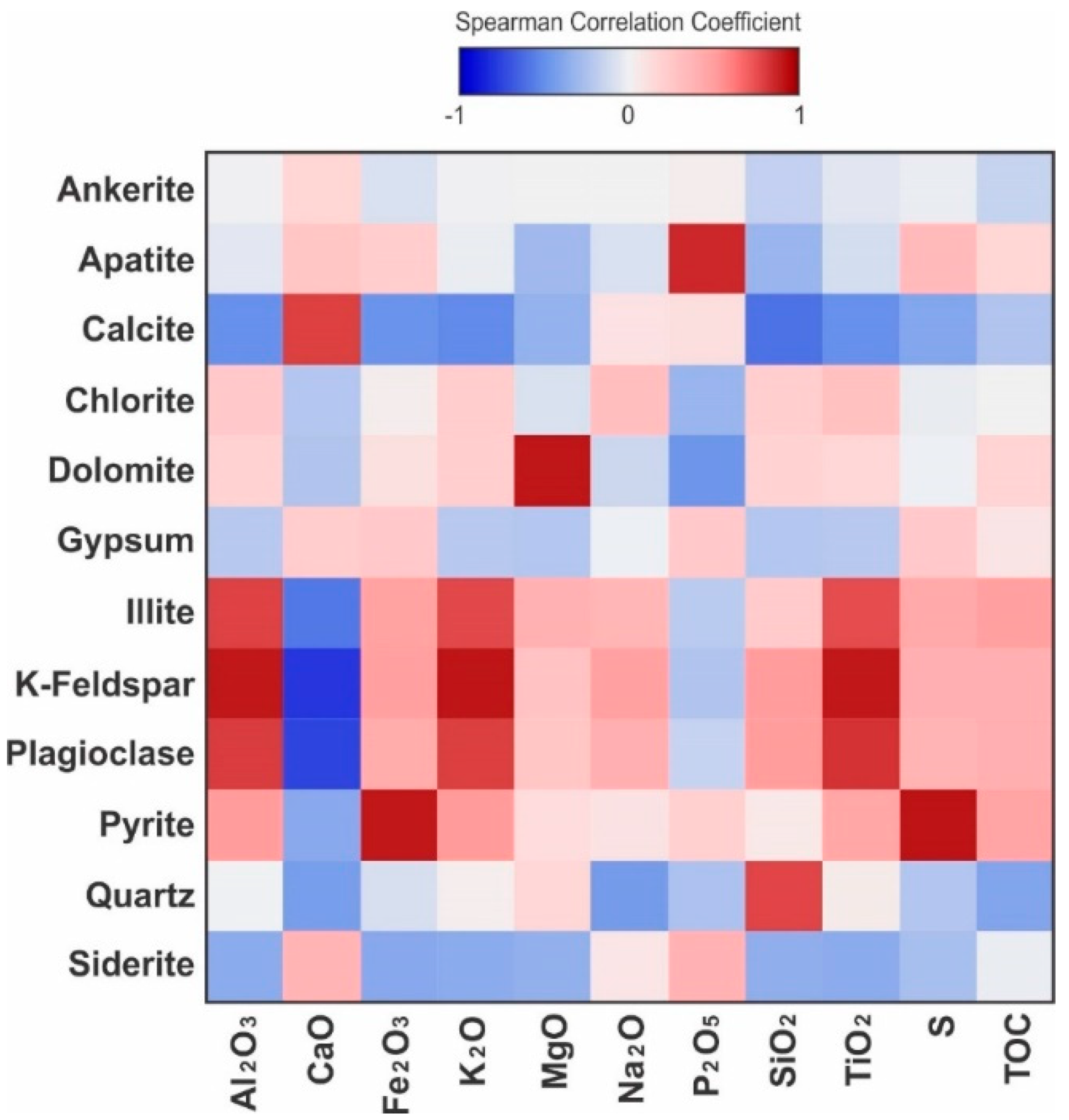
3.1. Sedimentology and Mineralogy of the Doig Phosphate Zone
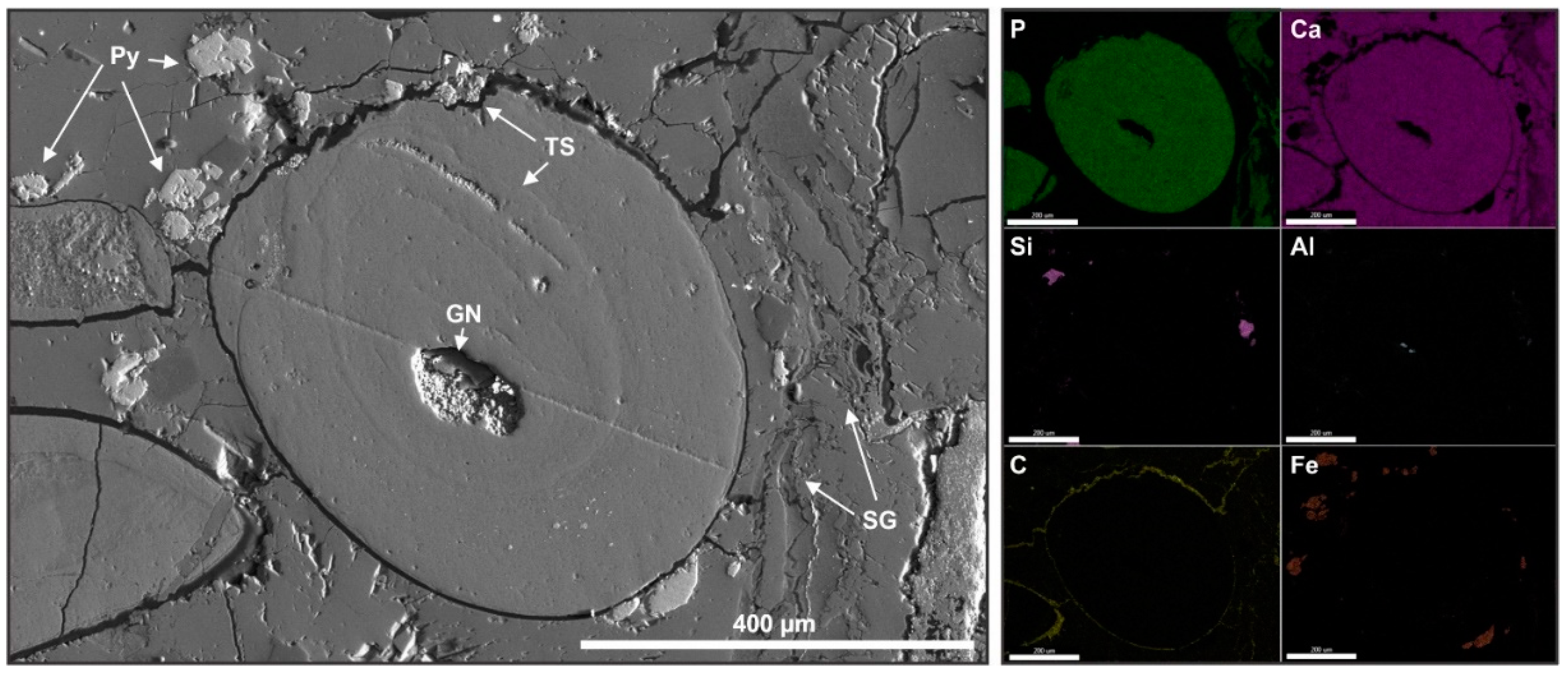
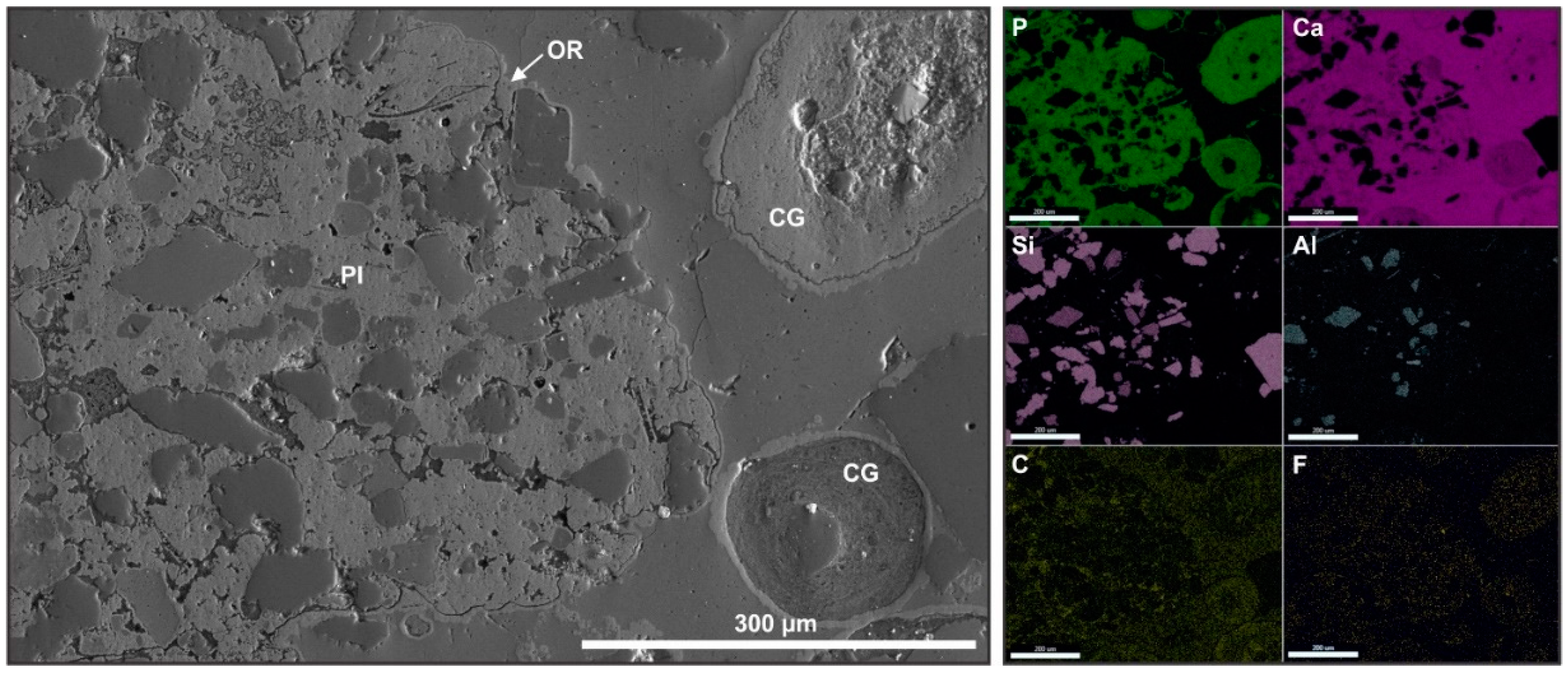
3.2. Structure, Composition, and Origin of Apatite Grains
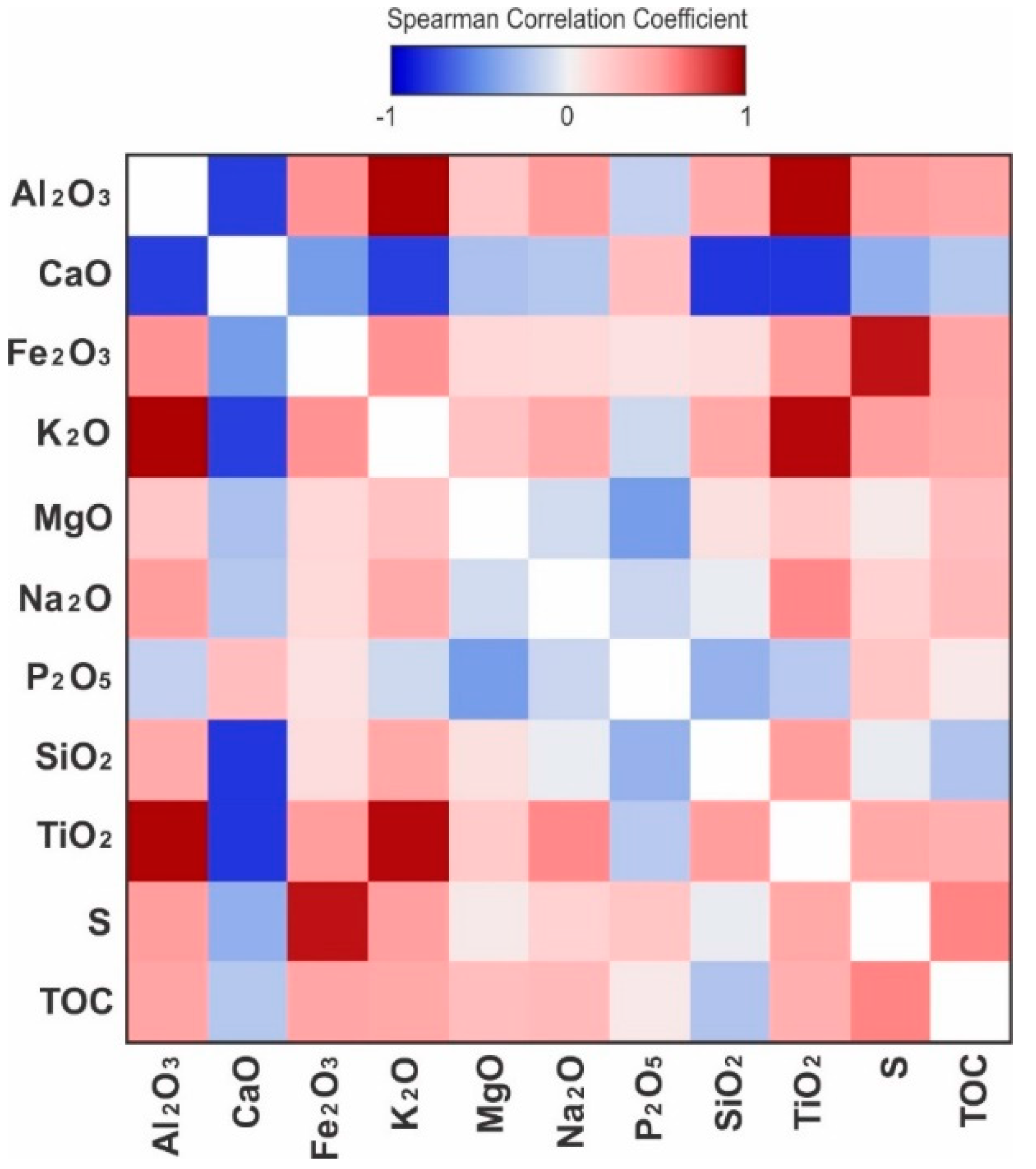
3.3. Lithogeochemistry
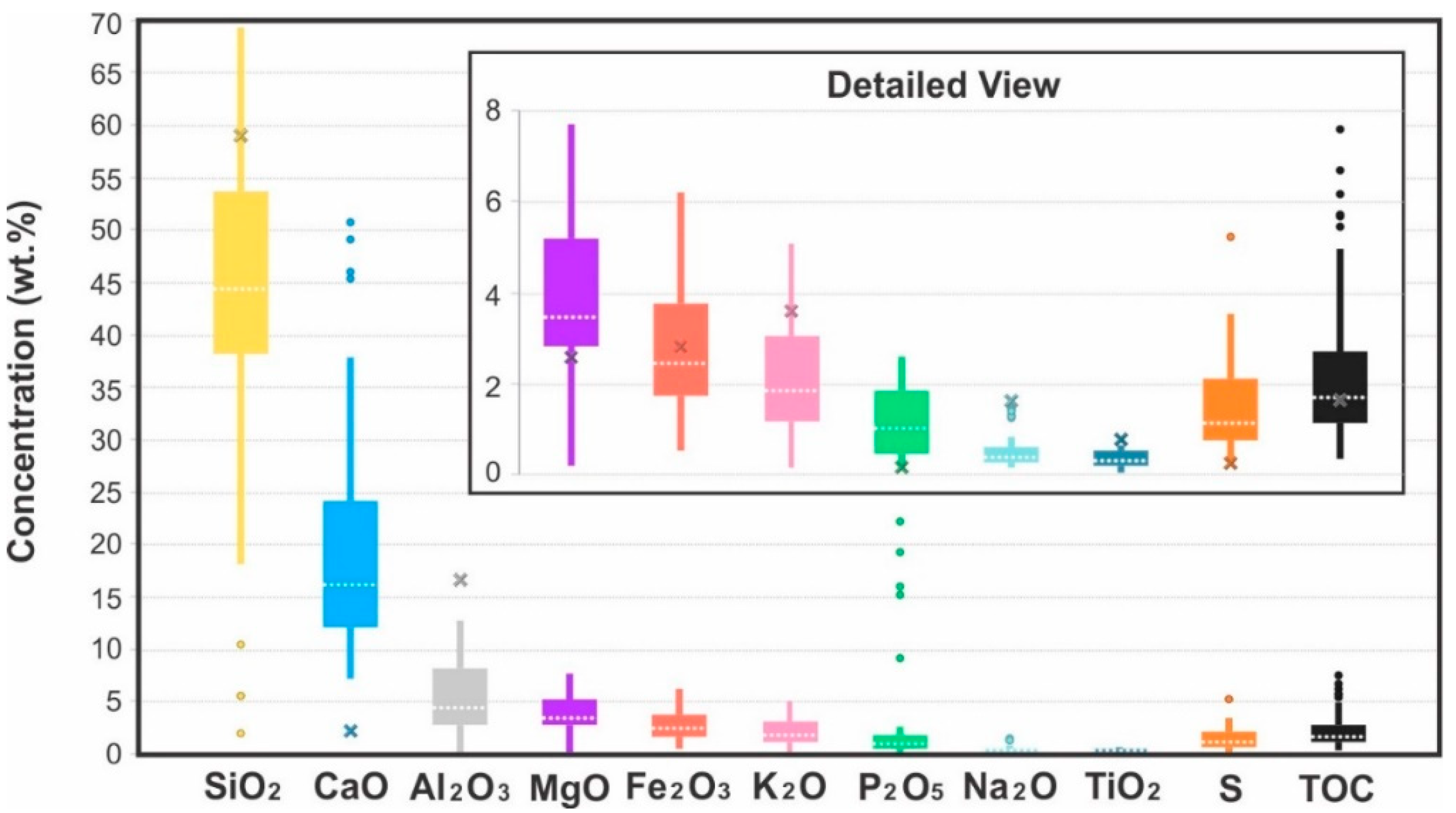
3.3.1. Major Elements
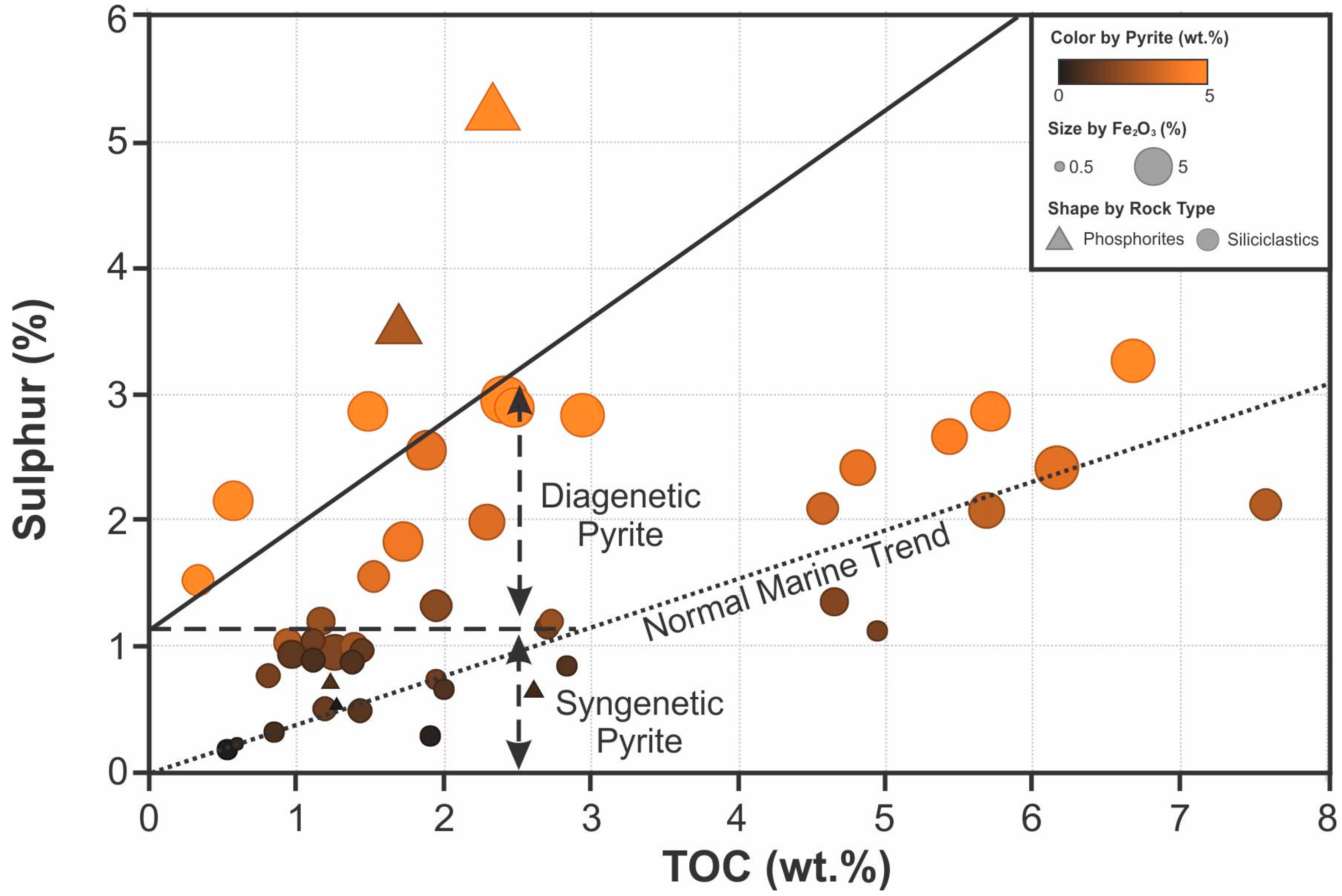
3.3.2. Sulfur and Organic Carbon
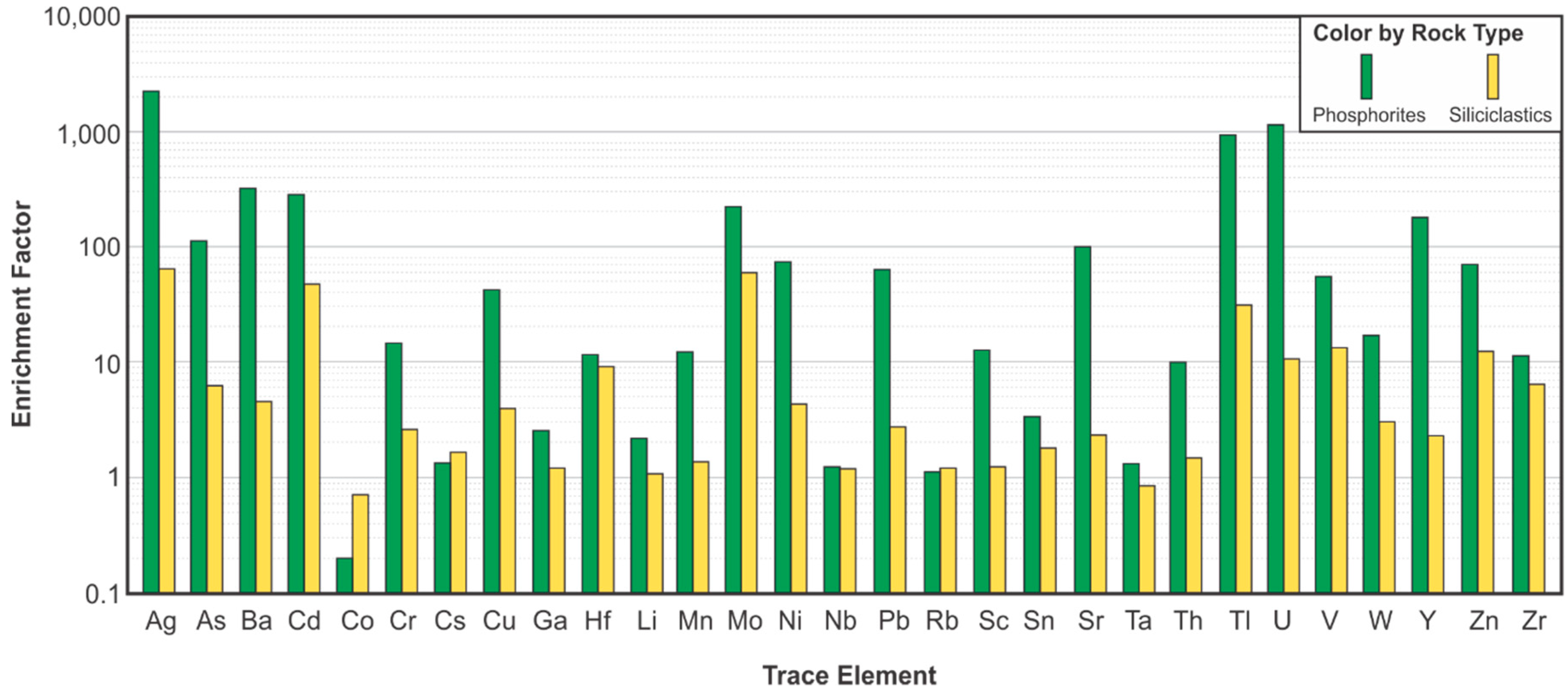
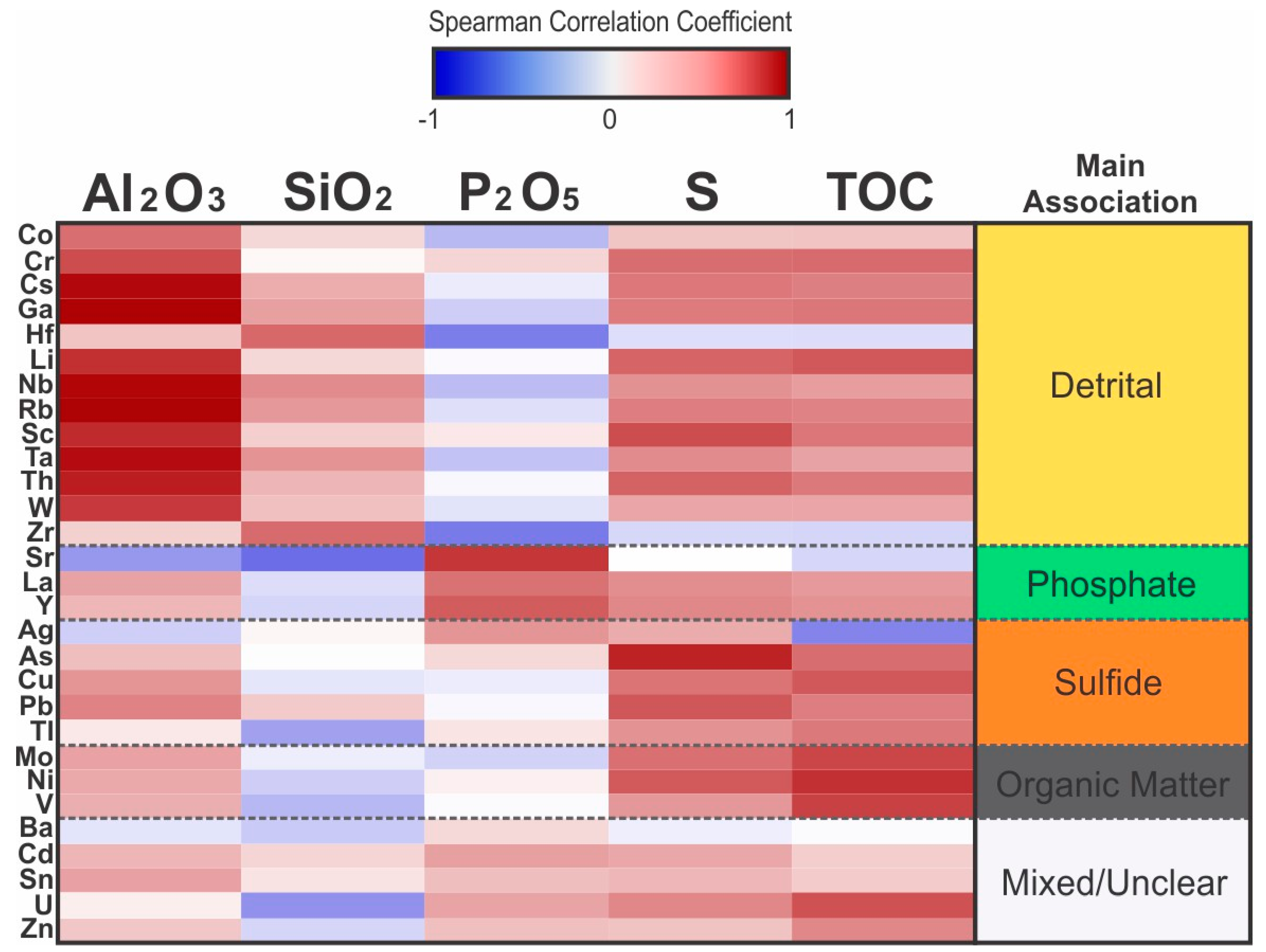
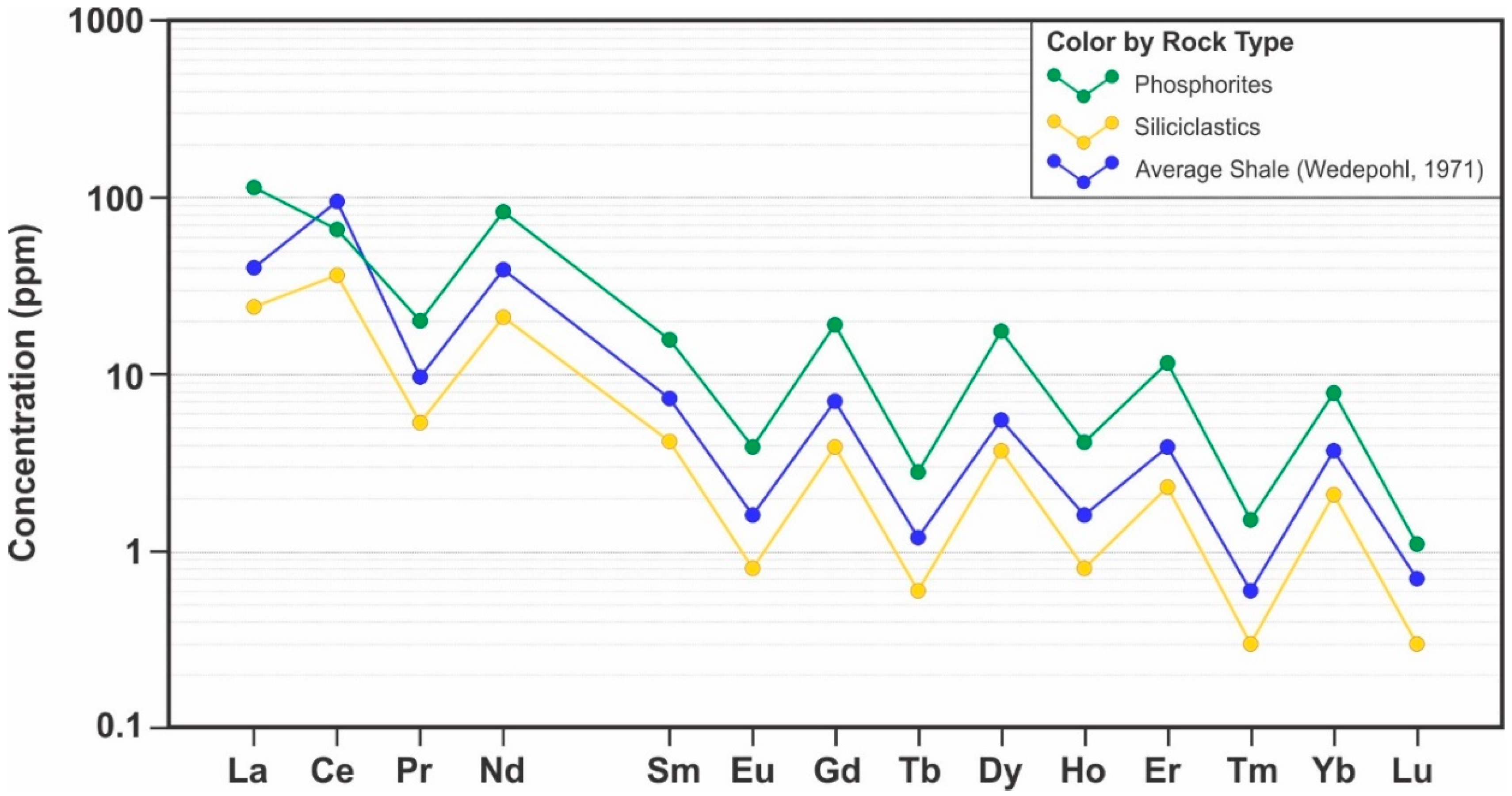
3.3.3. Trace Elements
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cook, P.J. Spatial and temporal controls on the formation of phosphate deposits—A review. In Phosphate Minerals, 1st ed.; Nriagu, J.O., Moore, P.B., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 242–274. [Google Scholar] [CrossRef]
- Trappe, J. Phanerozoic Phosphorite Depositional Systems: A Dynamic Model for a Sedimentary Resource System, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2005; p. 316. [Google Scholar] [CrossRef]
- Filippelli, G.M. Phosphate rock formation and marine phosphorus geochemistry: The deep time perspective. Chemosphere 2011, 84, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Slansky, M. Nomenclature descriptive des phosphorites. Sci. Géologiques Bull. Mémoires 1989, 42, 255–266. [Google Scholar] [CrossRef]
- Glenn, C.R.; Föllmi, K.B.; Riggs, S.R.; Baturin, G.N.; Grimm, K.A.; Trappe, J.; Abed, A.M.; Galli-Olivier, C.; Garrison, R.E.; Ilyin, A.V.; et al. Phosphorus and phosphorites: Sedimentology and environments of formation. Eclogae Geol. Helv. 1994, 87, 747–788. [Google Scholar]
- Föllmi, K.B.; Garrison, R.E.; Grimm, K.A. Stratification in phosphatic sediments: Illustrations from the Neogene of California. In Cycles and Events in Stratigraphy, 1st ed.; Einsele, G., Ricken, W., Seilacher, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 492–507. [Google Scholar]
- Creaney, S.; Allan, J. Hydrocarbon generation and migration in the Western Canada Sedimentary Basin. In Classic Petroleum Province: Geological Society Special Publications 50, 1st ed.; Brooks, J., Ed.; Geological Society of London: London, UK, 1990; pp. 189–202. [Google Scholar] [CrossRef]
- Butrenchuk, S. Phosphate Deposits in British Columbia; British Columbia Ministry of Employment and Investment, Energy and Minerals Division: West Vancouver, BC, Canada, 1996; p. 126.
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Golding, M.L.; Orchard, M.J.; Zonneveld, J.-P.; Wilson, N.S.F. Determining the age and depositional model of the Doig phosphate zone in northeastern British Columbia using conodont biostratigraphy. Bull. Can. Pet. Geol. 2015, 63, 143–170. [Google Scholar] [CrossRef]
- Golding, M.L.; Mortensen, J.K.; Ferri, F.; Zonneveld, J.-P.; Orchard, M. Determining the provenance of Triassic sedimentary rocks in northeastern British Columbia and western Alberta using detrital zircon geochronology, with implications for regional tectonics. Can. J. Earth Sci. 2016, 53, 140–155. [Google Scholar] [CrossRef]
- Crombez, V.; Baudin, F.; Rohais, S.; Riquier, L.; Euzen, T.; Pauthier, S.; Ducros, M.; Caron, B.; Vaisblat, N. Basin scale distribution of organic matter in marine fine-grained sedimentary rocks: Insight from sequence stratigraphy and multi-proxies analysis in the Montney and Doig Formations. Mar. Pet. Geol. 2017, 83, 382–401. [Google Scholar] [CrossRef] [Green Version]
- Crombez, V.; Rohais, S.; Euzen, T.; Riquier, L.; Baudin, F.; Hernandez-Bilbao, E. Trace metal element as paleoenvironmental proxies: Why should we account for sedimentation rates variations? Geology 2020, 48, 839–843. [Google Scholar] [CrossRef]
- Davies, G.R.; Hume, D. ‘Upper’ Montney-‘lower’ Doig: Quo vadis? In Proceedings of the CSPG CSEG CWLS GeoConvention, Calgary, AB, Canada, 9–11 May 2011. [Google Scholar]
- Furlong, C.M.; Gingras, M.K.; Moslow, T.F.; Zonneveld, J.-P. The Sunset Prairie Formation: Designation of a new Middle Triassic formation between the Lower Triassic Montney Formation and Middle Triassic Doig Formation in the Western Canada Sedimentary Basin, northeast British Columbia. Bull. Can. Pet. Geol. 2018, 66, 193–214. [Google Scholar]
- Gibson, D.W.; Barclay, J.E. Middle Absaroka Sequence—The Triassic stable craton. In Western Canada Sedimentary Basin—A Case History, 1st ed.; Ricketts, B.D., Ed.; Canadian Society of Petroleum Geologists: Calgary, AB, Canada, 1989; pp. 219–232. [Google Scholar]
- Edwards, D.E.; Barclay, J.E.; Gibson, D.W.; Kvill, G.E.; Halton, E. Triassic strata of the Western Canada Sedimentary Basin. In Geological Atlas of the Western Canada Sedimentary Basin, 1st ed.; Mossop, G.D., Shetsen, I., Eds.; Canadian Society of Petroleum Geologists and the Alberta Research Council: Calgary, AB, Canada, 1994; pp. 259–275. [Google Scholar]
- Davies, G.R. The Triassic of the Western Canada Sedimentary Basin: Tectonic and stratigraphic framework, paleogeography, paleoclimate and biota. Bull. Can. Pet. Geol. 1997, 45, 434–460. [Google Scholar]
- Chalmers, G.R.L.; Bustin, R.M. Geological evaluation of Halfway–Doig–Montney hybrid gas shale–tight gas reservoir, northeastern British Columbia. Mar. Pet. Geol. 2012, 38, 53–72. [Google Scholar] [CrossRef]
- Evoy, R.W.; Moslow, T.F. Lithofacies associations and depositional environments in the Middle Triassic Doig Formation, Buick Creek Field, northeastern British Columbia. Bull. Can. Pet. Geol. 1995, 43, 461–475. [Google Scholar]
- Ibrahimbas, A.; Riediger, C. Hydrocarbon Source Rock Potential as Determined by Rock-Eval 6/TOC Pyrolysis, Northeast British Columbia and Northwest Alberta. Resour. Dev. Geosci. Branch Summ. Act. 2004, 7–18. [Google Scholar]
- Faraj, B.; Harold, W.; Addison, G.; McKinstry, B.; Donaleshen, R.; Sloan, G.; Lee, J.; Anderson, T.; Leal, R.; Anderson, C.; et al. Shale Gas Potential of Selected Upper Cretaceous, Jurassic, Triassic and Devonian Shale Formations in the Western Canada Sedimentary Basin of Western Canada: Implications for Shale Gas Production, GRI-02/0233; Gas Technology Institute: Des Plains, IL, USA, 2002; p. 258. [Google Scholar]
- Riediger, C.L.; Fowler, M.G.; Brooks, P.W.; Snowdon, L.R. Triassic oils and potential Mesozoic source rocks, Peace River Arch area, Western Canada Basin. Org. Geochem. 1990, 16, 295–305. [Google Scholar] [CrossRef]
- Creaney, S.; Allan, J.; Cole, K.S.; Fowler, M.G.; Brooks, P.W.; Osadetz, K.G.; Macqueen, R.W.; Snowdon, L.R.; Riediger, C.L. Petroleum generation and migration in the Western Canada Sedimentary Basin. In Geological Atlas of the Western Canada Sedimentary Basin, 1st ed.; Mossop, G.D., Shetsen, I., Eds.; Canadian Society of Petroleum Geologists and the Alberta Research Council: Calgary, AB, Canada, 1994; pp. 455–468. [Google Scholar]
- Silva, P.L.; Bustin, R.M. Preliminary liquid hydrocarbon potential assessment of the Doig Formation, northeastern British Columbia and west-central Alberta, based on thickness, organic richness and maturity. Geosci. BC Summ. Act. 2018, 4, 39–50. [Google Scholar]
- Munson, E.O.; Chalmers, G.R.L.; Bustin, R.M.; Li, K. Utilizing smear mounts for X-ray diffraction as a fully quantitative approach in rapidly characterizing the mineralogy of shale gas reservoirs. J. Unconv. Oil Gas Resour. 2016, 14, 22–31. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Walsh, J.N. Inductively coupled plasma-atomic emission spectrometry (ICP-AES). In Modern Analytical Geochemistry: An Introduction to Quantitative Chemical Analysis Techniques for Earth, Environmental and Material Scientists, 1st ed.; Gill, R., Ed.; Routledge: London, UK, 1997; pp. 41–66. [Google Scholar]
- Rowland, A.P. Atomic absorption spectrometry and other solution methods. In Modern Analytical Geochemistry: An Introduction to Quantitative Chemical Analysis Techniques for Earth, Environmental and Material Scientists, 1st ed.; Gill, R., Ed.; Routledge: London, UK, 1997; pp. 67–86. [Google Scholar] [CrossRef]
- Espitalié, J.; Laporte, J.L.; Madec, M.; Marquis, F.; Leplat, P.; Paulet, J.; Boutefeu, A. Méthode rapide de caractérisation des roches mètres, de leur potentiel pétrolier et de leur degré d’évolution. Rev. Inst. Fr. Pet. 1977, 32, 23–42. [Google Scholar] [CrossRef]
- Peters, K.E. Guidelines for evaluating petroleum source rock using programmed pyrolysis. Am. Assoc. Pet. Geol. Bull. 1986, 70, 318–329. [Google Scholar]
- Dean, J.R. Practical Inductively Coupled Plasma Spectroscopy, 1st ed.; Wiley: Chichester, UK, 2005; p. 208. [Google Scholar]
- Huegi, T. Gesteinbildend wichtige karbonate und deren nachweis mittels farbmethoden. Schweiz. Mineral. Petrogr. Mitt. 1945, 25, 114–140. [Google Scholar]
- Warne, S.S.J. A quick field or laboratory staining scheme for the differentiation of the major carbonate minerals. J. Sediment. Res. 1962, 32, 29–38. [Google Scholar]
- Evamy, B.D. The application of a chemical staining technique to a study of dedolomitisation. Sedimentology 1963, 2, 164–170. [Google Scholar] [CrossRef]
- Dickson, J.A.D. A modified technique for carbonates in thin section. Nature 1965, 205, 587. [Google Scholar] [CrossRef]
- Bailey, E.H.; Stevens, R.E. Selective staining of K-feldspar and plagioclase on rock slabs and thin sections. Am. Mineral. 1960, 45, 1020–1025. [Google Scholar]
- Pufahl, P.K.; Grimm, K.A. Coated phosphate grains: Proxy for physical, chemical, and ecological changes in seawater. Geology 2003, 31, 801–804. [Google Scholar] [CrossRef]
- Schulz, H.N.; Schulz, H.D. Large sulfur bacteria and the formation of phosphorite. Science 2005, 307, 416–418. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 1st ed.; Elsevier Butterworth-Heinemann: Oxford, UK, 1997; p. 1600. [Google Scholar]
- Wilkin, R.T.; Barnes, H.L.; Brantley, S.L. The size distribution of framboidal pyrite in modern sediments: An indicator of redox conditions. Geochim. Cosmochim. Acta 1996, 60, 3897–3912. [Google Scholar] [CrossRef]
- Suits, N.S.; Wilkin, R.T. Pyrite formation in the water column and sediments of a meromictic lake. Geology 1998, 26, 1099–1102. [Google Scholar] [CrossRef]
- Wedepohl, K.H. Environmental influences on the chemical composition of shales and clays. In Physics and Chemistry of the Earth, 1st ed.; Ahrens, L.H., Ed.; Pergamon Press: Oxford, UK, 1971; Volume 8, pp. 307–333. [Google Scholar] [CrossRef]
- Hunt, J.M. Distribution of hydrocarbons in sedimentary rocks. Geochim. Cosmochim. Acta 1961, 22, 37–49. [Google Scholar] [CrossRef]
- Smith, J.V. Feldspar Minerals: 2 Chemical and Textural Properties, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1974; p. 692. [Google Scholar] [CrossRef]
- Morad, S.; Aldahan, A.A. Diagenetic ‘replacement’ of feldspars by titanium oxides in sandstones. Sediment. Geol. 1987, 51, 147–153. [Google Scholar] [CrossRef]
- Dolcater, D.L.; Syers, J.K.; Jackson, M.L. Titanium as free oxide and substituted forms in kaolinites and other soil minerals. Clays Clay Miner. 1970, 18, 71–79. [Google Scholar] [CrossRef]
- Gaudette, H.E.; Eades, J.L.; Grim, R.E. The nature of illite. Clays Clay Miner. 1964, 13, 33–48. [Google Scholar] [CrossRef]
- Zviagina, B.B.; Drits, V.A.; Dorzhieva, O.V. Distinguishing features and identification criteria for K-dioctahedral 1M micas (Illite-aluminoceladonite and illite-glauconite-celadonite series) from middle-infrared spectroscopy data. Minerals 2020, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, I.; Burnett, W.; Nathan, Y.; Almbaydin, F.S.M.; Attia, A.K.M.; Castro, L.N.; Flicoteaux, R.; Hilmy, M.E.; Husain, V.; Qutawnah, A.A.; et al. Phosphorite geochemistry: State-of-the-art and environmental concerns. Eclogae Geol. Helv. 1994, 87, 643–700. [Google Scholar]
- Kuehner, S.M.; Joswiak, D.J. Naturally occurring ferric iron sanidine from the Leucite Hills lamproite. Am. Mineral. 1996, 81, 229–237. [Google Scholar] [CrossRef]
- Shchipalkina, N.V.; Pekov, I.V.; Britvin, S.N.; Koshlyakova, N.N.; Vigasina, M.F.; Sidorov, E.G. A new mineral ferrisanidine, K[Fe3+Si3O8], the first natural feldspar with species-defining iron. Minerals 2019, 9, 770. [Google Scholar] [CrossRef] [Green Version]
- Raiswell, R.; Berner, R.A. Pyrite formation in euxinic and semi-euxinic sediments. Am. J. Sci. 1985, 285, 710–724. [Google Scholar] [CrossRef]
- Łukawska-Matuszewska, K.; Graca, B.; Brocławik, O.; Zalewska, T. The impact of declining oxygen conditions on pyrite accumulation in shelf sediments (Baltic Sea). Biogeochemistry 2019, 142, 209–230. [Google Scholar] [CrossRef] [Green Version]
- Canfield, D.E.; Raiswell, R.; Bottrell, S.H. The reactivity of sedimentary iron minerals towards sulfide. Am. J. Sci. 1992, 292, 659–683. [Google Scholar] [CrossRef]
- Sternbeck, J.; Sohlenius, G. Authigenic sulfide and carbonate mineral formation in Holocene sediments of the Baltic Sea. Chem. Geol. 1997, 135, 55–73. [Google Scholar] [CrossRef]
- Garrels, R.M.; Christ, C.L. Solution, Minerals, and Equilibria, 1st ed.; Harper & Row: New York, NY, USA, 1965; p. 450. [Google Scholar]
- de Baar, H.J.W.; Bacon, M.P.; Brewer, P.G.; Bruland, K.W. Rare earth elements in the Pacific and Atlantic Oceans. Geochim. Cosmochim. Acta 1985, 49, 1943–1959. [Google Scholar] [CrossRef]
- Wang, X.; Griffin, W.L.; Chen, J. Hf contents and Zr/Hf ratios in granitic zircons. Geochem. J. 2010, 44, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Fleet, M.E. Compositions of the apatite-group minerals: Substitution mechanisms and controlling factors. Rev. Mineral. Geochemistry 2019, 48, 13–49. [Google Scholar] [CrossRef]
- Hughes, J.M.; Cameron, M.; Mariano, A.N. Rare-earth-element ordering and structural variations in natural rare-earth-bearing apatites. Am. Mineral. 1991, 76, 1165–1173. [Google Scholar]
- Owens, C.L.; Nash, G.R.; Hadler, K.; Fitzpatrick, R.S.; Anderson, C.G.; Wall, F. Apatite enrichment by rare earth elements: A review of the effects of surface properties. Adv. Colloid Interface Sci. 2019, 265, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Baumer, A.; Caruba, R.; Bizzouard, H.; Peckett, A. Chlorapatite de synthésis: Substitution et inclusions de Mn, Ce, U et Th en traces. Can. Mineral. 1983, 21, 567–573. [Google Scholar]
- Gilinkskaya, L.G.; Zanin, Y.N.; Knubovets, R.G.; Korneva, T.A.; Fadeeva, V. Organophosphorus radicals in natural apatites Ca2(PO4)3(F,OH). J. Struct. Chem. 1993, 33, 859–870. [Google Scholar] [CrossRef]
- Bigi, A.; Foresti, E.; Marchetti, F.; Ripamonti, A.; Roveri, N. Barium calcium hydroxyapatite solid solutions. J. Chem. Soc. Dalt. Trans. 1984, 6, 1091–1093. [Google Scholar] [CrossRef]
- Rosenberg, P.E.; Foit, F.F. The stability of transition metal dolomites in carbonate systems: A discussion. Geochim. Cosmochim. Acta 1979, 43, 951–955. [Google Scholar] [CrossRef]
- Wen, H.; Wen, L.; Chen, H.; Zheng, R.; Dang, L.; Li, Y. Geochemical characteristics and diagenetic fluids of dolomite reservoirs in the Huanglong Formation, Eastern Sichuan Basin, China. Pet. Sci. 2014, 11, 52–66. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Diaz, M.A.; Morse, J.W. Pyritisation of trace metals in anoxic marine sediments. Geochim. Cosmochim. Acta 1992, 56, 2681–2702. [Google Scholar] [CrossRef]
- Morse, J.W.; Luther, G.W., III. Chemical influences on trace metal-sulfide interactions in anoxic sediments. Geochim. Cosmochim. Acta 1999, 63, 3373–3378. [Google Scholar] [CrossRef]
- Helz, G.R.; Miller, C.V.; Charnock, J.M.; Mosselmans, J.L.W.; Pattrick, R.A.D.; Garner, C.D.; Vaughan, D.J. Mechanisms of molybdenum removal from the sea and its concentration in black shales: EXAFS evidences. Geochim. Cosmochim. Acta 1996, 60, 3631–3642. [Google Scholar] [CrossRef]
- Tribovillard, N.; Riboulleau, A.; Lyons, T.; Baudin, F. Enhanced trapping of molybdenum by sulfurized organic matter of marine origin as recorded by various Mesozoic formations. Chem. Geol. 2004, 213, 385–401. [Google Scholar] [CrossRef]
- Emerson, S.R.; Huested, S.S. Ocean anoxia and the concentration of molybdenum and vanadium in seawater. Mar. Chem. 1991, 34, 177–196. [Google Scholar] [CrossRef]
- Morford, J.L.; Emerson, S.R.; Breckel, E.J.; Kim, S.H. Diagenesis of oxyanions (V, U, Re, and Mo) in pore waters and sediments from a continental margin. Geochim. Cosmochim. Acta 2005, 69, 5021–5032. [Google Scholar] [CrossRef]
- Sheldon, R.P. Ancient marine phosphorites. Annu. Rev. Earth Planet. Sci. 1981, 9, 251–284. [Google Scholar] [CrossRef]
- Hein, J.R.; Perkins, R.B.; McIntyre, B.R. Evolution of thought concerning the origin of the Phosphoria Formation, western US phosphate field. In Life Cycle of the Phosphoria Formation: From Deposition to the Post-Mining Environment, 1st ed.; Hein, J.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 19–42. [Google Scholar] [CrossRef]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide, 1st ed.; Cambridge University Press: Cambridge, UK, 2005; p. 1132. [Google Scholar] [CrossRef]
- Orr, W.L. Kerogen/asphaltene/sulfur relationships in sulfur-rich Monterey oils. Org. Geochem. 1986, 10, 499–516. [Google Scholar] [CrossRef]
- Martin, G. Pyrolysis of organosulphur compounds. In The Chemistry of Sulphur Containing Functional Groups, Supplement S, 1st ed.; Patai, S., Rappoport, Z., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 1993; pp. 395–437. [Google Scholar] [CrossRef]







| Unique Well Identifier | Core Interval Logged (m) | Number of Samples |
|---|---|---|
| 100/04-09-084-22W6/00 | 1631–1660.35 | 11 |
| 200/c-082-F 094-H-01/00 | 1045–1063 | 16 |
| 100/12-04-086-20W6/00 | 1593–1611.2 | 6 |
| 200/a-063-A 093-P-09/00 | 2420–2435.8 | 9 |
| 100/15-34-080-18W6/00 | 2045–2077.38 | 11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacerda Silva, P.; Bustin, R.M. Significance and Distribution of Apatite in the Triassic Doig Phosphate Zone, Western Canada Sedimentary Basin. Minerals 2020, 10, 904. https://doi.org/10.3390/min10100904
Lacerda Silva P, Bustin RM. Significance and Distribution of Apatite in the Triassic Doig Phosphate Zone, Western Canada Sedimentary Basin. Minerals. 2020; 10(10):904. https://doi.org/10.3390/min10100904
Chicago/Turabian StyleLacerda Silva, Pablo, and R. Marc Bustin. 2020. "Significance and Distribution of Apatite in the Triassic Doig Phosphate Zone, Western Canada Sedimentary Basin" Minerals 10, no. 10: 904. https://doi.org/10.3390/min10100904






