A Reconstitution Approach for Whole Rock Major and Trace Element Compositions of Granulites from the Kapuskasing Structural Zone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bulk Rock Major and Trace Element Analysis (X-Ray Fluorescence (XRF) and Solution Inductively Coupled Plasma Mass Spectrometry (ICPMS))
2.3. Scanning Electron Microscope Electron Dispersive Spectroscopy (SEM-EDS) Phase Mapping
2.4. EPMA Major Element Analysis
2.5. Laser Ablation ICPMS (LA-ICPMS) Trace Element Analysis
3. Results
4. Discussion
4.1. Evaluation of the Reconstitution Approach
4.2. Distribution of the Trace Elements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 1–64. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Fountain, D.M. Nature and composition of the continental crust: A lower crustal perspective. Rev. Geophys. 1995, 33, 267–309. [Google Scholar] [CrossRef] [Green Version]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Hacker, B.R.; Kelemen, P.B.; Behn, M.D. Continental Lower Crust. Annu. Rev. Earth Planet. Sci. 2015, 43, 167–205. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Chubakov, V.; Mantovani, F.; Rudnick, R.L.; McDonough, W.F. A reference Earth model for the heat-producing elements and associated geoneutrino flux. Geochem. Geophys. Geosystems 2013, 14, 2003–2029. [Google Scholar] [CrossRef] [Green Version]
- Bohlen, S.R.; Mezger, K. Origin of Granulite Terranes and the Formation of the Lowermost Continental Crust. Science 1989, 244, 326–329. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Presper, T. Geochemistry of Intermediate to High-Pressure Granulites. In Granulites and Crustal Evolution; Vielzeuf, D., Vidal, P., Eds.; Kluwer: Amsterdam, The Netherlands, 1990; pp. 523–550. [Google Scholar] [CrossRef]
- Chen, S.; O’Reilly, S.Y.; Zhou, X.; Griffin, W.L.; Zhang, G.; Sun, M.; Feng, J.; Zhang, M. Thermal and petrological structure of the lithosphere beneath Hannuoba, Sino-Korean Craton, China: Evidence from xenoliths. Lithos 2001, 56, 267–301. [Google Scholar] [CrossRef]
- Downes, H. The nature of the lower continental crust of Europe: Petrological and geochemical evidence from xenoliths. Phys. Earth Planet. Inter. 1993, 79, 195–218. [Google Scholar] [CrossRef]
- Griffin, W.L.; Sutherland, F.L.; Hollis, J.D. Geothermal profile and crust-mantle transition beneath east-central Queensland: Volcanology, xenolith petrology and seismic data. J. Volcanol. Geotherm. Res. 1987, 31, 177–203. [Google Scholar] [CrossRef]
- Korenaga, J. Urey ratio and the structure and evolution of Earth’s mantle. Rev. Geophys. 2008, 46. [Google Scholar] [CrossRef] [Green Version]
- Jaupart, C.; Labrosse, S.; Lucazeau, F.; Mareschal, J.-C. Temperatures, Heat, and Energy in the Mantle of the Earth. Treatise Geophys. 2015, 223–270. [Google Scholar] [CrossRef]
- Ireland, T.R.; Rudnick, R.L.; Spetsius, Z. Trace elements in diamond inclusions from eclogites reveal link to Archean granites. Earth Planet. Sci. Lett. 1994, 128, 199–213. [Google Scholar] [CrossRef]
- Jacob, D.E. Nature and origin of eclogite xenoliths from kimberlites. Lithos 2004, 77, 295–316. [Google Scholar] [CrossRef]
- Eggins, S.M.; Rudnick, R.L.; McDonough, W.F. The composition of peridotites and their minerals: A laser-ablation ICP–MS study. Earth Planet. Sci. Lett. 1998, 154, 53–71. [Google Scholar] [CrossRef]
- Barnhart, K.R.; Mahan, K.H.; Blackburn, T.J.; Bowring, S.A.; Dudas, F.O. Deep crustal xenoliths from central Montana, USA: Implications for the timing and mechanisms of high-velocity lower crust formation. Geosphere 2012, 8, 1408–1428. [Google Scholar] [CrossRef]
- Barth, M.G.; Rudnick, R.L.; Horn, I.; McDonough, W.F.; Spicuzza, M.J.; Valley, J.W.; Haggerty, S.E. Geochemistry of xenolithic eclogites from West Africa, part I: A link between low MgO eclogites and archean crust formation. Geochim. Cosmochim. Acta 2001, 65, 1499–1527. [Google Scholar] [CrossRef]
- Babechuk, M.G.; Kamber, B.S.; Greig, A.; Canil, D.; Kodolányi, J. The behaviour of tungsten during mantle melting revisited with implications for planetary differentiation time scales. Geochim. Cosmochim. Acta 2010, 74, 1448–1470. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Qian, S.-P.; Ren, Z.-Y.; Zhang, L.; Hong, L.-B.; Liu, J.-Q. Chemical and Pb isotope composition of olivine-hosted melt inclusions from the Hannuoba basalts, North China Craton: Implications for petrogenesis and mantle source. Chem. Geol. 2015, 401, 111–125. [Google Scholar] [CrossRef]
- Jones, I.; Ubide, T.; Crossingham, T.; Wilding, B.; Verdel, C. Evidence of a common source component for east Australian Cenozoic mafic magmatism. Lithos 2020, 354–355, 105254. [Google Scholar] [CrossRef]
- Boland, A.V.; Ellis, R.M.; Northey, D.J.; West, G.F.; Green, A.G.; Forsyth, D.A.; Mereu, R.F.; Meyer, R.P.; Morel-à-I’Huissier, P.; Buchbinder, G.G.R.; et al. Seismic delineation of upthrust Archaean crust in Kapuskasing, Northern Ontario. Nature 1988, 335, 711–713. [Google Scholar] [CrossRef]
- Fountain, D.M.; Salisbury, M.H.; Percival, J. Seismic structure of the continental crust based on rock velocity measurements from the Kapuskasing Uplift. J. Geophys. Res. Solid Earth 1990, 95, 1167–1186. [Google Scholar] [CrossRef]
- Percival, J.A.; West, G.F. The Kapuskasing uplift: A geological and geophysical synthesis. Can. J. Earth Sci. 1994, 31, 1256–1286. [Google Scholar] [CrossRef]
- Benn, K.; Kamber, B.S. In Situ U/Pb Granulite-Hosted Zircon Dates, Kapuskasing Structural Zone, Ontario: A Late Archean Large Igneous Province (LIP) as a Substrate for Juvenile Crust. J. Geol. 2009, 117, 519–541. [Google Scholar] [CrossRef]
- Hanes, J.A.; Archibald, D.A.; Queen, M.; Farrar, E. Constraints from 40Ar/39Ar geochronology on the tectonothermal history of the Kapuskasing uplift in the Canadian Superior Province. Can. J. Earth Sci. 1994, 31, 1146–1171. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of Reference Values for NIST SRM 610–617 Glasses Following ISO Guidelines. Geostand. Geoanalytical Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Ulrich, T.; Kamber, B.S. Natural Obsidian Glass as an External Accuracy Reference Material in Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry. Geostand. Geoanalytical Res. 2013, 37, 169–188. [Google Scholar] [CrossRef]
- Wiedenbeck, M.; Hanchar, J.M.; Peck, W.H.; Sylvester, P.; Valley, J.; Whitehouse, M.J.; Kronz, A.; Morishita, Y.; Nasdala, L.; Fiebig, J.; et al. Further Characterisation of the 91500 Zircon Crystal. Geostand. Geoanalytical Res. 2004, 28, 9–39. [Google Scholar] [CrossRef]
- Chew, D.M.; Babechuk, M.G.; Cogné, N.; Mark, C.; O’Sullivan, G.J.; Henrichs, I.A.; Doepke, D.; McKenna, C.A. (LA,Q)-ICPMS trace-element analyses of Durango and McClure Mountain apatite and implications for making natural LA-ICPMS mineral standards. Chem. Geol. 2016, 435, 35–48. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Wu, F.-Y.; Yang, J.-H.; Chew, D.M.; Xie, L.-W.; Chu, Z.-Y.; Zhang, Y.-B.; Huang, C. Sr and Nd isotopic compositions of apatite reference materials used in U–Th–Pb geochronology. Chem. Geol. 2014, 385, 35–55. [Google Scholar] [CrossRef]
- Ma, Q.; Evans, N.J.; Ling, X.-X.; Yang, J.-H.; Wu, F.-Y.; Zhao, Z.-D.; Yang, Y.-H. Natural Titanite Reference Materials for In Situ U-Pb and Sm-Nd Isotopic Measurements by LA-(MC)-ICP-MS. Geostand. Geoanalytical Res. 2019, 43, 355–384. [Google Scholar] [CrossRef]
- Spandler, C.; Hammerli, J.; Sha, P.; Hilbert-Wolf, H.; Hu, Y.; Roberts, E.; Schmitz, M. MKED1: A new titanite standard for in situ analysis of Sm–Nd isotopes and U–Pb geochronology. Chem. Geol. 2016, 425, 110–126. [Google Scholar] [CrossRef]
- Percival, J.A.; Peterman, Z.E. Rb–Sr biotite and whole-rock data from the Kapuskasing uplift and their bearing on the cooling and exhumation history. Can. J. Earth Sci. 1994, 31, 1172–1181. [Google Scholar] [CrossRef]
- Halls, H.C.; Zhang, B. Crustal uplift in the southern Superior Province, Canada, revealed by paleomagnetism. Tectonophysics 2003, 362, 123–136. [Google Scholar] [CrossRef]
- Thakurdin, Y.; Bolhar, R.; Horváth, P.; Rocholl, A.; Collerson, K. Characterization of crustal xenoliths from the Bearpaw Mountains, Montana (USA), using U-Pb geochronology, whole-rock geochemistry and thermobarometry, with implications for lower crustal processes and evolution of the Wyoming Craton. Chem. Geol. 2019, 524, 295–322. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Taylor, S.R. The composition and petrogenesis of the lower crust: A xenolith study. J. Geophys. Res. Solid Earth 1987, 92, 13981–14005. [Google Scholar] [CrossRef]
- Scherer, E.E.; Cameron, K.L.; Johnson, C.M.; Beard, B.L.; Barovich, K.M.; Collerson, K.D. Lu-Hf geochronology applied to dating Cenozoic events affecting lower crustal xenoliths from Kilbourne Hole, New Mexico. Chem. Geol. 1997, 142, 63–78. [Google Scholar] [CrossRef]
- Boyd, F.R.; McCallister, R.H. Densities of fertile and sterile garnet peridotites. Geophys. Res. Lett. 1976, 3, 509–512. [Google Scholar] [CrossRef]
- Eggins, S.M.; Woodhead, J.D.; Kinsley, L.P.J.; Mortimer, G.E.; Sylvester, P.; McCulloch, M.T.; Hergt, J.M.; Handler, M.R. A simple method for the precise determination of ≥ 40 trace elements in geological samples by ICPMS using enriched isotope internal standardisation. Chem. Geol. 1997, 134, 311–326. [Google Scholar] [CrossRef]
- McFarlane, C.R.M.; McCulloch, M.T. Coupling of in-situ Sm–Nd systematics and U–Pb dating of monazite and allanite with applications to crustal evolution studies. Chem. Geol. 2007, 245, 45–60. [Google Scholar] [CrossRef]
- Fisher, C.M.; Bauer, A.M.; Luo, Y.; Sarkar, C.; Hanchar, J.M.; Vervoort, J.D.; Tapster, S.R.; Horstwood, M.; Pearson, D.G. Laser ablation split-stream analysis of the Sm-Nd and U-Pb isotope compositions of monazite, titanite, and apatite—Improvements, potential reference materials, and application to the Archean Saglek Block gneisses. Chem. Geol. 2020, 539, 119493. [Google Scholar] [CrossRef]
- Fisher, C.M.; Bauer, A.M.; Vervoort, J.D. Disturbances in the Sm–Nd isotope system of the Acasta Gneiss Complex—Implications for the Nd isotope record of the early Earth. Earth Planet. Sci. Lett. 2020, 530, 115900. [Google Scholar] [CrossRef]
- Hammerli, J.; Kemp, A.I.S.; Whitehouse, M.J. In situ trace element and Sm-Nd isotope analysis of accessory minerals in an Eoarchean tonalitic gneiss from Greenland: Implications for Hf and Nd isotope decoupling in Earth’s ancient rocks. Chem. Geol. 2019, 524, 394–405. [Google Scholar] [CrossRef]
- Petrus, J.A.; Chew, D.M.; Leybourne, M.I.; Kamber, B.S. A new approach to laser-ablation inductively-coupled-plasma mass-spectrometry (LA-ICP-MS) using the flexible map interrogation tool ‘Monocle’. Chem. Geol. 2017, 463, 76–93. [Google Scholar] [CrossRef]
- Burger, M.; Schwarz, G.; Gundlach-Graham, A.; Käser, D.; Hattendorf, B.; Günther, D. Capabilities of laser ablation inductively coupled plasma time-of-flight mass spectrometry. J. Anal. At. Spectrom. 2017, 32, 1946–1959. [Google Scholar] [CrossRef]

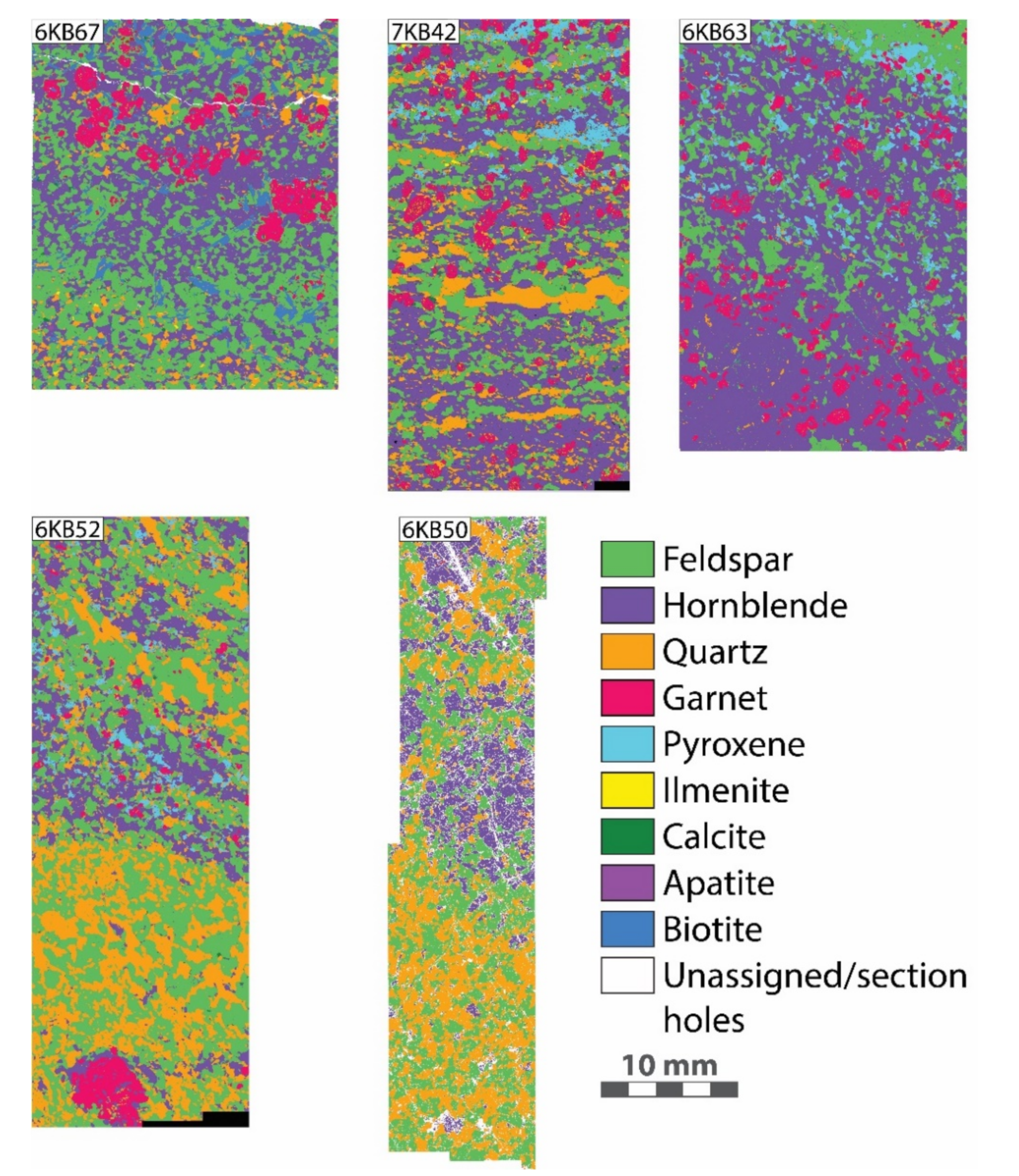
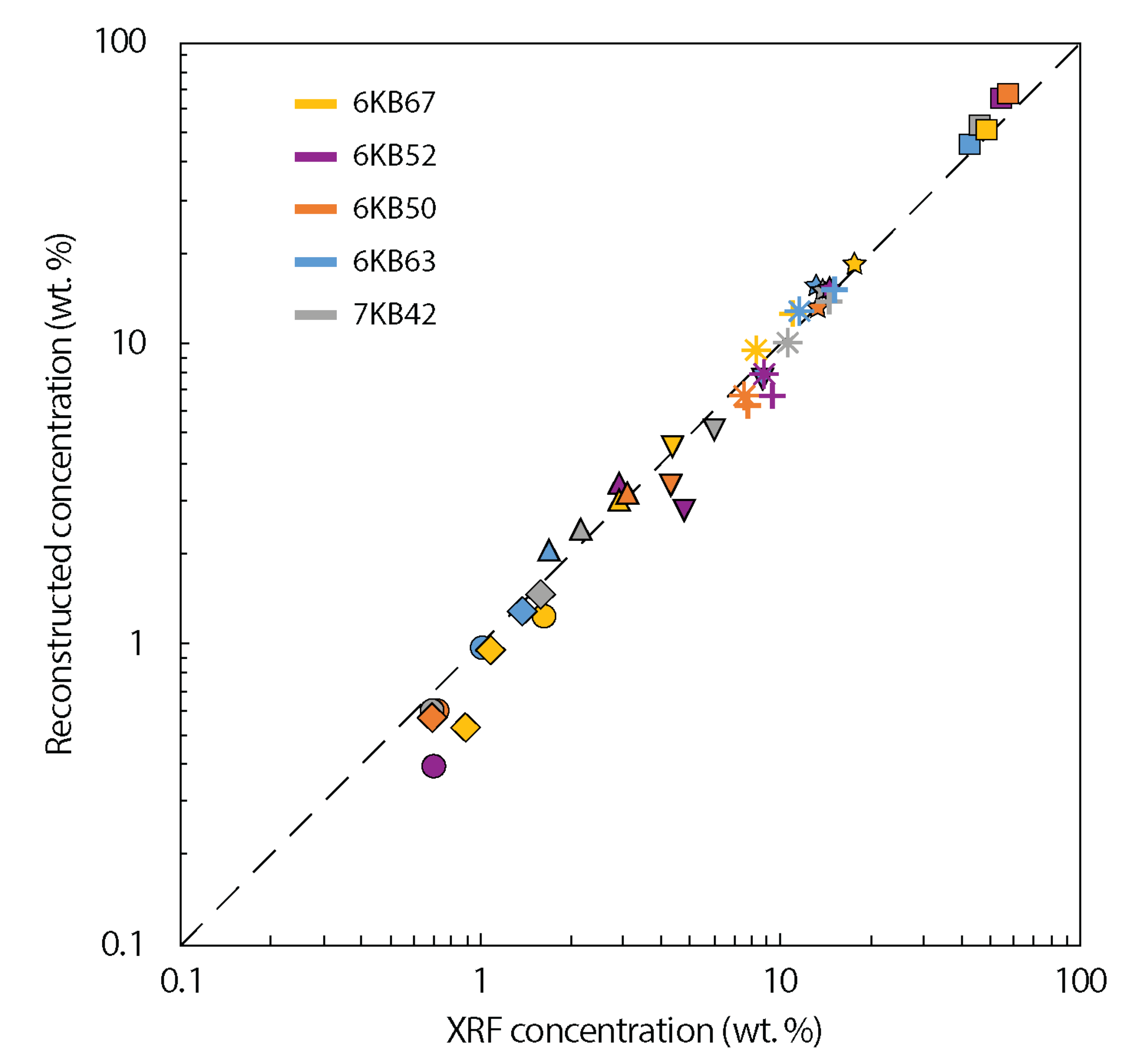
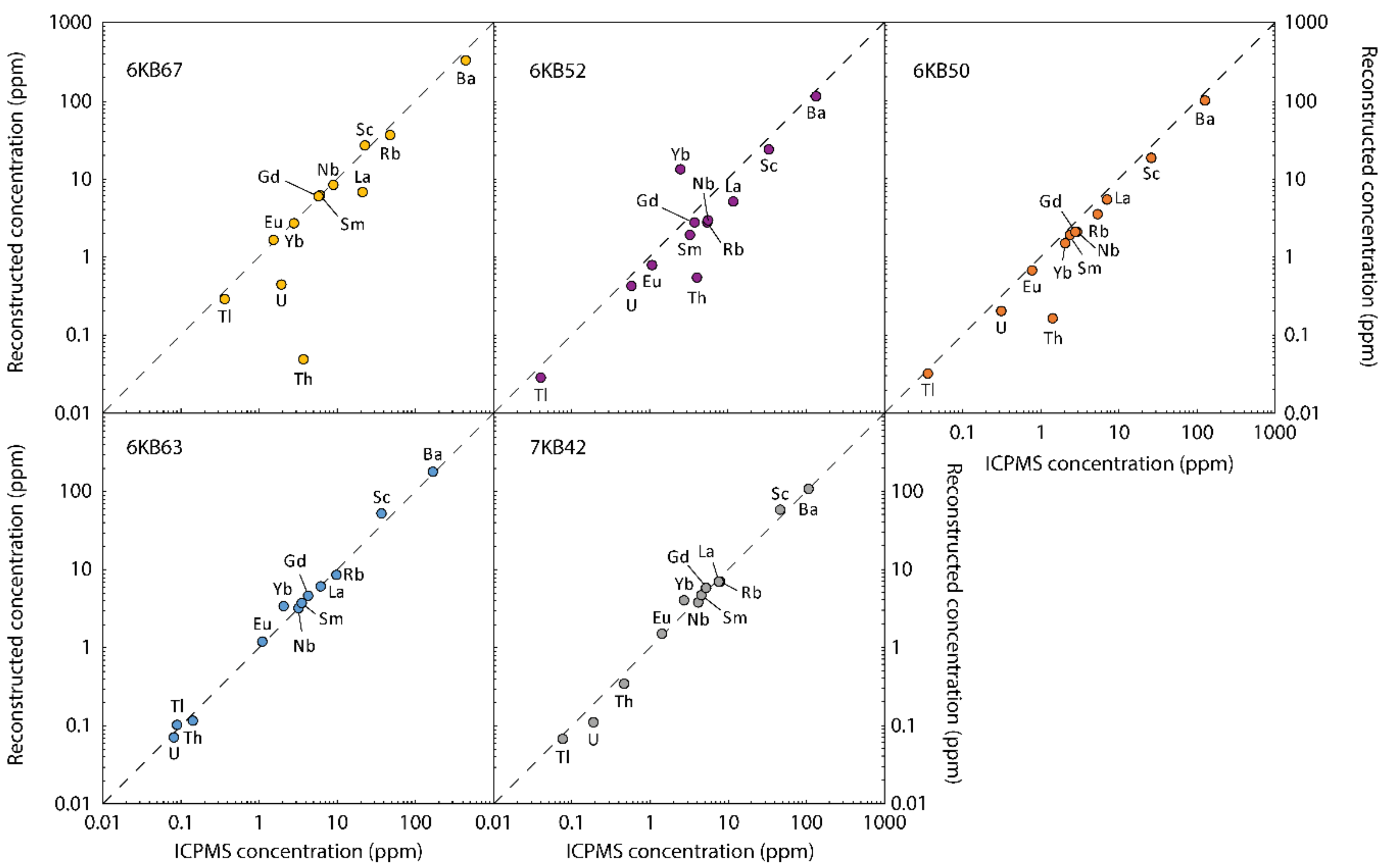
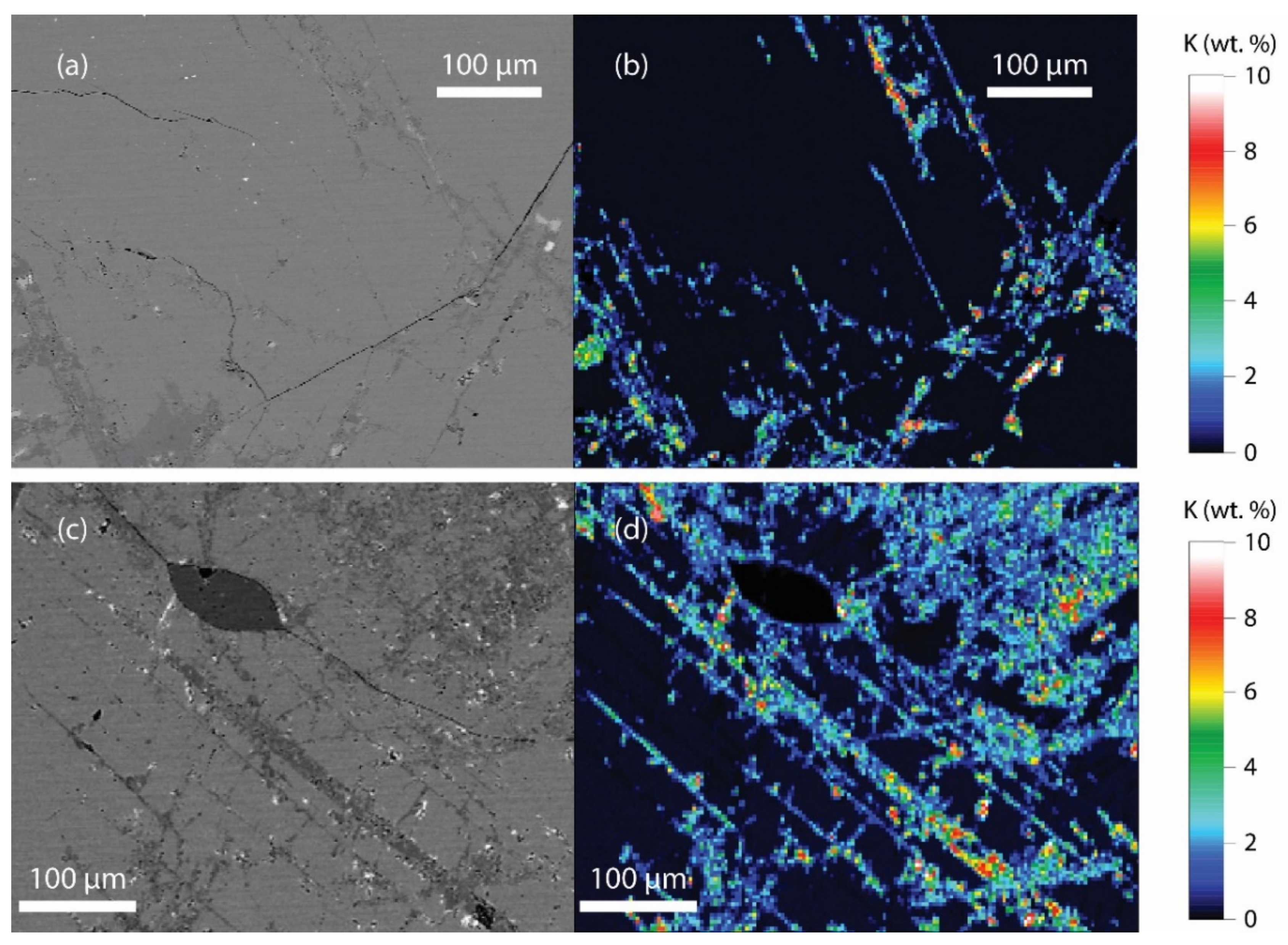
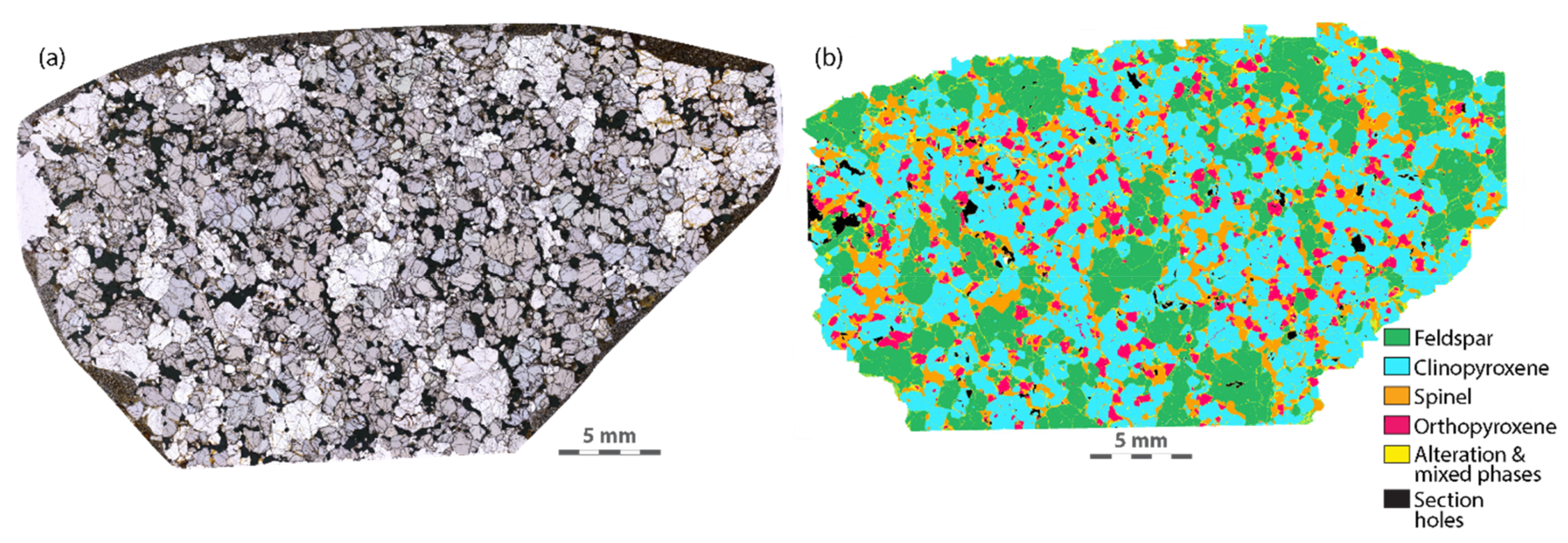
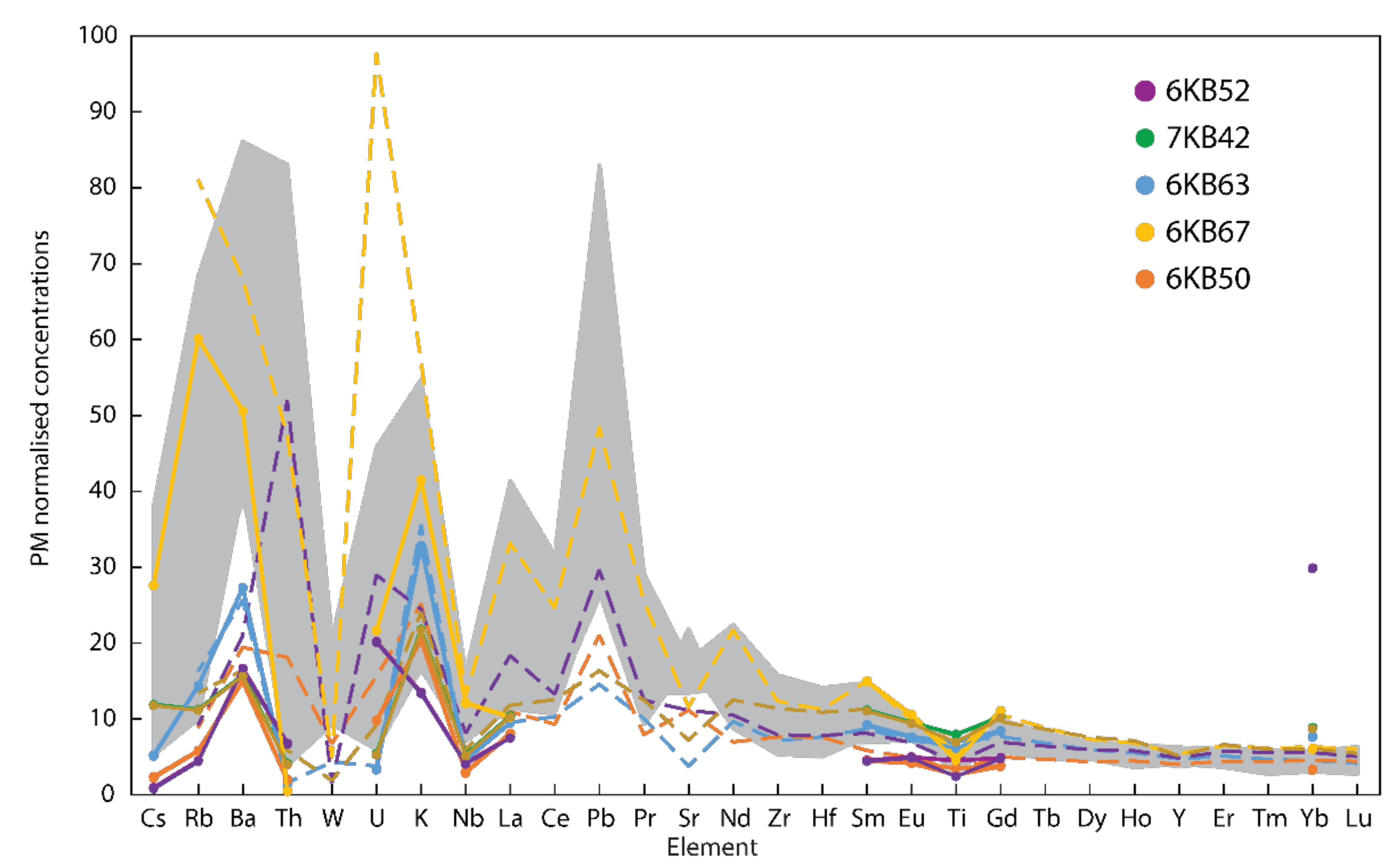
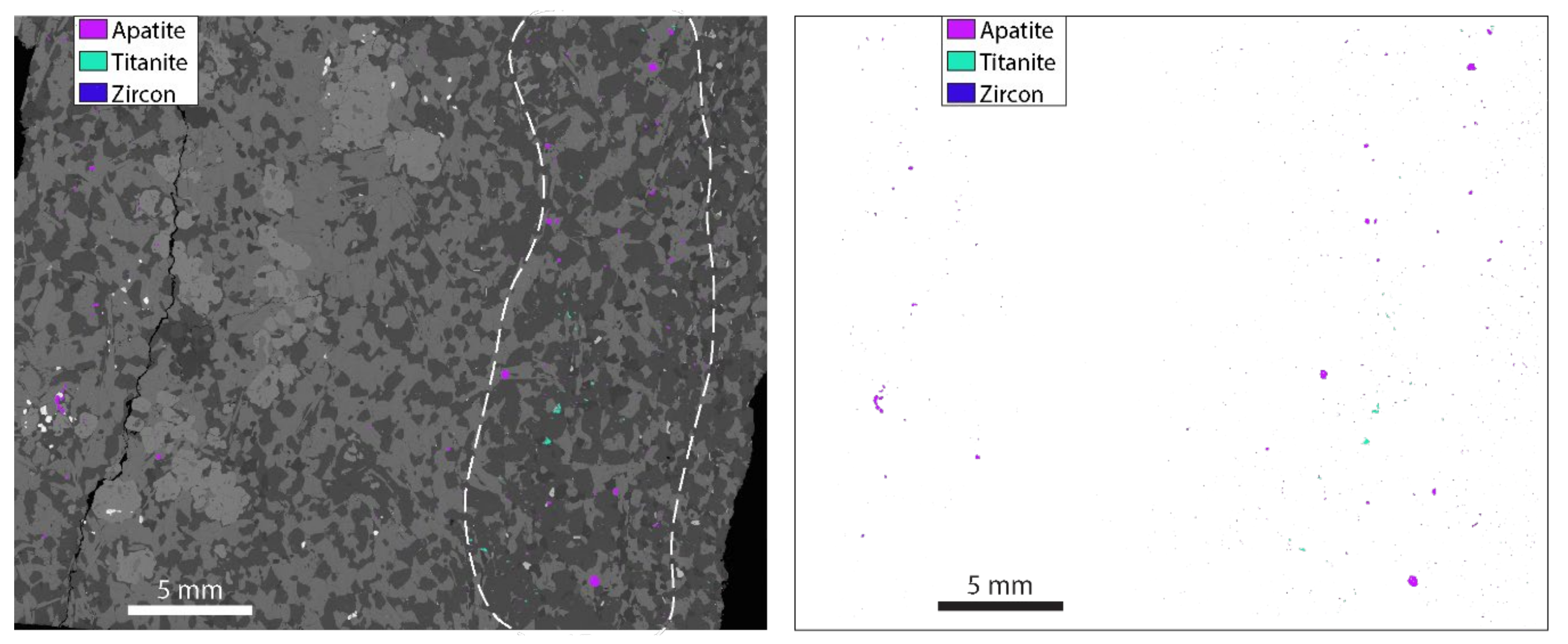
| Sample | 6KB63 | 6KB50 | 6KB52 | 7KB42 | 6KB67 |
|---|---|---|---|---|---|
| quartz | 0.88 | 33.48 | 27.00 | 14.24 | 6.18 |
| plagioclase | 20.21 | 39.05 | 43.12 | 27.90 | 39.50 |
| hornblende | 63.32 | 27.18 | 22.07 | 43.47 | 38.26 |
| garnet | 8.70 | 0.10 | 3.47 | 8.30 | 7.14 |
| clinopyroxene | 4.99 | 0.00 | 3.09 | 4.39 | 0.00 |
| biotite | 0.83 | 0.00 | 0.00 | 0.33 | 6.90 |
| K-feldspar | 0.03 | <0.01 | 0.01 | 0.00 | 0.01 |
| calcite | 0.69 | 0.02 | 0.10 | 0.55 | 1.16 |
| epidote | 0.00 | 0.00 | 0.82 | 0.00 | 0.00 |
| ilmenite | 0.05 | 0.00 | 0.00 | 0.63 | 0.17 |
| Fe-oxide | 0.00 | 0.01 | 0.09 | 0.04 | 0.00 |
| pyrite | 0.09 | 0.10 | 0.05 | 0.03 | 0.16 |
| apatite | 0.22 | 0.06 | 0.07 | 0.12 | 0.22 |
| zircon | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| titanite | <0.01 | <0.01 | 0.09 | <0.01 | 0.03 |
| chlorite | 0.00 | 0.00 | 0.00 | 0.00 | 0.28 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Sample | 6KB63 | 6KB50 | 6KB52 | 7KB42 | 6KB67 |
|---|---|---|---|---|---|
| SiO2 (wt%) | 43.39 | 58.55 | 55.17 | 46.59 | 49.41 |
| TiO2 | 1.40 | 0.70 | 0.90 | 1.60 | 1.10 |
| Al2O3 | 13.35 | 13.59 | 14.86 | 14.06 | 17.82 |
| FeO | 15.40 | 7.91 | 9.58 | 14.70 | 11.15 |
| MnO | 0.18 | 0.14 | 0.17 | 0.22 | 0.34 |
| MgO | 8.84 | 4.38 | 4.84 | 6.08 | 4.42 |
| CaO | 11.70 | 7.72 | 8.92 | 10.79 | 8.46 |
| Na2O | 1.73 | 3.15 | 2.96 | 2.20 | 2.95 |
| K2O | 1.03 | 0.73 | 0.71 | 0.70 | 1.65 |
| P2O5 | 0.12 | 0.08 | 0.11 | 0.25 | 0.27 |
| LOI | 1.06 | 2.19 | 0.90 | 1.04 | 1.05 |
| Total | 99.90 | 100.02 | 100.17 | 99.86 | 99.87 |
| Li (ppm) | 16.5 | 13.4 | 9.79 | 12.4 | 19.8 |
| Be | 0.67 | 0.75 | 1.30 | 0.68 | 1.59 |
| Sc | 37.4 | 26.3 | 34.1 | 47.1 | 23.2 |
| V | 361 | 196 | 249 | 441 | 321 |
| Cr | 190 | 112 | 217 | 210 | 20 |
| Co | 60.0 | 32.0 | 38.9 | 58.3 | 32.4 |
| Ni | 127 | 59 | 124 | 117 | 44 |
| Cu | 91.2 | 106 | 131 | 160 | 58.1 |
| Zn | 273 | 74.94 | 90.60 | 175 | 122 |
| Ga | 22.3 | 16.9 | 18.6 | 22.2 | 22.2 |
| Ge | 1.09 | 0.49 | 0.62 | 1.01 | 0.75 |
| Rb | 9.84 | 5.39 | 5.60 | 8.13 | 48.6 |
| Sr | 77.9 | 224 | 224 | 147 | 233 |
| Y | 20.9 | 17.9 | 21.6 | 22.8 | 23.8 |
| Zr | 76.36 | 81.37 | 84.58 | 121 | 131 |
| Nb | 3.28 | 2.94 | 5.65 | 4.19 | 9.01 |
| Mo | 0.48 | 0.27 | 0.39 | 0.33 | 0.49 |
| Cd | 0.09 | 0.04 | 0.05 | 0.08 | 0.09 |
| Sn | 1.73 | 1.03 | 1.48 | 1.16 | 1.23 |
| Ba | 170 | 129 | 139 | 109 | 449 |
| La | 6.29 | 7.18 | 11.96 | 7.73 | 21.53 |
| Ce | 17.44 | 15.83 | 22.41 | 21.25 | 41.52 |
| Pr | 2.59 | 2.05 | 3.20 | 3.21 | 6.51 |
| Nd | 12.25 | 8.88 | 13.31 | 15.80 | 27.27 |
| Sm | 3.59 | 2.43 | 3.35 | 4.64 | 6.14 |
| Eu | 1.11 | 0.78 | 1.08 | 1.45 | 1.54 |
| Gd | 4.26 | 2.80 | 3.85 | 5.33 | 5.80 |
| Tb | 0.69 | 0.48 | 0.65 | 0.87 | 0.89 |
| Dy | 4.11 | 3.05 | 4.11 | 5.21 | 5.05 |
| Ho | 0.85 | 0.69 | 0.89 | 1.08 | 1.04 |
| Er | 2.31 | 2.01 | 2.57 | 2.96 | 2.90 |
| Tm | 0.33 | 0.31 | 0.39 | 0.42 | 0.43 |
| Yb | 2.07 | 2.07 | 2.52 | 2.75 | 2.85 |
| Lu | 0.29 | 0.31 | 0.35 | 0.38 | 0.42 |
| Hf | 2.20 | 2.19 | 2.26 | 3.12 | 3.22 |
| Ta | 0.17 | 0.20 | 0.44 | 0.23 | 0.61 |
| W | 0.13 | 0.20 | 0.05 | 0.06 | 0.15 |
| Tl | 0.09 | 0.04 | 0.04 | 0.08 | 0.36 |
| Pb | 2.20 | 3.17 | 4.46 | 2.47 | 7.27 |
| Bi | 0.13 | 0.13 | 0.04 | 0.03 | 0.08 |
| Th | 0.14 | 1.45 | 4.13 | 0.48 | 3.77 |
| U | 0.08 | 0.32 | 0.59 | 0.19 | 1.98 |
| Sample | 6KB63 | 6KB50 | 6KB52 | 7KB42 | 6KB67 |
|---|---|---|---|---|---|
| SiO2 (wt%) | 45.04 | 66.10 | 63.43 | 52.35 | 50.07 |
| TiO2 | 1.26 | 0.56 | 0.52 | 1.42 | 0.93 |
| Al2O3 | 15.27 | 13.06 | 14.98 | 14.41 | 18.16 |
| FeO | 14.84 | 6.36 | 6.57 | 13.64 | 12.39 |
| MnO | 0.24 | 0.11 | 0.22 | 0.24 | 0.49 |
| MgO | 7.44 | 3.29 | 2.73 | 5.02 | 4.42 |
| CaO | 12.53 | 6.51 | 7.71 | 9.51 | 8.98 |
| Na2O | 2.01 | 3.12 | 3.33 | 2.49 | 2.98 |
| K2O | 0.95 | 0.59 | 0.39 | 0.63 | 1.20 |
| P2O5 | 0.09 | 0.03 | 0.04 | 0.05 | 0.10 |
| Total | 99.68 | 99.74 | 99.92 | 99.76 | 99.72 |
| Sc (ppm) | 52.7 | 18.3 | 23.0 | 55.8 | 26.6 |
| Rb | 8.63 | 3.50 | 2.72 | 6.76 | 36.07 |
| Nb | 3.21 | 2.06 | 2.88 | 3.62 | 8.33 |
| Cs | 0.11 | 0.05 | 0.02 | 0.25 | 0.58 |
| Ba | 180 | 99 | 110 | 104 | 334 |
| La | 6.18 | 5.26 | 4.94 | 6.75 | 6.68 |
| Sm | 3.76 | 1.87 | 1.85 | 4.54 | 6.09 |
| Eu | 1.18 | 0.66 | 0.77 | 1.47 | 1.63 |
| Gd | 4.61 | 2.09 | 2.67 | 5.60 | 6.02 |
| Yb | 3.40 | 1.48 | 13.18 | 3.91 | 2.70 |
| Tl | 0.10 | 0.03 | 0.03 | 0.07 | 0.28 |
| Th | 0.12 | 0.16 | 0.54 | 0.33 | 0.05 |
| U | 0.07 | 0.20 | 0.41 | 0.11 | 0.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emo, R.B.; Kamber, B.S. A Reconstitution Approach for Whole Rock Major and Trace Element Compositions of Granulites from the Kapuskasing Structural Zone. Minerals 2020, 10, 573. https://doi.org/10.3390/min10060573
Emo RB, Kamber BS. A Reconstitution Approach for Whole Rock Major and Trace Element Compositions of Granulites from the Kapuskasing Structural Zone. Minerals. 2020; 10(6):573. https://doi.org/10.3390/min10060573
Chicago/Turabian StyleEmo, Robert B., and Balz S. Kamber. 2020. "A Reconstitution Approach for Whole Rock Major and Trace Element Compositions of Granulites from the Kapuskasing Structural Zone" Minerals 10, no. 6: 573. https://doi.org/10.3390/min10060573





