Experimental Etching of Diamonds: Extrapolation to Impact Diamonds from the Popigai Crater (Russia)
Abstract
:1. Introduction
2. Materials and Methods
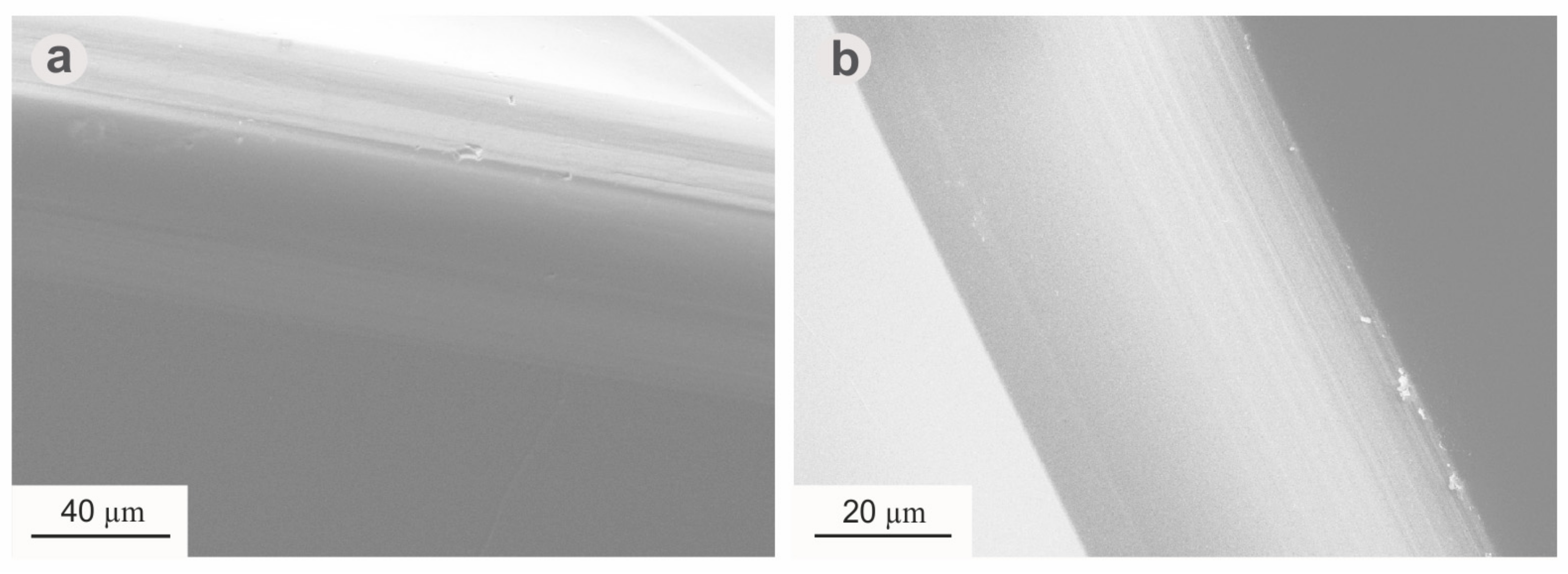
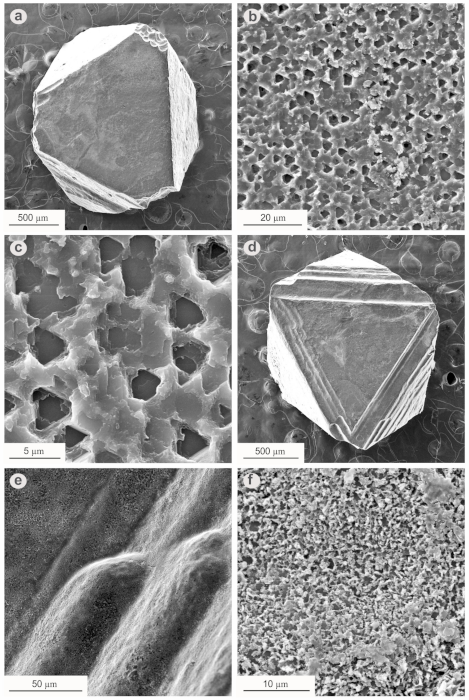
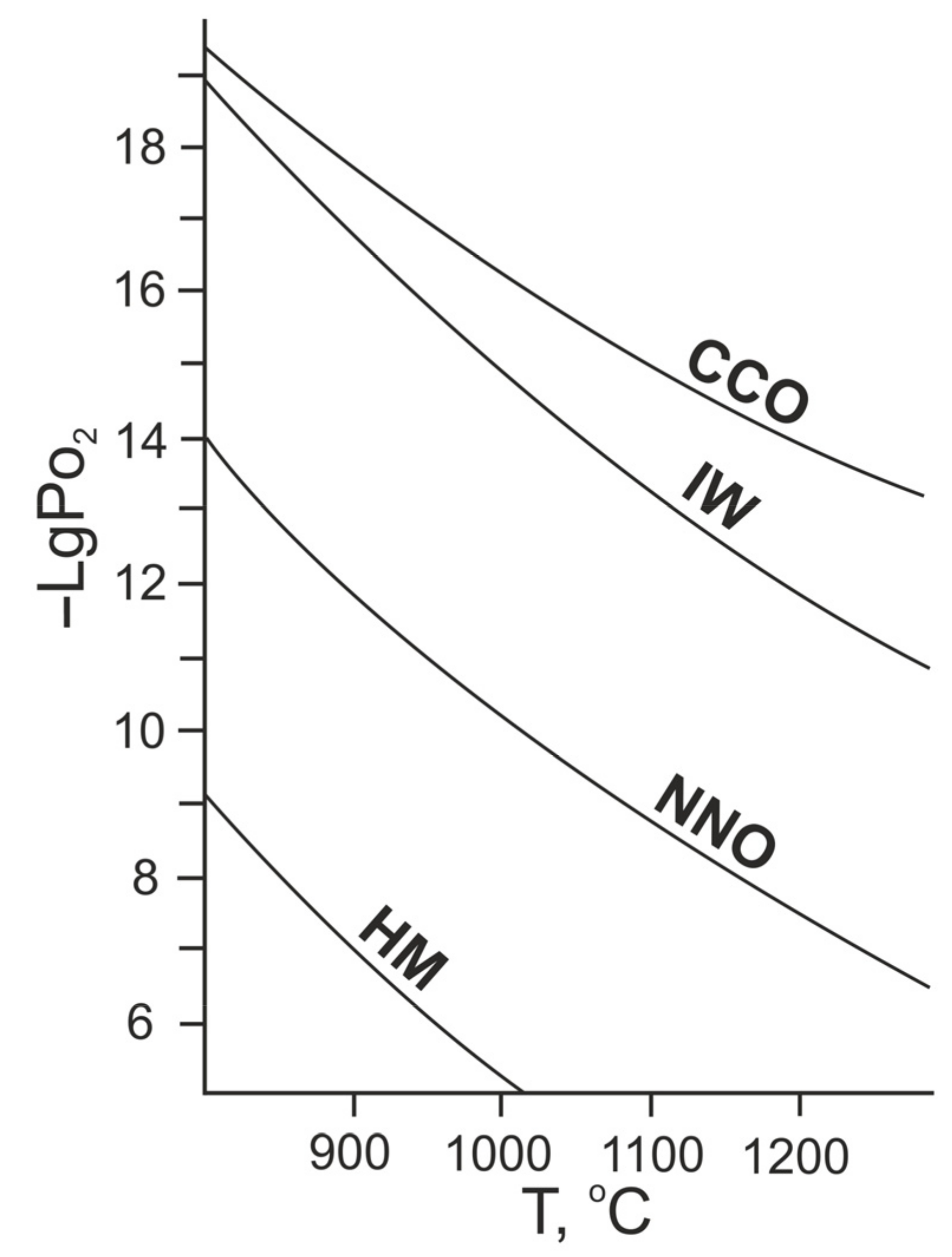
3. Results
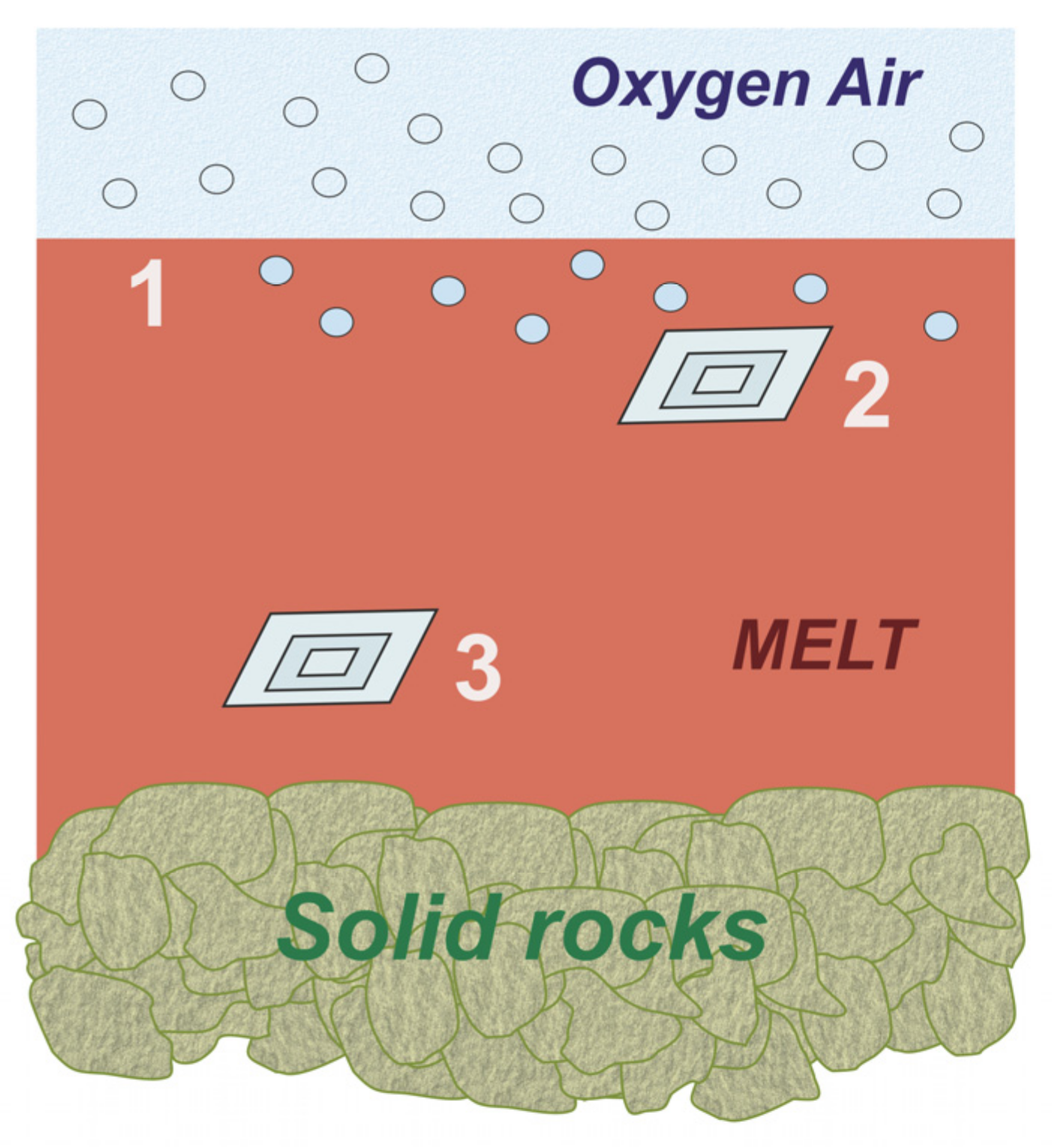
4. Discussion
5. Conclusions
- Diamond crystals inevitably experience natural oxidation and surface graphitization during pressure decrease at high residual temperatures within the molten target rocks after an impact event.
- Natural etching of diamonds in silicate melt can occur in a wide range of oxidation conditions controlled by O2 diffusion.
- Some impact diamonds located near the surface of the molten rock were oxidized at the highest rates.
- The preservation of diamonds deep within the melt was possible due to protection from oxidizing agents.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arima, M. Experimental study of growth and resorption of diamond in kimberlitic melts at high pressure and temperatures. In Proceedings of the 3rd NIRIM International Symposium on Advanced Materials (ISAM′96), Tsukuba, Japan, 4–8 March 1996; pp. 223–228. [Google Scholar]
- Sonin, V.M.; Zhimulev, E.I.; Chepurov, A.I.; Afanasiev, V.P. Morphology of diamond crystals etched in a kimberlite melt at high PT parameters. Izvestiya Vysshykh Uchebnykh Zavedeniy Geologiya Razvedka 2002, 1, 60–69. (In Russian) [Google Scholar]
- Sonin, V.M.; Zhimulev, E.I.; Tomilenko, A.A.; Chepurov, S.A.; Chepurov, A.I. Chromatographic study of diamond etching in kimberlitic melts in the context of diamond natural stability. Geol. Ore Deposit. 2004, 46, 182–190. [Google Scholar]
- Kozai, Y.; Arima, M. Experimental study on diamond dissolution in kimberlitic and lamproitic melts at 1300–1420 °C and 1 GPa with controlled oxygen partial pressure. Am. Mineral. 2005, 90, 1759–1766. [Google Scholar]
- Fedortchouk, Y.; Canil, D.; Semenets, E. Mechanism of diamond oxidation and their bearing on the fluid composition in kimberlitic magmas. Am. Mineral. 2007, 92, 1200–1212. [Google Scholar]
- Khokhryakov, A.F.; Pal’yanov, Y.N. The evolution of diamond morphology in the process of dissolution: Experimental data. Am. Mineral. 2007, 92, 909–917. [Google Scholar] [CrossRef]
- Arima, M.; Kozai, Y. Diamond dissolution rates in kimberlitic melts at 1300–1500 °C in the graphite stability field. Eur. J. Mineral. 2008, 20, 357–364. [Google Scholar] [CrossRef]
- Fedortchouk, Y. A new approach to understanding diamond surface features based on a review of experimental and natural diamond studies. Earth Sci. Rev. 2019, 193, 45–65. [Google Scholar] [CrossRef]
- Chepurov, A.I.; Sonin, V.M.; Zhimulev, E.I.; Chepurov, A.A.; Pomazansky, B.S.; Zemnukhov, A.L. Dissolution of diamond crystals in a heterogeneous (metal–sulfide–silicate) medium at 4 GPa and 1400 °C. J. Mineral. Petrol. Sci. 2018, 113, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Chepurov, A.I.; Sonin, V.M.; Zhimulev, E.I.; Chepurov, A.A. Preservation conditions of CLIPPIR diamonds in the earth’s mantle in a heterogeneous metal–sulphide–silicate medium (experimental modeling). J. Mineral. Petrol. Sci. 2020, 115, 236–246. [Google Scholar] [CrossRef]
- Sonin, V.M.; Zhimulev, E.I.; Pomazanskiy, B.S.; Zemnuhov, A.L.; Chepurov, A.A.; Afanasiev, V.P.; Chepurov, A.I. Morphological features of diamond crystals dissolved in Fe0.7S0.3 melt at 4 GPa and 1400 °C. Geol. Ore Deposit. 2018, 60, 82–92. [Google Scholar] [CrossRef]
- Sonin, V.M.; Zhimulev, E.I.; Chepurov, A.A.; Lindenblot, E.S.; Logvinova, A.M.; Shcheglov, D.V.; Pomazanskii, B.S.; Afanas′ev, V.P.; Chepurov, A.I. Dissolution of natural octahedral diamonds in an Fe–S melt at high pressure. Geol. Ore Deposit. 2020, 62, 497–507. [Google Scholar] [CrossRef]
- Gryaznov, I.A.; Zhimulev, E.I.; Sonin, V.M.; Lindenblot, E.S.; Chepurov, A.A.; Sobolev, N.V. Morphological features of diamond crystals resulting from dissolution in a Fe–Ni–S melt under high pressure. Dokl. Earth Sci. 2019, 489, 1449–1452. [Google Scholar] [CrossRef]
- Smith, E.M.; Shirey, S.B.; Nestola, F.; Bullock, E.S.; Wang, J.; Richardson, S.H.; Wang, W. Large gem diamonds from metallic liquid in Earth’s deep mantle. Science 2016, 35, 1403–1405. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.M.; Shirey, S.D.; Wang, W. The very deep origin of the World′s biggest diamond. Gems Gemol. 2017, 53, 388–403. [Google Scholar] [CrossRef]
- Grieve, R.A.F. Economic natural resource deposits at terrestrial impact structures. Mineral Deposits and Earth Evolutions. Geol. Soc. Spec. Publ. 2005, 248, 1–29. [Google Scholar]
- Vishnevsky, S.A.; Afanasiev, V.P.; Argunov, K.P.; Palchik, N.A. Impact Diamonds: Their Features, Origin and Significance; SB RAS, UIGGM: Novosibirsk, Russia, 1997; 53p. (In Russian) [Google Scholar]
- Masaitis, V.L.; Mashchak, M.S.; Raichlin, A.I.; Selivanovskaya, T.V.; Shafranovsky, G.I. Diamond-Bearing Impactites of the Popigai Astrobleme; VSEGEI: Saint Petersburg, Russia, 1998; 179p. (In Russian) [Google Scholar]
- Kvasnytsya, V.N.; Zintchouk, N.N.; Koptil, V.I. Typomorfism of Diamond Microcrystals; Nedra Business Center: Moscow, Russia, 1999; 224p. (In Russian) [Google Scholar]
- Masaitis, V.L. Impact diamonds of the Popigai Astrobleme: Main properties and practical use. Geol. Ore Deposit. 2013, 55, 607–612. [Google Scholar] [CrossRef]
- Pokhilenko, N.P. Mineral budget of the republic of Sakha (Yakutia) Arctic Zone. Econ. Russ. East 2015, 2, 12–20. (In Russian) [Google Scholar]
- Shafranovsky, G.I. Crystallomorphology of diamonds on graphite. Zap. Vsesoyuznogo Mineral. Obs. 1985, 114, 30–34. (In Russian) [Google Scholar]
- Erskine, D.J.; Nellis, W.J. Shock-induced martensitic phase transformation of oriented graphite to diamond. Nature 1991, 349, 317–319. [Google Scholar] [CrossRef]
- Kurdyumov, A.V.; Britun, V.F.; Yarosh, V.V.; Danilenko, A.I.; Zelyavskii, V.B. The influence of the shock compression conditions on the graphite transformations into lonsdaleite and diamond. J. Superhard. Mater. 2012, 34, 19–27. [Google Scholar] [CrossRef]
- Valter, A.A.; Yeremenko, G.K.; Kvasnytsya, V.N.; Polkanov, Y.A. Shock-Metamorphic Minerals of Carbon; Nauk. Dumka: Kiev, Ukraine, 1992; 170p. (In Russian) [Google Scholar]
- Kaminsky, F.V.; Blinova, T.K.; Galimov, E.M.; Klyuev, Y.A.; Kodina, L.A.; Koptil’, V.I.; Krivonos, V.S.; Frolova, L.N.; Khrenov, A.Y. Polycrystalline aggregates of diamond with lonsdaleite from placers of Yakutia. Mineral. J. 1985, 7, 27–35. (In Russian) [Google Scholar]
- Masaitis, V.L.; Shafranovsky, G.I.; Grieve, R.; Perederi, U.V.; Balmasov, E.L.; Fedorova, I.G. Diamonds from suevite in the Sudbury impact structure, Ontario, Canada. Zap. Rossiiskogo Mineral. Obs. 1997, 126, 1–6. (In Russian) [Google Scholar]
- Pratesi, G.; Guidice, A.L.; Vishnevsky, S.; Manfredotti, C.; Cipriani, C. Cathodoluminescence investigation on the Popigai, Ries, and Lappajärvi impact diamonds. Am. Mineral. 2003, 88, 1778–1787. [Google Scholar]
- Kvasnytsya, V.; Wirth, R.; Piazolo, S.; Jacob, D.T.; Trimby, P. Surface morphology and structural types of natural impact apographitic diamonds. J. Superhard Mater. 2016, 38, 71–84. [Google Scholar] [CrossRef]
- Valter, A.A.; Kvasnytsya, V.N.; Yeremenko, G.K. Structure, composition, and optical properties of diamond paramorphs after graphite. Mineral. J. 1990, 12, 3–16. (In Russian) [Google Scholar]
- Ohfuji, H.; Irifune, T.; Litasov, K.D.; Yamashita, T.; Isobe, F.; Afanasiev, V.P.; Pokhilenko, N.P. Natural occurrence of pure nano-polycrystalline diamond from impact crater. Sci. Rep. 2015, 5, 14702. [Google Scholar] [CrossRef] [PubMed]
- Yelisseyev, A.; Gromilov, S.; Afanasiev, V.; Sildos, I.; Kiisk, V. Effect of lonsdaleite on the optical properties of impact diamonds. Diam. Rel. Mater. 2020, 101, 107640. [Google Scholar] [CrossRef]
- Gromilov, S.A.; Nikolaev, R.E.; Cherepanova, S.V. Formation of compressed and mixed-layer graphite upon heating impact diamonds. J. Struct. Chem. 2018, 59, 355–364. [Google Scholar] [CrossRef]
- Kenkmann, T.; Hornemann, U.; Stöffler, D. Experimental shock synthesis of diamonds in a graphite gneiss. Meteorit. Planet. Sci. 2005, 40, 1299–1310. [Google Scholar] [CrossRef]
- Afanasiev, V.; Gromilov, S.; Sonin, V.; Zhimulev, E.; Chepurov, A. Graphite in rocks of the Popigai impact crater: Residual or retrograde? Turk. J. Earth Sci. 2019, 28, 470–477. [Google Scholar] [CrossRef]
- Masaitis, V.L.; Shafranovsky, G.I.; Fedorova, I.G. The apographitic impact diamonds from astroblemes Ries and Popigai. Zap. Rossiiskogo Mineral. Obs. 1995, 124, 12–19. (In Russian) [Google Scholar]
- Valter, A.A.; Yeremenko, G.K.; Polkanov, Y.A.; Siebenschock, M.; Schmitt, R.T.; Stöffler, D. Impact diamond from zuvites of the Ries crater (Germany): New data. Mineral. J. 1998, 20, 3–12. (In Russian) [Google Scholar]
- Vishnevsky, S.A. Astroblemes; Nonparel: Novosibirsk, Russia, 2007; 288p. (In Russian) [Google Scholar]
- Huebner, J.S. Buffering techniques for hydrostatic systems at elevated pressures. In Research Techniques for High Pressures and Temperatures; Ulmer, G.C., Ed.; Springer: Berlin/Heidelberg, Germany, 1971; pp. 123–177. [Google Scholar]
- Gramenitsky, E.N.; Kotel′nikov, A.R. Experimental Petrography; Moscow University Press: Moscow, Russia, 1984; 252p. (In Russian) [Google Scholar]
- Sonin, V.M.; Afanas′ev, V.P.; Chepurov, A.I. Stability of diamond under reducing conditions (at the level of the CCO buffer) in the presence of silicate melt. In Materials on Genetic and Experimental Mineralogy; SB RAS: Novosibirsk, Russia, 1995; Volume 11, pp. 98–110. (In Russian) [Google Scholar]
- Sonin, V.M.; Zhimulev, E.I.; Naberukhina, A.V. Peculiarities of diamond etching in basalt melt at atmospheric pressure. Izvestiya Vysshykh Uchebnykh Zavedeniy Geologiya Razvedka 2000, 1, 60–69. (In Russian) [Google Scholar]
- Sonin, V.M.; Fedorov, I.I.; Pokhilenko, L.N.; Pokhilenko, N.P. Diamond oxidation rate as related to oxygen fugacity. Geol. Ore Deposit. 2000, 42, 496–502. [Google Scholar]
- Sonin, V.M.; Zhimulev, E.I.; Fedorov, I.I.; Chepurov, A.I. Effect of oxygen fugacity on etching rate of diamond crystals in silicate melt. Geol. Ore Deposit. 2006, 48, 499–501. [Google Scholar] [CrossRef]
- Kukharenko, A.A.; Titova, V.M. New data on the solution of diamond crystals. Uch. Zap. Leningr. Gos. Univ. Ser. Geol. Nauk. 1957, 215, 108–134. (In Russian) [Google Scholar]
- Evans, T.; Sauter, D.H. Etching of diamond surfaces with gases. Phil. Mag. 1961, 6, 429–440. [Google Scholar]
- Patel, A.R.; Agarwal, M.K. Fast etching of diamond surfaces. Surf. Sci. 1965, 3, 502–505. [Google Scholar]
- Patel, A.R.; Agarwal, M.K. Studies of etch rates of different faces on natural diamond. Ind. Diamond Rev. 1966, 26, 334–336. [Google Scholar]
- Varshavsky, A.V. Thermal etching of diamond crystals in air. Zap. Rossiiskogo Mineral. Obs. 1965, 94, 471–475. (In Russian) [Google Scholar]
- Uspenskaya, K.S.; Tolmachev, Y.N.; Fedoseev, D.V. Oxidation and graphitization of diamond at low pressures. Russ. J. Phys. Chem. 1982, 56, 495–496. (In Russian) [Google Scholar]
- Bartoshinsky, Z.V.; Kvasnytsya, V.N. Crystalomorphology of Diamonds from Kimberlites; Nauk. Dumka: Kiev, Ukraine, 1991; 172p. (In Russian) [Google Scholar]
- Sonin, V.M.; Zhimulev, E.I.; Afanas′ev, V.P.; Chepurov, A.I. Genetic aspects of the diamond morphology. Geol. Ore Deposit. 2002, 44, 291–299. [Google Scholar]
- Nardov, V.M. Crystal photograms etched in a kimberlite melt. Zap. Rossiiskogo Mineral. Obs. 1958, 87, 612–614. (In Russian) [Google Scholar]
- Titova, V.M. New data on the dissolution of diamond. Zap. Rossiiskogo Mineral. Obs. 1962, 91, 334–337. (In Russian) [Google Scholar]
- Seal, M. Graphitization and plastic deformation of diamond. Nature 1958, 182, 1264–1267. [Google Scholar] [CrossRef]
- Howes, V.R. The graphitization of diamond. Proc. Phys. Soc. 1962, 80, 648–661. [Google Scholar] [CrossRef]
- Titova, V.M.; Futergendler, S.I. On orientation of graphite formed on diamond as a result of its allotropic transformation on heating. Kristallografiya 1962, 7, 925–929. (In Russian) [Google Scholar]
- Kadik, A.A.; Lukanin, O.A. Outgassing of the Upper Mantle Upon Melting; Nauka: Moscow, Russia, 1986; 97p. (In Russian) [Google Scholar]
- Frank, F.C.; Puttic, K.E. Etch pits and trigons on diamond. II. Phill. Mag. 1958, 3, 1273–1279. [Google Scholar]
- Harris, J.W.; Vance, E.R. Studies of the reaction between diamond and kimberlite. Contrib. Mineral. Petrol. 1974, 47, 237–244. [Google Scholar]
- Sonin, V.M.; Naberukhina, A.V.; Fedorova, E.N.; Turkin, A.I. Etching of diamond in a silicate melt at atmospheric pressure. Zap. Rossiiskogo Mineral. Obs. 2000, 129, 76–81. (In Russian) [Google Scholar]
- Epelbaum, M.B. Silicate Melts with Volatile Components; Nauka: Moscow, Russia, 1980; 255p. (In Russian) [Google Scholar]
- Dunn, T. Oxygen chemical diffusion in three basaltic liquids at elevated temperatures and pressures. Geochim. Cosmochim. Acta 1983, 47, 1923–1930. [Google Scholar] [CrossRef]
- Chepurov, A.I.; Fedorov, I.I.; Sonin, V.M. Experimental Modelling of the Diamond Formation Processes; SB RAS, SPC UIGGM: Novosibirsk, Russia, 1997; 196p. (In Russian) [Google Scholar]
- Osovetsky, B.M.; Naumova, O.B. The micro- and nanoforms of impact diamonds surface. Bull. Perm Univ. Geol. 2014, 2, 8–19. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Sangwal, K. Etching of Crystals: Theory, Experiment, and Application; Science: Amsterdam, The Netherlands, 1987; 497p. [Google Scholar]
- Sonin, V.M.; Gryaznov, I.A.; Chepurov, A.I.; Pokhilenko, N.P. H2O—As a possible initiator of surface graphitization of impact diamonds. Dokl. Earth Sci. 2021, 498, 388–391. [Google Scholar] [CrossRef]
- Zhdankina, O.Y.; Kulakova, I.I.; Rudenko, A.P. Oxidation of kimberlite diamonds with mixtures of carbon dioxide and water vapor. Mosc. Univ. Chem. Bull. 1985, 26, 497–501. (In Russian) [Google Scholar]
- Masaitis, V.L.; Futergendler, S.I.; Gnevushev, M.A. Diamonds in impactites of the Popigai meteoritic crater. Zap. Vsesoyuznogo Mineral. Obs. 1972, 101, 108–112. (In Russian) [Google Scholar]
- Dolgov, Y.A.; Vishnevsky, S.A.; Shugurova, N.A. Gas inclusions in impactites. In Thermobarogeochemistry and Genetic Mineralogy; Dolgov, Y.A., Ed.; IGG SB AS USSR: Novosibirsk, Russia, 1975; pp. 129–140. (In Russian) [Google Scholar]
- Vishnevsky, S.A. Water and diamond potential of the Popigai impactites-tagamites. In Proceedings of the Magmatism and Metamorphism in the Earth’s History, Book of Abstracts, XIth Petrographic Conference, Yekaterinburg, Russia, 24–28 August 2010; Volume 1, pp. 120–121. [Google Scholar]




| Etchant | T, °C | Etching Sculptures |
|---|---|---|
| Na2O–B2O3–SiO2, Ar | 1100 | None |
| Na2O–B2O3–SiO2, H2 | 1100 | None |
| Na2O–B2O3–SiO2, air | 1000 | Discoids, negative trigons |
| Na2O–SiO2, air | 1000 | Irregular etch pits and negative trigons (matted surfaces) |
| Na2O–B2O3–SiO2 and basalt (1:1), air | 1000 | Positive trigons, hexagonal etch pits |
| Basalt, air | 1130 | Positive trigons, hexagonal etch pits |
| Basalt, HM buffer | 1130 | Negative trigons, often with canted corners |
| Basalt, NNO buffer | 1130 | Negative trigons, irregular etch pits |
| Basalt, CCO buffer | 1200 | Round pits with a spherical bottom; corrosion textures with irregular nonaligned etch pits |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonin, V.; Zhimulev, E.; Chepurov, A.; Gryaznov, I.; Chepurov, A.; Afanasiev, V.; Pokhilenko, N. Experimental Etching of Diamonds: Extrapolation to Impact Diamonds from the Popigai Crater (Russia). Minerals 2021, 11, 1229. https://doi.org/10.3390/min11111229
Sonin V, Zhimulev E, Chepurov A, Gryaznov I, Chepurov A, Afanasiev V, Pokhilenko N. Experimental Etching of Diamonds: Extrapolation to Impact Diamonds from the Popigai Crater (Russia). Minerals. 2021; 11(11):1229. https://doi.org/10.3390/min11111229
Chicago/Turabian StyleSonin, Valeri, Egor Zhimulev, Aleksei Chepurov, Ivan Gryaznov, Anatoly Chepurov, Valentin Afanasiev, and Nikolai Pokhilenko. 2021. "Experimental Etching of Diamonds: Extrapolation to Impact Diamonds from the Popigai Crater (Russia)" Minerals 11, no. 11: 1229. https://doi.org/10.3390/min11111229






